Abstract
Quantifying carbon and biomass is relevant information needed in the fight against global warming. Since in Mediterranean ecosystems the agroforestry surface is very large, estimating carbon stocks and their distribution in the different compartments (above ground biomass, root, litter and soil) of these ecosystems is very important. In this work, fixed carbon was quantified in the two most abundant systems of thermophilic shrub of southwestern Iberian Peninsula: Rockroseland (Cistus ladanifer L.) and Broomland (Retama sphaerocarpa L.). Biomass was estimated through regression functions from morphology parameters. The results showed that the distribution of carbon among the compartments depends on the species. It was estimated that 34.7 Mg ha−1 of carbon retained in the C. ladanifer system, distributed among the different reservoirs. The shrub system of R. sphaerocarpa stores 24.3 Mg ha−1 of carbon. The carbon stored in biomass was differently also distributed among its components in each species. In C. ladanifer, carbon in above ground biomass is more than 85 % of the total biomass, and 15 % corresponds to carbon in root. However, in R. sphaerocarpa carbon stored in roots goes up to 48 %. These values show that it is important to quantify the carbon stored in all the components of the ecosystem (including the root), and show how important it is to maintain shrubs as reservoirs of carbon in Mediterranean agroforestry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A key climate change mitigation strategy is to reduce the atmospheric concentrations of greenhouse gases, particularly CO2, through the process of carbon sequestration (UNFCC 2007). Carbon sequestration involves primarily the uptake of atmospheric CO2 during photosynthesis and transfer of fixed C into vegetation, detritus, and soil pools for ‘‘secure’’ storage. It occurs in two major segments aboveground and belowground. The total amount sequestered in each part differs greatly depending on a number of factors including the region and the type of system (Nadelhoffer and Raich 1992). Patterns of aboveground biomass distribution in terrestrial ecosystems are reasonably well understood, whereas knowledge of belowground biomass and its distribution is still quite limited (McNaughton et al. 1998). This disparity in knowledge is essentially because of methodological difficulties associated with observing and measuring root biomass (Titlyanova et al. 1999). Knowledge of root biomass dynamics is fundamental to improve our understanding of carbon allocation and storage in terrestrial ecosystems (Cairns et al. 1997). The importance of below-ground biomass in the determination of carbon storage is emphasized by studies like the ones performed by Fan et al. 2008, which showed that, as average for all the grassland types in China, 88.1 % of their carbon density is contained in their below-ground biomass. The root-to-shoot ratio is therefore commonly used to estimate below ground living biomass. The ratios differ considerably among species and ecological conditions, supposing estimate errors in some cases.
Biomass data is a basic requirement for the estimation of carbon storage and can be acquired in different ways but field-measured data is the most basic, direct and authentic (Fang et al. 2001). In forests, shrubs are a small component of the overall carbon budget, estimated as 2 % of total forest carbon (Kimble et al. 2002). However, shrubs often dominate early successional stages of many forest types, particularly following fires, and in some cases vigorous shrub communities constitute a primary land management objective for wildlife cover and forage. Furthermore, together with deforestation and desertification, woody shrub encroachment has been pointed to as one of the major aspects of global land-cover and land-use change (Asner 2004).
Carbon storage in above and belowground biomass, in litter, and in mineral soil is one of the several important functions of shrub communities in agroforestry, native or productive (Nilsson and Wardle 2005). These communities accumulate carbon both in biomass and in soil organic matter, except if disturbance occurs. Estimating carbon stocks and their distribution in different compartments of these ecosystems is essential for understanding the amount at which carbon is allocated to labile and stable components (Sierra et al. 2007). Actually, the knowledge of shrub communities’ effects on carbon stocks is still scarce around the world. However, shrubs agroforestry may require much closer attention if set-aside (meaning conversion of agricultural land use to native vegetation) is included as a carbon sink under the international policy core acts (MacClaran et al. 2008).
Since shrubs represent an important percentage of land cover in Spain (18 million ha-36 % of the total) (San Miguel et al. 2008), they could play a significant role in the carbon balance of this country.
Two shrub species widely represented in the Mediterranean basin were selected: Retama sphaerocarpa (L.) Boiss and Cistus ladanifer (L.). In Spain, C. ladanifer communities cover 2.8 million ha and R. sphaerocarpa 1.7 million ha (Ruiz de la Torre 1990). Retama is a resprouting, N-fixing leafless shrub, which often forms monospecific patches and develops a dimorphic root system, with both shallow lateral roots and a main tap root (Haase et al. 1996). On the other hand, Cistus is a nonresprouting, drought-tolerant semideciduous shrub with a dense shallow-rooted system, mostly in the first meter, often forming extensive clusters of pure stands (Silva and Rego 2004).
The objective of this study was to quantify the biomass (above- and below-ground), litter, and soil carbon storage for these two shrub species (C. ladanifer and R. sphaercarpa), aimed at using these data to evaluate their contribution to carbon stocks.
Materials and methods
Study area
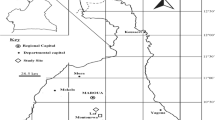
The study area is located in the county of Jerez de los Caballeros, the Extremadura region (southwest Spain) UTM, X: 670438.70, Y: 4238179.27. The topography is not very pronounced (5–30 % slopes), with elevations ranging from 150 to 745 m asl. The climate is mediterranean and the bioclimatic floor is mesomediterranean, with a mean annual temperature of 15.7 °C and mean annual precipitation exceeding 645 mm (Cabezas and Escudero 1989). The native vegetation is Quercus rotundifolia but its dramatic reduction in recent centuries led to the degradation of soils that are now basically covered by shrub species. Currently the studied shrub communities are considered stable components of the landscape (Figueiredo 1990; Devesa 1995). The two shrub species in the study area form monospecific shrub systems (Fig. 1).
Generation of estimate functions of biomass
In order to obtain estimate regression functions of biomass, 100 specimens were selected in the study area, from both C. ladanifer and R. sphaerocarpa, of different sizes. Data were obtained in May 2011, the time of the year that, for most species, corresponds to maximum production of biomass, either aboveground or belowground.
The morphological parameters measured were height, trunk diameter (at 10 cm from the ground), and maximum and minimum crown diameter. All specimens were extracted with root. Each specimen was divided in three components: roots, trunk-stems and leaves. These samples were oven-dried (72 h × 60 °C) until constant weight was reached. The dried material was then weighed on the same balance in order to determine the dry weight of each fraction.
The age of each specimen was estimated by growth ring counting following the method proposed by Yamaguchi (1991).
Regression analyses were performed to relate the morphological variables and age to the biomass of each component. The analyses involved the calculation and interpretation of linear, exponential, logarithmic, quadratic and cubic models. The best predictive models were selected based on the Sperman´s Rank determination coefficient (r 2) and its significance level (p) (Sokal and Rohlf 1995).
Samplings for the calculation of biomass and necromass
The dimensions and the number of sampling units depend on the specific and structural complexity of the study community (Pastor-López and Martín 1995). In this work, 60 plots of land were established within the distribution area of each species as follows: the study area was divided into squares of 2 × 2 km (Fig. 1). Each square with presence of C. ladanifer or R sphaerocarpa had a number assigned and 60 squares are selected at random. One plot is established in each square. These plots of land were designed with a surface extension of 5 × 5 m for C. ladanifer and 20 × 20 m for R. sphaerocarpa.
In each plot, or sampling unit, the number of individuals was counted. There was a total of 4367 individuals of C. ladanifer and 9401 of R. sphaerocarpa. Each individual of C. ladanifer had its trunk diameter measured and those of R. sphaerocarpa had their trunk diameter and height measured. These parameters were selected as they are the morphological variables that adjust best to biomass and age.
The biomass of each component was calculated for each specimen, applying the regression function selected.
Likewise, density and coverage were calculated in the 60 plots established for each species. Coverage was measured using linear transects. Starting at the center of the plot, and always in the same direction, vertical projection of the specimens was measured over a 50 m long rope. The percentage was expressed as the relation between projected length and total length considered.
The litter was defined as organic material deposited over soil. Quantification was peformed in the 60 plots established for each species. The litter included in a 50 × 50 cm wooden square, thrown randomly inside each sampling plot, was collected.
Carbon content in biomass
The C concentration in dry matter of the different biomass components (roots, stems and leaves) of each specimen was measured by dry combustion (ISO 1994) using an Elemental Leco CHNS-932 autoanalyser. Two groups were defined for each species (Young and Old) considering the maximum ages found in the study area. For C. ladanifer: Young between 0 and 5 years and Old between 30 and 55 years; And for R. sphaerocarpa: Young between 0 and 3 years and Old between 15 and 18 years. 20 specimens of each age group were analysed. Carbon per plant was calculated by multiplying biomass values by the C concentration in dry matter.
Root:shoot ratio carbon was computed, for each individual, with the amount in belowground and that of aboveground carbon.
Carbon content in soil
Assuming that the amount of carbon in soil is equivalent to the amount of organic carbon in it, the total carbon content in soil (Ct, expressed as g m−2 C) is obtained by applying the following formula (Rovira et al. 2007):
Being C the carbon concentration of fine ground, D a is the apparent density of soil (g cm−3), Gauge (cm) is the horizon thickness and V is the percentage of horizon volume occupied by stones and gravels. For the calculation of these variables, a soil sample was collected at random in each of the 60 sampling plots of each species. The method followed to collect the soil samples is known as the “known volume cylinder method” (MacDiken 1997). By this method, a metal cylinder of known volume (5 cm deep and 10 cm diameter) is pierced into the soil, and, without compressing, the volumetric sample of soil is extracted unchanged, thus obtaining the D a . The samples were air dried and sieved to determine the V fraction (>2 mm). The percentage of organic carbon in soil was calculated using the method of Walkley and Black (1934), by dichromate oxidation (Nelson and Sommers 1996).
The statistical analysis
Differences between species were tested using t-Student test.
The C concentration in dry matter was studied by two-way ANOVA, using age and components as fixed factors. Significant differences (p < 0.05) between groups were studied using Tukey´s HSD test. Homocedasticity assumption was verified in all cases using Levene´s test (p > 0.05) and variable transformation was not needed.
All statistical analyses were made using statistical program 19.0 version of the SPSS software for Windows.
Results
Biomass and age estimation equations
The best parameter to estimate the biomass of C. ladanifer is trunk diameter, and for R. sphaerocarpa the best parameter is height. The parameter that best estimates the age of both species is trunk diameter. The functions selected (Table 1) show determination coefficients (r 2) that explain more than 85 % of the variability of the data in the case of the root biomass of C. ladanifer, around 90 % for age and above 95 % for the rest of the parameters, in both species.
C concentration in biomass
The mean value of the percentage of carbon for C. ladanifer is 43.8 and 46.27 % for R. sphaerocarpa (Table 2). In all components, the amount of carbon is greater in R. sphaerocarpa than in C. ladanifer. There are significant differences among the percentages of carbon of the different components. The lowest percentages of carbon are found in the roots of both species: 40.02 % in C. ladanifer and 43.39 % in R. sphaerocarpa. There are no significant differences between the carbon percentages of stems and leaves of C. ladanifer, although in R. sphaerocarpa there are. Broom leaves contain 3 % more carbon than stems.
The statistical analysis in both species did not show significant differences in carbon percentage between the age groups studied.
Carbon stored
The shrubland of C. ladanifer in the study area had a density of 2.23 ± 0.95 individuals m−2 and 85 % coverage. R. sphaerocarpa had a density of 0.17 individuals m−2 and 32 % coverage.
The carbon stored in the C. ladanifer shrubland biomass is 9.46 Mg ha−1 of carbon (C). This value is more than 4 times greater than the 2.44 Mg ha−1 of C stored in the shrubland of R. sphaerocarpa (Table 3). The distribution of carbon among the different biomass components is different in the two species (Fig. 2). The carbon stored in the roots of C. ladanifer and R. sphaerocarpa corresponds to 14.9 and 48 % of the biomass carbon, respectively. The carbon stored in the leaves of C. ladanifer corresponds to 9.1 % of the biomass carbon, and 13.4 % in the case of R. sphaerocarpa. And finally, the carbon stored in the stems of C. ladanifer is 76 % of the biomass carbon, and 38.5 % in the case of R. sphaerocarpa. In all cases, there are significant differences in the amount of carbon stored in the different components of the biomass of both species (Table 3).
There are also significant differences between the amount of carbon stored in the litter of rockroseland and broomlands. The carbon stored in the litter of rockroseland is 2.07 Mg ha−1 of C, three times greater than the 0.69 Mg ha−1 of C stored in broomlands (Table 4).
The carbon stored in the soil of Cistus is 23.14 Mg ha−1 of C, slightly higher than the 21.14 Mg ha−1 of C accumulated in broomland soil. There are no significant differences between these values (Table 4).
Adding up all the components analysed, the total carbon present in rockrose is 34.67 and 24.27 Mg ha−1 of C in broomlands. The percentage analysis shows the great importance of the soil compartment in both shrubland formations. The carbon stored in the first 5 cm of soil in rockrose and broomlands is 66.7 and 87.1 %, respectively, of the total carbon stored in each system (Fig. 3). Likewise, 27.3 and 10 % correspond to the carbon stored in the biomass of each species. Necromass provides the lowest percentage with 6 and 2.8 % in rockrose and broomlands, respectively. It is important to highlight that the root, as a biomass component, represents 4.1 and 4.8 % of all the carbon stored in the shrubland systems of C. ladanifer and R. sphaerocarpa, respectively (Fig. 3).
Root:shoot ratio
The root: shoot ratio obtained in both species is below 1 in all ages studied. Upon performance of a dynamic or temporal analysis of this index, clear differences appear between the Root: shoot ratios of both species. In C. ladanifer, this index is constant and approximately around 0.2 in all ages (Fig. 4). On the contrary, in R. sphaerocarpa it varies and increases with increasing specimen age (from 0.4 to 0.97). In broomland, the young specimens show greater amount of carbon aboveground than belowground, whereas those of greater age show almost the same amount of carbon in roots as in aboveground components.
Discussion
In order to know the amount of carbon fixed in forests, it must always be taken into account that the shrubland provides a relevant contribution to the maintenance of the atmospheric gas equilibrium, especially in Mediterranean ecosystems where the distribution of shrubland is high (Kowalski et al. 2004). When conducting biomass studies to quantify carbon storage, since the increase of species depends on the environmental conditions, it is a mistake to use allometric functions that were already established in other studies, because incorrect estimates could be obtained (Buras et al. 2012). This is shown in the studies of Patón et al. (1998), Simões (2002), Navarro and Blanco (2006) and Ruíz-Peinado et al. (2013), in which the functions provided for C. ladanifer and R. sphaerocarpa are different among the study areas. In this study, trunk diameter and height are the parameters that show the best adjustment to determine the biomass of the individual of C. ladanifer and R. sphaerocarpa, respectively. For the present study, it is considered that the methodology proposed for the estimation of biomass by trunk diameter or height is valid, as confirmed by their determination coefficients. However, these data cannot be generalised for all the distribution areas of both species; it is recommended to perform the appropriate samplings in each case in order to obtain the functions that adjust best.
Despite the variations in the carbon percentage of dry matter among different woody species (Ragland et al. 1991; Lamlom and Savidge 2003), the IPCC recommends to use 50 % to calculate the amount of carbon present in the biomass of those species whose actual percentage is not available. In studies performed by authors like Ortiz (1997), Lopera and Gutiérrez (2000) and Gayoso and Schlegel (2001) it is stated that the percentage of carbon varies among species and components analysed, obtaining values that range between 41 and 53 %. Following the recommendations of the IPCC involves, inevitably, some estimate error. Therefore, in this study the actual percentage of carbon was quantified in the two species selected at different growth states and in each of the biomass components (roots, stems and leaves). The mean value of the carbon percentage for C. ladanifer is 43.8 and 46.27 % for R. sphaerocarpa. The amount of carbon does not depend on age, but on the component analysed. Thus, roots show lower amount of carbon than the rest of components, with a maximum of 6 % less than leaves in both species. This tendency was observed in studies conducted by Fonseca et al. 2012, in which the carbon quantified in shrubland species like C. ladanifer, Cytisus multiflorus and Erica australis show, on average, a difference of 3 % between aboveground and root.
Regarding the percentage of carbon calculated in both species, the amount of carbon accumulated in the biomass in C. ladanifer is 9.46 Mg ha−1. The aboveground side represents 8.05 Mg ha−1and roots 1.41 Mg ha−1. These amounts are significantly greater than those of R. sphaerocarpa, whose amount of total accumulated carbon is 2.44 Mg ha−1 (aboveground 1.27 Mg ha−1and root 1.17 Mg ha−1). These differences were also detected in studies conducted by Fonseca et al. 2012, in typical Mediterranean shrubland species. The amount of carbon accumulated aboveground ranged between 4.8 (C. multiflorus) and 6.9 Mg ha−1 of C (E. australis), and the amounts of belowground carbon ranged between 2.3 and 17.1 Mg ha−1 for the same species.
The carbon stock per ha in R. sphaerocarpa is significantly lower than that of C. ladanifer. This is because the density of R. sphaerocarpa in the study area, 0.17 individuals m−2, is much lower than the density of C. ladanifer, 2.33 individuals m−2. This could be explained by the ecology and growth of each species. Brooms are a multiple-trunk species capable of re-sprouting after losing its aboveground biomass. Besides, the wide and deep set of roots of R. sphaerocarpa, (Haase et al. 1996), prevents the individuals from growing in nearby areas; in turn, they tend to grow at a certain distance, spreading all over the territory (Rolo and Moreno 2012). On the contrary, rockrose is a single-trunk species, non re-sprouting; its root system is not as complex as that of brooms and due to its opportunistic and colonising nature, individuals are not found scattered in space but they tend to grow very close from one another, which is fostered by the high production of seeds (Metcalfe and Kunin 2006). The density obtained in this study for C. ladanifer, 2.33 individuals m−2, is consistent with that reported for the same species in a study conducted in Montes de Propio– Jerez de la Frontera (Cádiz), in which case it was 2.30 individuals m−2 (Navarro and Blanco 2006). The difference in density between these two systems is also evident in coverage, being 85 % for C. ladanifer and 32 % for R. sphaerocarpa. These very low values for brooms are in line with the studies conducted by Rolo and Moreno (2012), in which in plots encroached with R. sphaerocarpa, an average of 40 ± 10 % of the area was covered by shrubs. On the other hand, in plots encroached with C. ladanifer the average shrub cover was 70.0 ± 1.6 %. These values reflect the typical growth pattern of these shrub species.
If we consider the contribution of each of the biomass components (Table 2), the differences between the two species are even higher. These differences may be due to physiological, biological and ecological characteristics of each of these species or to structural parameters like density and coverage, previously mentioned. However, it is important to highlight that the differences are not only interspecific, but they are also intraspecific, related to the occupation of different environments. For instance, the rockrose formations studied in northern Portugal (Fonseca et al. 2012) show an aboveground carbon amount of 5.3 Mg ha−1 and a root carbon amount of 3.2 Mg ha−1; in northern of Extremadura (Spain) (Ruíz-Peinado et al. 2013) show an aboveground carbon amount of 4,35 Mg ha−1 and a root carbon amount of 2.42 Mg ha−1. In the rockrose formations studied in this work, the aboveground part represents 8 Mg ha−1 and the root part represents 1.41 Mg ha−1 of carbon. In brooms studied for Ruiz-Peinado et al. (2013) the aboveground carbon amount is 1.82 Mg ha−1 and the root carbon amount is 2.65 Mg ha−1. In the brooms studied in this work the aboveground part represents 1.27 Mg ha−1 and the root part represents 1.17 Mg ha−1 of carbon. These differences may be explained by the different growth states (average age of the specimens, cover, density) in each of the study areas and the environment conditions under which they grow (climatic condition, livestock density). For example, the age of the individuals of C. ladanifer and R. sphaerocaprpa studied by Ruiz-Peinado et al. (2013) ranged between 6 and 11 years for Cistus and between 4 and 22 years for broom. In our study, the range is between 0.6 and 55 years for Cistus, and between 0.5 and 18 years for broom. This shows that the age structure of both systems is different and thus also the biomass accumulated.
Whereas in the case of rockrose specimens the contribution of roots to total carbon is independent of age, representing a sixth of such carbon, this relation in brooms is clearly dependent of age. The amount of carbon in roots at young ages is lower than that of the aboveground part and they become equal from the age of 16 years, ranging from 0.4 to 1. These values are lower to those documented for other shrubby ecosystems in Spain like heathlands and kermes oak formations, with values of 2.3 and between 2.6 and 4.7, respectively, but more similar to those obtained in shrublands in Chile, which range from 0.3 to 0.7, and to those of Chaparral in California, with values between 0.4 and 0.8 (Kummerow et al. 1977; Miller 1977; Hoffmann and Kummerow 1978; Martínez García and Merino 1987; Cañellas and San Miguel 2000). In a wide revision carried out by Mokany et al. (2006) about the belowground/aboveground ratio of different terrestrial biomasses, these authors showed that the values in the shrubland systems ranged between 0.3 and 4.25. The results obtained in all these shrubland systems seem to indicate that the amount of carbon in roots does not depend exclusively on the age of the specimen but it also depends on the characteristics of the soil and other factors like competition intensity, the species itself, rainfall or regeneration strategy.
Plants give off some of their total carbon to the soil as organic carbon through dead leaves and radical exudates. The estimated annual amount of carbon that enters the soil from plant remains represent 7 % of the carbon contained in vegetation (Schlesinger 1977). Despite its minor contribution to total carbon, litter is an important component of the carbon biogeochemical cycle (Ordóñez et al. 2008), as it is the interface between vegetation and soil. The carbon accumulated in necromass in this study is 2.07 Mg ha−1 of C for C. ladanifer and 0.69 Mg ha−1 of C for R. shaerocarpa. These amounts represent 6 and 2.8 % of the carbon storage in rockrose and broom, respectively. This value should not be despised as it corresponds to 22 % of the carbon accumulated in the aboveground part in rockrose, and 28 % in brooms. In rockrose formations studied in southern Portugal by Simões et al. (2009), the amount of carbon accumulated in necromass is 2.05 Mg ha−1, which is very similar to the values reported in the present study.
The soil constitutes the main reservoir of carbon in an ecosystem and its accumulation depends on the type of vegetation, climatic conditions and soil properties. Besides, some factors like soil fertility, management or irrigation affect plant production and, therefore, the content of organic matter (Bravo 2007). Jackson et al. (1997) point out the methodological difficulty to estimate carbon in soils, since generally the analysis is only performed on the first few centimeters of the profile, thus greatly underestimating the content of carbon. It must be taken into account that organic carbon is present not only in the horizons closer to the surface. Soil works in the study area (Rozas 1993) show that the average soil depth is 1 meter and that the carbon proportion of the different horizons is: (0–20: 43 %; 20–40: 21.5 %; 40–60: 16.5 %; 60–80: 14 %; 80–100: 5 %). For C. ladanifer an amount of 23.14 Mg ha−1 of C (5 cm thick) was obtained, whereas for R. sphaerocarpa the value was of 21.14 Mg ha−1 of C (5 cm thick), thus showing no significant differences between species. If we assume the distribution of carbon in the different horizons according to Rozas (1993) in the first 20 cm of soil, there would be 90.45 Mg ha−1 of C stored for rockrose and 82.63 Mg ha−1 of C for brooms. These values are not different from those provided by Fonseca et al. (2012) in systems of Mediterranean shrubland at which the carbon accumulated was analysed in the first 20 cm of soils (95 Mg ha−1 of C for C. ladanifer; 96 Mg ha−1 of C for C. multiflorus; and 103 Mg ha−1 of C for E. australis).
Finally, it is important to highlight that the soil is the main reservoir of carbon in the systems studied and that shrubland communities can play a relevant role as carbon stocks and, therefore, their involvement in climate change mitigation policies must be taken into account.
References
Asner GP (2004) Biophysical remote sensing signatures of arid and semi-arid regions. In: Ustin SL (ed) Remote sensing for natural resources management and environmental monitoring: manual of remote sensing. Wiley, New York, pp 53–109
Bravo F (2007) El papel de los bosques españoles en la mitigación del cambio climático. Fundación Gas Natural, Barcelona
Buras A, Wucherer W, Zerbe S, Noviskiy Z, Muchitdinov N, Shimshikov B, Zverev N, Schmidt S, Wilmking M, Thevs N (2012) Allometric variability of Haloxylon species in Central Asia. For Ecol Manag 274:1–9
Cabezas J, Escudero JC (1989) Estudio termométrico de la provincia de Badajoz. Dirección General de Investigación, Extensión y Capacitación Agrarias, Badajoz
Cairns MA, Winjum JK, Phillips DL, Kolchugina TP, Vinson TS (1997) Terrestrial carbon dynamics: case studies in the former Soviet Union, the conterminous United States, Mexico and Brazil. Mitig Adapt Strat Glob Change 1:363–383
Canellas I, San Miguel A (2000) Biomass of root and shoot sistemas of Quercus coccifera shrublands in Eastern Spain. Ann For Sci 57:803–810
Devesa JA (1995) Vegetación y Flora de Extremadura. Universitas Editorial, Badajoz
Fan JW, Zhong HP, Harris W, Yu GR, Wang SQ, Hu ZM, Yue YZ (2008) Carbon storage in the grasslands of China based on field measurements of above-and below-ground biomass. Clim Change 86:375–396
Fang JY, Chen AP, Peng CH, Zhao SQ, Ci LJ (2001) Changes in forerst biomass carbon storage in China between 1949 and 1998. Sci 292(5525):2320–2322
Figueiredo T (1990) Aplicaçao da equaçao universal de perda de solo na estimativa da erosao potencial: o caso do Parque Natural de Montesinho. ESAB, Bragança
Fonseca W, Alice FE, Rey-Benayas JM (2012) Carbon accumulation in aboveground and belowground biomass and soil of different age native forest plantations in the humid tropical lowlands of Costa Rica. New For 43:197–211
Gayoso J, Schlegel B (2001) Proyectos Forestales para la mitigación de gases efecto invernadero Una tarea pendiente. Ambient Desarro 17(1):41–49
Haase P, Pugnaire FI, Fernández EM, Puigdefábregas J, Clark SC, Incoll LD (1996) Investigation of rooting depth in the semiarid shrub Retama sphaerocarpa (L.) Boiss. by labelling of ground water with a chemical tracer. J Hydrol 177:23–31
Hoffman A, Kummerow J (1978) Root studies in the Chilean matorral. Oecologia 32:57–69
ISO (1994) Organic and total carbon after dry combustion. In: Oostra S, Majdi H, Olsson M (eds) Environment soil quality. ISO/DIS 10694, Geneva
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci 94:7362–7366
Kimble JM, Lal R, Follett RR (2002) Agricultural practices and policy options for carbon sequestration: what we know and where we need to go. In: Kimbel JM, Lal R, Follett RF (eds) Agricultural practices and policies for carbon sequestration in soil. Lewis Publishers, New York, p 512
Kowalski AS, Loustau D, Berbigier P, Manca G, Tedeschi V, Borghetti M, Valentini R, Kolari P, Berninger F, Rannik E, Hari P, Rayment M, Mencuccini M, Moncrieff J, Grace J (2004) Paired comparisons of carbon exchange between undisturbed and regenerating stands in four managed forests in Europe. Glob Change Biol 10:1707–1723
Kummerow J, Kraus D, Jow W (1977) Root systems of chaparral shrubs. Oecologia 29:163–177
Lamlom SH, Savidge RA (2003) A reassessment of carbon content in wood: variation within and between 41 North American species. Biomass Bioenerg 25:381–388
Lopera G, Gutierrez V (2000) Viabilidad técnica y económica de la utilización de plantaciones de Pinus patula como sumideros de Carbono. Tesis Ingeniero Forestal, Universidad Nacional de Colombia, Medellín
MacClaran MP, Moore-Kucera J, Martens DA, Van Haren J, Marsh SE (2008) Soil carbon and nitrogen in relation to shrub size and death in a semi-arid grassland. Geoderma 145:60–68
MacDiken K (1997) A Guide to monitoring carbon storage in forestry and agroforestry projects. Winrock International, Arlington
Martínez García F, Merino J (1987) Evolución estacional de la biomasa subterránea del matorral del P.N. de Doñana. VIII Bienal Real Sociedad Esp History. Natural 47:263–570
McNaughton SJ, Banyikawa FF, McNaughton MM (1998) Root biomass and productivity in a grazing ecosystem: the Serengeti. Ecology 79:587–592
Metcalfe DB, Kunin WE (2006) The effects of plant density upon pollination success, reproductive effort and fruit parasitism in Cistus ladanifer L. (Cistaceae). Plant Ecol 185:41–47
Miller PC (1977) Root-shoot biomass ratios in shrubs in southern California and Chile. Madroño 24(4):215–223
Mokany K, Raison J, Prokushkin AS (2006) Critical analysis of root: shoot ratios in terrestrial biomes. Glob Change Biol 12:84–96
Nadelhoffer KJ, Raich JW (1992) Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecology 73:1139–1147
Navarro RM, Blanco P (2006) Estimation of above-ground biomass in shrubland ecosystems of southern Spain. Invest Agrar Sist Recur For 15(2):197–207
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Page AL et al (eds) Methods of soil analysis, Part 2, 2nd agronomy. American Society of Agronomy Inc, Madison, pp 961–1010
Nilsson MC, Wardle DA (2005) Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Front Ecol Environ 3:421–428
Ordóñez JAB, de Jong BHJ, García-Oliva F, Aviña FL, Pérez JV, Guerrero G, Martínez R, Masera O (2008) Carbon content in vegetation, litter, and oil under 10 different land-use and land-cover classes in the Central Highland of Michoacan, Mexico. For Ecol Manage 255:2074–2084
Ortíz E (1997) Costa Rican secondary forest: an economic option for joint implementation initiatives to reduce atmospheric CO2. Draft paper presented for inclusion in the Beijer Seminar in Punta Leona, Mexico
Pastor-López A, Martín J (1995) Ecuaciones de fitomasa para Pinus halepensis en repoblaciones de la provincia de Alicante. Stud Oecol 12:79–88
Patón D, Azocar P, Tovar J (1998) Growth and productivity in forage biomass in relation to the age assessed by dendrochronology in the evergreen shrub Cistus ladanifer L using different regression models. J Arid Environ 38:221–235
Ragland KW, Aerts DJ, Baker AJ (1991) Properties of wood for combustion analysis. Bioresour Technol 37:161–168
Rolo V, Moreno G (2012) Interspecific competition induces asymmetrical rooting profile adjustments in shrub-encroached open oak woodlands. Trees 26:997–1006
Rovira P, Romanya J, Rubio A, Roca N, Alloza JA, Vallejo R (2007) Estimación del carbono orgánico en los suelos peninsulares españoles. In: Bravo F (ed) El papel de los bosques españoles en la mitigación del cambio climático. Fundación Gas Natural, Barcelona, pp 197–292
Rozas MA (1993) Estudio edafológico de la comarca de Jerez de los Caballeros (Spain) PhD thesis. Universidad de Extremadura. Badajoz
Ruiz de la Torre J (1990) Memoria General del Mapa Forestal de España a escala 1:200000. Ministerio de Agricultura, Pesca y Alimentación, Madrid
Ruíz-Peinado R, Moreno R, Juárez E, Montero G, Roig S (2013) The contribution of two common shrub species to aboveground and belowground carbon stock in Iberian dehesas. J Arid Environ 91:22–30
San Miguel A, Roig S, Cañellas I (2008) Fruticeticultura. Gestión de arbustedos y matorrales. In: Serrada R, Montero G, Reque J (eds) Compendio de selvicultura aplicada en España. Arco/Libros, Madrid, pp 877–907
Schlesinger WH (1977) Carbon balance in terrestrial detritus. Ann Rev Ecol Syst 8:51–81
Sierra CA, Harmon ME, Moreno FH, Orrego SA, del Valle JI (2007) Spatial and temporal variability of net ecosystem production in a tropical forest: testing the hypothesis of a significant carbon sink. Glob Change Biol 13:838–853
Silva JS, Rego FC (2004) Root to shoot relationships in Mediterranean woody plants from Central Portugal. Biologia 59(Suppl 13):109–115
Simões MP (2002) Dinâmica de biomassa (carbono) e nutrientes em Cistus salvifolius L. e Cistus ladanifer L. Influência nas características do solo. PhD thesis. Universidade de Évora, Évora
Simões MP, Madeira M, Gazarini L (2009) Ability of Cistus L shrubs to promote soil rehabilitation in extensive oak woodlands of Mediterranean areas. Plant Soil 323:249–265
Sokal RR, Rohlf FJ (1995) Biometry. Freeman, New York, p 887
Titlyanova AA, Romanova IP, Kosykh NP (1999) Pattern and process in above-ground and below-ground components of grassland ecosystems. J Veg Sci 10:307–320
UNFCC (2007) United nations framework convention on climate change, 13th conference of parties to the UNFCCC.
Walkley A, Black IA (1934) An examination of the Degtjareff method for etermining organic carbon in soils: effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–263
Yamaguchi DK (1991) A simple method for cross-dating increment core from living trees. Con. J. For Res 21:414–416
Acknowledgments
We thank the Consejería de Economía, Comercio e Innovación de la Junta de Extremadura for their assistance in funding this work (GRU09038) as part of the “Plan for the Consolidation of and Support to Research Groups”. Special thanks to Manuel Mota (statistician) for valuable statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alías, J.C., García, M., Sosa, T. et al. Carbon storage in the different compartments of two systems of shrubs of the southwestern Iberian Peninsula. Agroforest Syst 89, 575–585 (2015). https://doi.org/10.1007/s10457-015-9792-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-015-9792-z