Abstract
The fixed-film sequencing batch reactor, or F-SBR, was developed to treat high organic compound levels and toxic cyanide concentrations in cassava wastewater. The performance of the F-SBR was compared with that of a conventional sequencing batch reactor, or SBR, that was operated with organic compound contents of 16,266.67–26,666 mg COD/L and 132.92–252.66 mg CN−/L. The cyanide and chemical oxygen demand removal efficiencies of the conventional SBR system were 42.61% and 36.83%, respectively, while those of the F-SBR were 77.95% and 74.43%, respectively; the cyanide removal efficiency reached 95.45% when the hydraulic retention time was increased to 5 days, and the F-SBR was very effective for the complete removal of cyanide when the hydraulic retention time was increased to 10 days. This effectiveness was similar to the effectiveness of chemical oxygen demand removal, which reached 40–78% efficiency with the F-SBR system. These results showed that the immobilization of cyanide-degrading bacteria such as Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2 carried out with a polypropylene ring in a fixed-film aerobic system enhanced the performance of the reactor and can be successfully applied for cyanide and chemical oxygen demand removal from industrial wastewater with high cyanide and chemical oxygen demand concentrations. This study may provide a promising alternative technique that reduces economic operation costs in solving wastewater contamination problems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cassava (Manihot esculenta) starch, or tapioca starch, is an important economic product in Thailand (El-Sharkawy 2003; Chavalparit and Ongwandee 2009). Nevertheless, in starch processing, 1 kg of fresh cassava root yields approximately 0.2 kg of starch, 0.4–0.9 kg of residue, and 5–7 l of wastewater (Peters and Ngai 2005; Sangyoka et al. 2007). Cassava wastewater is a carbohydrate-rich starch waste that contains high chemical oxygen demand (COD), high biochemical oxygen demand (BOD), high total solids (TS), and very high organic cyanide (Hien et al. 1999; Kaewkannetra et al. 2009, 2011). The cyanide concentration in cassava mill wastewater has been reported to range between 10.4 and 274 mg/L depending on the cyanoglycoside content of the cassava varieties (Siller and Winter 1998; Balagopalan and Rajalekshmy 1998), and cyanide is highly toxic because it has a strong effect on many organisms at relatively low concentrations (1–2 mM). It has the ability to bind iron in blood by forming complexes and can inhibit oxygen transfer to cells by blocking electron transport and energy release in cells (Ripley et al. 1996), thereby causing suffocation and human and animal health hazards. Since cyanide is a toxic compound as a metabolic inhibitor, it is a serious threat to discharge into the environment, and aquatic life in the receiving waters must be protected. Hence, many countries have implemented a statutory limit of approximately 0.2 mg/L for cyanide discharge into natural water basins (ATSDR 1997; Patil and Paknikar 2000a, b).
Cyanide can be removed by several techniques, including chemical and physical methods, but these processes entail high operational costs or large amounts of land for treatment and involve the use of additional hazardous reagents (Watanabe et al. 1998; Mpongwana et al. 2019). In contrast, a biological approach has been developed to greatly reduce costs and perform highly as an environmentally acceptable method for cyanide removal. Under aerobic conditions, biological processes may consume hydrogen cyanide and generate hydrogen cyanate and hydrolyze the latter compound into ammonia and carbon dioxide (Baxter and Cummings 2006; Dzombak et al. 2006). Furthermore, the enhancement of the biological treatment systems has been reported. For example, the immobilization of bacterium Rhodococcus rhodochrous BX2 with biofilm-forming bacterium Bacillus mojavensis M1 in fluidized bed reactors reached 99.8% of cyanide removal efficiency under optimal conditions (An et al. 2018), and the technology of immobilized cell systems of Klebsiella oxytoca revealed the efficiency better than the suspended systems (Chen et al. 2007), and also the alginate-entrapped cells of Serratia marcescens RL2b immobilized in the packed bed reactor exhibited the complete cyanide degradation (Kumar et al. 2015). However, in many instances, complete degradation of some cyanide complexes is not achieved (Figueira et al. 1996; Yanase et al. 2000). The sequencing batch reactor (SBR) system is an alternative biological system used for cyanide treatment (Sirianuntapiboon et al. 2008). However, this system has some disadvantages, such as high excess sludge production and a high sludge volume index (Barnett et al. 1994; Dangcong et al. 2000; Kargi and Uygur 2002; Wilen and Balmer 1998). Fixed-film sequencing batch reactors (F-SBRs) were developed to support biological reactors with a high degradation performance. F-SBR systems have attracted a great deal of attention due to their ability to take advantage of both fixed biofilms and SBRs, which show improved biomass concentrations in reactors with increased specific removal efficiencies and are capable of covering small areas. Therefore, the main idea of developing an F-SBR system was to achieve a system that has the advantages of biofilm systems, including (1) the capability for the treatment of all domestic and industrial wastewaters, (2) shockability, (3) compactness (small footprint), (4) no need to return sludge, and (5) a stable ecological system (Nicolella et al. 2000; Han et al. 2012).
Therefore, this study aimed to evaluate the feasibility and advantage of a fixed-film sequencing batch reactor with a mixed culture of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2 immobilized on polypropylene ring carriers as a biofilm medium applied to remove high concentrations of cyanide and organic matter from cassava wastewater.
1 Materials and Methods
1.1 Microorganisms
A culture of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2 with a high efficiency for cyanide degradation (Potivichayanon and Kitleartpornpairoat 2014) was isolated from a wastewater treatment system in the cassava starch industry. A previous study found that the mixed culture was able to degrade hydrogen cyanide and metal-cyanide complexes. In this study, bacterial cells were cultured in a buffer medium containing 2.7 g of KH2PO4, 3.5 g of K2HPO4, 10 ml of a mineral salt solution (300 mg of FeSO4·7H2O, 180 mg of MgCl2·6H2O, 130 mg of Co(NO3)2·6H2O, 40 mg of CaCl2, 40 mg of ZnSO4 and 20 mg of MoO3 in 1 L of deionized water), and 25 mg/L potassium cyanide (KCN). An inoculum of 10 ml of mixed culture was added into an Erlenmeyer flask containing 90 ml of buffer medium and the cyanide complexes and incubated at room temperature at 150 rpm on a rotary shaker. The growth of the mixed culture was studied for 7 days by the colony count technique on buffer medium agar and incubated at 30 °C (Potivichayanon and Kitleartpornpairoat 2014; Supromin et al. 2015; Potivichayanon et al. 2017), and the cells were used as a starter for studies of cyanide degradation on a flask scale and in the F-SBR system.
1.2 Cyanide Degradation on the Flask Scale
Inocula of the mixed culture were transferred to flasks containing 30 ml of buffer medium and 70 ml of cassava wastewater for acclimatization. The cell growth in terms of total cells was monitored every 24 h for 5 days of incubation. The microorganism cell growth was analyzed by the colony counting technique on buffer medium agar and incubated at 30 °C, and the wastewater characterization, such as determinations of COD and cyanide content, as a function of total cyanide was performed in triplicate by following the standard methods for the examination of water and wastewater (APHA 2005).
1.3 Immobilization of Mixed Culture Bacteria
The mixed culture bacteria of SUTS 1 and SUTS 2 grown in the exponential phase (Potivichayanon and Kitleartpornpairoat 2014) were immobilized on packing materials [polypropylene Pall (PP) ring]. The diameter of the polypropylene Pall ring was 3 in. The PP rings were sterilized with ultraviolet light using laminar airflow cabinets (ScanLaf/Mars 1500, Labogene Aps, Denmark) and transferred into F-SBR containing buffer medium (30 L). After that, the mixed culture bacteria were added to the F-SBR system to become immobilized cells at a ratio of 10:100 v/v and prepared for 30 days.
1.4 Wastewater Source and Sample Characteristics
The cassava wastewater in this study was collected from a primary sedimentation tank in a cassava industrial site at Nakhon Ratchasima, Thailand. The chemical and physical characterization was carried out in triplicate. The experiments were carried out with various concentrations of pollutant in the wastewater that are given as average values 132.92–252.66 mg CN−/L and 16,266.67–26,666 mg COD/L; for nitrogen compound content (pH 4.3–5.1), NH3-N was 7.81–8.96 mg/L, and NO3−-N was 3.59–4.21 mg/L.
1.5 Experimental Design
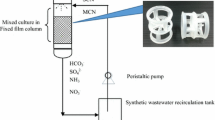
The experiments were performed in two types of sequencing batch reactor (SBR): a conventional sequencing batch reactor (SBR) was a control, and a fixed-film sequencing batch reactor (F-SBR) was also used. Each reactor was fabricated from transparent acrylic material (5 mm thick). The diameter of each reactor was 30 cm, and a depth of 50 cm corresponded to an effective volume of 35 L; they had a working volume of 30 L. The conventional SBR was operated in parallel with the F-SBR, but the F-SBR had fixed-film packing medium installed as 30% of the working volume in the reactor (Fig. 1). The influent (cassava wastewater) was fed into a reactor at the bottom of the system, while the effluent was drawn at the top of a packing medium layer using a peristaltic pump. One set of air pumps was used to supply air to each reactor, and the aeration system was equipped with an air diffuser installed at the bottom of each reactor. The conventional SBR system and the F-SBR system were installed with an automatic timer to control the intermittent operation of each step.
1.6 Reactor Start-Up and Operation
The typical cycles in the operation program consisted of five steps that were controlled by an automatic timer: fill (instantaneous), react (aeration), settle (sedimentation/clarification), draw (decant), and idle (Metcalf and Eddy 1991). The fixed-film bacterium was added to cassava wastewater to replace the buffer medium at a fill rate of 10% (v/v) every day into the wastewater to achieve a final working volume. Initially, a single start-up cycle was carried out over 24 h, including 1 h of feeding (fill), 21 h of aeration, 1 h of settling, and 1 h of effluent withdrawal (drawing and idle) under each set of operation conditions, as shown in Table 1. The wastewater was fed into the reactor within 1 h (fill step) by a peristaltic pump. The aeration was then continued for another 21 h (reaction step), and the required oxygen was maintained as evidenced by the dissolved oxygen in the system of approximately 0.35–1.5 mg/L. Then, the aeration was shut down for 1 h (settling step) during which sludge settled down to the bottom of reactor. Subsequently, the clarified effluent had to be removed (the volume of effluent was based on the operation program) for 1 h (drawing and idle step). The excess sludge was drawn during the drawing and idle steps. After that, the raw wastewater was filled into the reactors to a final volume of 30 L, and the operation was repeated. Each experiment was carried out for 10 days, and buffer medium was added as an essential nutrient source with a fill rate of 10% (v/v) to support the growth of microorganisms after the completion of each experiment. The influent was collected daily every 2 days, and the effluent was obtained once a day for biological and chemical analyses. In addition, the conventional SBR, as a control, was operated similar to F-SBR with a hydraulic retention time (HRT) of 3 days.
1.7 Analytical Methods
The growth of the mixed culture was estimated using the colony counting technique with tenfold serial dilutions with sterile saline solution (0.85% w/v NaCl). The cassava wastewater and effluent were analyzed in terms of the cyanide (CN−), chemical oxygen demand (COD), ammonia (NH3-N) and nitrite (NO2−-N) levels according to the standard methods for the examination of water and wastewater (APHA 2005) and nitrate (NO3−-N) (APHA 1998). All chemical ingredients were analytical grade and purchased from Merck (Darmstadt, Germany), Sigma-Aldrich (St. Louis, USA), or Ajax (Auckland, New Zealand). Dissolved oxygen (DO) and pH were measured routinely by using a DO meter and a pH meter (Jenway 3510, Keison, England). Biofilm concentrations were calculated as dry mass and expressed in grams (of biomass).
2 Results and Discussion
2.1 Cyanide Degradation on the Flask Scale
The preliminary studies of cyanide degradation were studies in buffer medium supplemented with 70% (v/v) cyanide-contaminated wastewater collected from the cassava industrial site; this wastewater contained an initial concentration of cyanide and an initial COD of 132.92 mg/L and 26,133.33 mg/L, respectively. The results demonstrated that the growth pattern of the mixed culture showed a clear exponential phase on day 4, with a maximum cell count of approximately 108 CFU/ml (Fig. 2). As growth occurred, the levels of cyanide removal efficiency were maintained at approximately 36–74% over the same periods. The increased rate of cyanide and COD removal from the medium was accompanied by an increase in the bacterial population. In other words, when the cells reached the exponential phase, the rate of cyanide degradation reached 70.95 and 73.88%. Meanwhile, the rate of COD removal as time progressed sharply increased from 26 to 66% with increasing cell growth in the exponential phase at 4 days of incubation. The results showed that organic matter consumption was dramatically faster in the exponential phase than in the lag phase. The cell population at the higher cyanide concentration showed that in the initial period, the cells needed some time for acclimatization. Particularly, the period corresponding to the initial cell adaptation phase occurred before the biodegradation started, which implies that the rates of cyanide and COD degradation are related to the metabolic activity of microorganisms. The results indicated that the mixed cultures are able to survive under the very high cyanide and COD concentrations typically found in the cassava industry and are capable of eliminating cyanide and COD in wastewater. The next process was conducted by applying a conventional sequencing batch reactor integrated with fixed-film bacteria to improve the efficiency in the treatment and removal of pollutants.
2.2 Evaluation of SBR and F-SBR Performances
The performances of SBR and F-SBR were determined regularly after operating at an HRT of 3 days and a wastewater flow rate of 168 ml/min. The reactor performance was evaluated continuously for 10 days under influent cyanide and COD concentrations of approximately 208.93 mg/L and 16,266.67 mg/L, respectively, or under organic loading of 69.64 mg CN−/L d and 5422.22 mg COD/L d. The results revealed that the conventional SBR system (without inoculum seeding) could perform, with an average removal efficiency of 42.61% for cyanide and 36.83% for COD; however, the system showed low removal efficiency. This might be due to the very high concentrations of cyanide and COD. The use of the control system to remove cyanide and COD resulted in effluent concentrations of these compounds of 90 mg/L and 7626.67 mg/L, respectively, which were attributed to the ability of indigenous microorganisms to remove cyanide from wastewater. As a result, it was necessary to improve the treatment system by installing a fixed-film mixed culture of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2. The ability of the F-SBR to include packing media significantly increased the biodegradation efficiency on the first day and effected more than 70.68% removal after 4 days of operation (Fig. 3a). The residual cyanide in the effluent was 61.25 mg/L. After that, this F-SBR system was 77.95% and 74.43% effective at degrading cyanide and COD, respectively, within a period of 10 days, with effluent containing 46.06 mg CN−/L and 6400 mg COD/L, whereas the efficiencies of the conventional SBR were 45.85% and 46.56%, respectively (Fig. 3a and Fig. 4). As time progressed, the maximum cell concentration in the F-SBR was found to be 107 CFU/ml, while the maximum cell concentration in the conventional SBR was 106 CFU/ml (Fig. 5). As a result, the total biomass of the F-SBR system due to an increase in the amount of biofilm mass on the medium in the system supported an efficiency higher than that of the SBR system. Nevertheless, a previous study reported that a mixed culture of SUTS 1 and SUTS 2 was capable of degrading cyanide and metal-cyanide complexes (Potivichayanon et al. 2017) because the efficiencies of bioreactors can be improved by inoculating a specific microorganism with enhanced metabolic capabilities (Li et al. 2015). Mekuto et al. (2013) reported that mixed culture mainly dominated by Bacillus spp. (Bacillus safensis, Bacillus licheniformis, and Bacillus tequilensis) was able to tolerate and degrade cyanide broth. These microorganisms were able to degrade 131 (65.5%) and 177 (44.3%) mg CN−/L in cultures containing 200 and 400 mg CN−/L over a period of 8 days, respectively.
Furthermore, cyanide was degraded by many microbes under aerobic conditions and transformed into less toxic forms by extracellular enzymes found on the cell wall of cyanide-oxidizing bacteria into byproducts such as ammonia (NH3-N), nitrite (NO2−-N), nitrate (NO3−-N), and bicarbonate (HCO3−) (Akcil and Mudder 2003; Sirianuntapiboon and Chuamkaew 2007; White and Schnabel 1998; Watanabe et al. 1998). Thus, this study investigated the byproducts ammonia (NH3-N), nitrite (NO2−-N) and nitrate (NO3−-N). The results showed that the byproduct concentrations increased with increasing cyanide removal efficiency. At an HRT of 3 days, the accumulation of ammonia occurred in the system after the first day of the operating period. In the control experiment (SBR), ammonia and nitrite concentrations exhibited stable values, with average values of approximately 31.35 mg/L and 0.03 mg/L, respectively, but nitrate exhibited high concentrations on days 2–3 (approximately 0.69 mg/L), conforming to residual cyanide concentrations and decreasing afterward. Although the F-SBR revealed an increase in ammonia concentrations to 17–26 mg/L, the nitrate concentration was 0.34–0.61 mg/L, and nitrite was found at low levels of approximately 0.02–0.11 mg/L (Fig. 6a). This behavior may be because the mixed culture was able to consume ammonia as a nitrogen source better than the indigenous microbes, which can be observed from this ammonia concentration being less than that in the SBR system because Pseudomonas spp. could degrade cyanide and convert it to ammonia under both aerobic and/or anaerobic conditions (Watanabe et al. 1998), similar to Agrobacterium tumefaciens SUTS 1, which was able to degrade cyanide to ammonia and nitrate as a final byproduct (Potivichayanon and Kitleartpornpairoat 2014). These could be easily utilized by microbes. Furthermore, immobilized cells are advantageous because they support a higher cell density than planktonic cells, eliminate cell recovery and offer the possibility of maintaining the cells under viable conditions with a high specific surface area for microbial proliferation (Bohn 1992; Potivichayanon et al. 2006; Terada et al. 2006; Dursan and Aksu 2002; Zhou et al. 2007; Chen et al. 2008; Dash et al. 2009a, b). The experiment suggested that the immobilized cells exhibited a high tolerance for the extreme environment.
2.3 Cyanide Removal Efficiency under Different HRTs
According to the above results, the F-SBR system can treat a very high concentration of organic contaminants that is greater than the concentrations treated by the conventional SBR because immobilized cells in the reactor are more suitable for cyanide treatment than the cells in the SBR. Therefore, the HRT is a critical design parameter for biological treatment systems whose proper selection guarantees the removal of substrates since establishing an optimal HRT significantly affects microbial and substrate reaction times (Romero Aguilar et al. 2013). In this study, the F-SBR system was applied to obtain the optimal conditions with various HRTs of 3, 5, 7, and 10 days. The results illustrated that the removal efficiency increased when the HRT was increased to 5 days with an organic loading of 3253.22 mg COD/L d and cyanide loading of 41.79 mg CN−/L d and initial cyanide and COD concentrations of 208.93 mg/L and 16,266.67 mg/L, respectively. The results showed that the system was able to degrade cyanide and COD better than when the HRT was 3 days. The cyanide removal efficiency remained at an average of 93.27% at a DO concentration of 0.51 mg/L and improved to 95.21% within 8 days (Fig. 3a) even though the COD exhibited the highest removal efficiency of approximately 77.05% on day 5 of the operating cycle, but then the capability decreased below 69.67% on day 10 of the experiment (Fig. 4). The residual cyanide concentration in the effluent was 9.50 mg/L, and the organic matter in terms of COD was less than 3733.33 mg/L. This retention time might be appropriate for the immobilized microorganisms in the system to promote degradation by utilizing cyanide compounds as carbon and nitrogen sources that can be degraded and/or eliminated into ammonia, nitrite, and nitrate, as mentioned above (Petrozzi and Dunn 1994; Dzombak et al. 2006). Nevertheless, the concentrations of byproducts, especially ammonia, increased sharply to 53.95 mg/L at first and then decreased afterwards while the nitrate concentration increased. An interesting observation on days 3–4 was an apparent link between the DO value and the nitrification reaction (Fig. 6b). When oxygen decreased, ammonia and nitrate concentrations decreased, but nitrite increased to 0.1767 mg/L and then decreased. This trend can explain why oxygenation is a major challenge in the biodegradation of cyanide in the degradation pathway and immobilization approach: living cells require sufficient oxygen to keep cells highly available and productive throughout a culture period (Meuwly et al. 2005). As a result of cell growth, the cell concentration tended to increase when the degradation rate increased; for example, the cyanide and COD removal efficiencies were in the ranges of 92–94% and 70–73%, respectively, and cell growth usually increased during 3–7 days of operation (Fig. 5). Furthermore, a previous study reported that Agrobacterium tumefaciens SUTS 1 was able to degrade cyanide to ammonia and nitrate as final byproducts; thus, the byproduct concentration was dependent on the rate of cyanide degradation (Potivichayanon and Kitleartpornpairoat 2014; Supromin et al. 2015; Potivichayanon et al. 2017). In addition, Ibrahim et al. (2015) reported that Pseudomonas spp. have a great capability for cyanide removal.
However, in the experiments with HRTs of 7 and 10 days, the concentrations of COD and cyanide in the cassava wastewater dropped to 10,906.67 mg/L and 62.46 mg/L, respectively, or the organic loading was 3635.56 mg COD/L d and 20.82 mg CN−/L d, respectively. At the HRT of 7 days and when the experiment was started with organic loadings of 1558.10 mg COD/L d and 8.92 mg CN−/L d, the cyanide degradation tended to drop. The results demonstrated that the highest cyanide removal efficiency occurred after 7 days of operation (approximately 89.21%), and the residual cyanide concentration in the effluent was 6.74 mg/L (Fig. 3b). However, the COD had the better removal efficiency of 77.87% after 3 days of operation, with a COD concentration in the effluent of approximately 2413.33 mg/L. This value decreased to below 53.30% afterward, with a final effluent concentration of 6400 mg/L (Fig. 4).Analysis of byproducts in the effluent revealed that ammonia accumulation in this cycle reached above 128.61 mg/L, but very low nitrite (average of 0.0081 mg/L) was present, sometimes not being detected. In contrast, the nitrate concentration in effluent increased with increasing ammonia and remained at a relatively high level of more than 0.2851 mg/L (Fig. 6c). This result indicated that the nitrogen from ammonia directly accumulated as an intermediate product before nitrate instead of being completely nitrified (Ma et al. 2017). Nevertheless, the accumulation of ammonia was affected by decreasing cyanide degradation because ammonia may inhibit bio-oxidation due to the participation of microbial enzymes in cyanide degradation, nitrification, and denitrification in the treating system (Bian et al. 2015; Zheng et al. 2016; Naveen et al. 2011; Suh et al. 1994). However, a fluctuating concentration of ammonia was observed; the high ammonia concentration in the broth could inhibit cyanide biodegradation, because the organisms prefer ammonia as a nitrogen source rather than cyanide, thus reducing cyanide degradation by the microbial species (Mekuto et al. 2013).
The F-SBR system exhibited better removal of cyanide when operating with a high retention time at 10 days, a wastewater flow rate of 54 ml/min and organic loadings of approximately 1090.67 mg COD/L d and 6.25 mg CN−/L d than under other conditions. The cyanide removal efficiency of the system gradually increased during the first period of the operation cycle and then reached a steady state after 4 days of operation (Fig. 3b), while the initial period presented the highest efficiency of COD removal, which was 74.21% (Fig. 4). In the steady state, it proved possible to achieve 94.42–96.70% (Fig. 3b). The residual cyanide decreased to approximately 2.07–3.51 mg/L. Towards the end of operation, the system could completely degrade the cyanide after 10 days of running. In addition, residual cyanide was not detected in the effluent on day 10 of an experiment, possibly because the cyanide in the reactor was oxidized rapidly by nitrification and transformed into ammonia and nitrate (Chapatwala et al. 1998; Sirianuntapiboon and Chuamkaew 2007). Furthermore, the nearly complete removal of cyanide increased the ammonia concentration in the effluent, corresponding to a cyanide removal efficiency that increased from 106.87 to 170.99 mg/L. Meanwhile, nitrite was not detected but there was accumulation of nitrate from 0.3406 to 0.7958 mg/L during that time (Fig. 6d). This might be because these compounds could be utilized as electron accepters to stimulate the denitrification reaction when insufficient DO was present (Third et al. 2003). In addition, Holt et al. (1994) reported that Agrobacterium spp. could utilize ammonia salts and nitrate as the sole nitrogen sources, and ammonia can be oxidized by microbes and converted to nitrite and nitrate. Furthermore, the formation of byproducts that are produced through cyanide degradation, especially ammonia, is hazardous to aquatic life if the concentration is in very high (EPA 2013).
2.4 Quantification of Bacterial Community
Biofilm formation of mixed-culture bacteria in the F-SBR system was observed after operation (Fig. 7) by random sampling of the packing medium from this reactor (top, middle, and bottom position). The microbial community attached to the carrier medium was characterized by its dry weight (Table 2). After the operation period, the total microorganisms attached to the medium averaged 2.95×107 CFU/ml and presented a specific growth rate (μ) of 0.005 h−1 (Fig. 8). This specific growth rate presented the cell growth (mass/volume) in a specific time (h) (Maier et al. 2009). In addition, the total number of suspended cells in the reactor was 6.65×107 CFU/ml; this indicated that the mixed culture, either immobilized or suspended, is tolerant to high organic loading and cyanide toxicity. Furthermore, immobilization of bacteria as biofilms on the surface of the packing material can reduce the chance of biomass wash-out because a combination of suspended cells and biofilms of immobilized cells were in the reactor (Magri et al. 2012; Masłoń and Tomaszek 2015). The performance of the reactor will be promoted to remove the very high levels of pollutant compounds contaminating the environment. However, the types of microorganisms and texture of packing materials for the biofilm system are the most important factors in the performance of wastewater treatment processes because they affect the weight, activities, and composition of the biofilm in the system (Felföldi et al. 2015; Masłoń and Tomaszek 2015). On the other hand, the operation cost of wastewater treatment system should be considered for the industrial plant. The SBR with biofilm system has been reported the cost-effectiveness because it could be operated in a different mode in the same tank and also it requires only the nutrient for supporting microbial growth and activating removal rate (White et al. 2000; Maniyam et al. 2015; Mpongwana et al. 2019). This is similar to this study, the F-SBR system was operated and added with 10% (v/v) of buffer medium as nutrient sources in every 10 days under the optimal conditions which the cost was estimated at 3.15 TH Baht or 0.11 US Dollar per 1 m3 of wastewater containing cyanide and organic matter with high initial concentration of 208.93 mg/L and 16,266.67 mg/L, respectively, whereas the traditional chemical treatments such as alkaline chlorination or hydrogen peroxide could be more expensive to operate due to high reagent usage and also undesirable toxic byproducts requiring further treatment (Akcil 2003; Parga et al. 2003; Dash et al. 2009a, 2009b; Botz et al. 2016; Pueyo et al. 2016); for example, if the usage of chlorine for alkaline chlorination process is in the range 3.0–8.0 g Cl2 per gram of cyanide oxidized under pH 10.5, it will generate toxic byproduct compounds or excess chlorine in the effluent, and if that of hydrogen peroxide process is 50 mg/L H2O2 for oxidizing 4.1 mg/L of initial cyanide under pH 10.9, the excess dosages of hydrogen peroxide are required for slurry treatment (Botz et al. 2016).
3 Conclusions
The bioremediation of cyanide and COD contaminating cassava wastewater by mixed microbial culture of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2 with indigenous microorganisms demonstrated that the F-SBR can treat high concentrations of cyanide and organic matter in cassava wastewater. The cyanide removal efficiency increased with increasing hydraulic retention time (HRT); the cyanide removal efficiency reached 95% with an HRT of 5 days. The use of the F-SBR was very effective for the complete removal of cyanide when the HRT was increased to 10 days, and it provided 40–78% COD removal efficiency. Therefore, these results showed that the fixed-film aerobic system can be successfully applied for cyanide and COD removal from industrial wastewater better than conventional SBR systems.This study contributes a sufficient method that is economical for use as a promising alternative technique, offering an applicable solution to the problem. However, the further studies are required to enhance the methods for the bioremediation of cyanide; for example, the idea of a fixed-film system must be developed to maintain the performance of such a system to increase the amount of packing medium because this increase should increase the surface area available for microbial adhesion, which will reduce the loss of microorganisms within the treatment system and make treatment with the system more effective.
References
Agency for Toxic Substances and Disease Registry ATSDR (1997) Toxicological profile for cyanide. Atlanta, GA, United States Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
Akcil, A. (2003). Destruction of cyanide in gold mill effluents: Biological versus chemical treatments. Biotechnol Adv, 21(6), 501–511 https://doi.org/10.1016/S0734-9750(03)00099-5.
Akcil, A., & Mudder, T. (2003). Microbial destruction of cyanide wastes in gold mining: Process review. Biotechnol Lett, 25, 445–450. https://doi.org/10.1023/A:1022608213814.
An, X., Cheng, Y., Huang, M., Sun, Y., Wang, H., Chen, X., Wang, J., Li, D., & Li, C. (2018). Treating organic cyanide-containing groundwater by immobilization of a nitrile-degrading bacterium with a biofilm-forming bacterium using fluidized bed reactors. Environ Pollut, 237, 908–916. https://doi.org/10.1016/j.envpol.2018.01.087.
APHA, AWWA, WPCF. (1998). Standards method for the examination of water and wastewater (20th ed.). Washington DC: American Public Health Association.
APHA, AWWA, WPCF. (2005). Standards method for the examination of water and wastewater (21st ed.). Washington DC: American Public Health Association.
Balagopalan, C., & Rajalekshmy, L. (1998). Cyanogen accumulation in environment during processing of cassava (Manihot esculenta Crantz) for starch and sago. Water Air Soil Pollut, 102, 407–413. https://doi.org/10.1023/A:1004992611810.
Barnett, J. W., Kerridge, G. J., & Russell, J. M. (1994). Effluent treatment system for the dairy industry. Austtralian Biotechnology, 4, 26–30.
Baxter, J., & Cummings, S. P. (2006). The current and future applications of microorganism in the bioremediation of cyanide contamination. Antonie Van Leeuwenhoek, 90, 1–17. https://doi.org/10.1007/s10482-006-9057-y.
Bian, D., Zhou, D., Huo, M., Ren, Q., Tian, X., Wan, L., Zhu, S., & Ai, S. (2015). Improving oxygen dissolution and distribution in a bioreactor with enhanced simultaneous cod and nitrogen removal by simply introducing micro-pressure and swirl. Appl Microbiol Biotechnol, 99, 8741–8749. https://doi.org/10.1007/s00253-015-6714-y.
Bohn, H. (1992). Consider biofiltration for decontaminating gases. Chem Eng Prog, 88, 35–40.
Botz, M. M., Mudder, T. I., & Akcil, A. U. (2016). Cyanide treatment: Physical, chemical, and biological processes. Gold Ore Processing: Project development and operations (pp. 619–645) (2nd ed.). Amsterdam: Elsevier Science.
Chapatwala, K. D., Babu, G. R. V., Vijiya, O. K., Kumar, K. P., & Wolfram, J. H. (1998). Biodegradation of cyanides, cyanates and thiocyanates to ammonia and carbon dioxide by immobilized cells of Pseudomonas putida. J Ind Microbiol Biotechnol, 20, 28–33. https://doi.org/10.1038/sj.jim.2900469.
Chavalparit, O., & Ongwandee, M. (2009). Clean technology for the tapioca starch industry in Thailand. J Clean Prod, 17(2), 105–110. https://doi.org/10.1016/j.jclepro.2008.03.001.
Chen, C. Y., Kao, C. M., Chen, S. C., Chien, H. Y., & Lin, C. E. (2007). Application of immobilized cells to the treatment of cyanide wastewater. Water Sci Technol, 56(7), 99–107. https://doi.org/10.2166/wst.2007.699.
Chen, H., Shao, M., & Li, Y. (2008). The characteristics of soil water cycle and water balance on steep grassland under natural and simulated rainfall conditions in the Loess Plateau of China. J Hydrol, 360(1–4), 242–251. https://doi.org/10.1016/j.jhydrol.2008.07.037.
Dangcong, P., Bernet, N., Delgenes, J.-P., & Moletta, R. (2000). Effects of oxygen supply methods on the performance of a sequencing batch reactor for high ammonia nitrification. Water Environ Res, 72(2), 195–200. https://doi.org/10.2175/106143000X137284.
Dash, R. R., Balomajumder, C., & Kumar, A. (2009a). Removal of metal cyanides from aqueous solutions by suspended and immobilized cells of Rhizopus oryzae (MTCC 2541). Eng Life Sci, 9(1), 53–59. https://doi.org/10.1002/elsc.200700024.
Dash, R. R., Gaur, A., & Balomajumder, C. (2009b). Cyanide in industrial wastewaters and its removal: A review on biotreatment. J Hazard Mater, 163(1), 1–11. https://doi.org/10.1016/j.jhazmat.2008.06.051.
Dursan, A. Y., & Aksu, Z. (2002). Effect of internal diffusivity of ferrous (II) cyanide complex ions in Ca-alginate immobilized Pseudomonas fluorescens gel beads on the biodegradation rate. Process Biochem, 37(7), 747–752. https://doi.org/10.1016/S0032-9592(01)00268-0.
Dzombak, D. A., Ghosh, R. S., & Wong-Chong, G. M. (2006). Cyanide in water and soil: Chemistry, risk, and management. United States: CRC Press. https://doi.org/10.1201/9781420032079.
El-Sharkawy, M. A. (2003). Cassava biology and physiology. Plant Mol Biol, 53, 621–641. https://doi.org/10.1023/B:PLAN.0000019109.01740.c6.
EPA. (2013). Aquatic life ambient water quality criteria for ammonia-freshwater. United States Environmental Protection Agency EPA-822-R-13-001.
Felföldi, T., Jurecska, L., Vajna, B., Barkács, K., Makk, J., Cebe, G., Szabó, A., Záray, G., & Márialigeti, K. (2015). Texture and type of polymer fiber carrier determine bacterial colonization and biofilm properties in wastewater. Chem Eng J, 264, 824–834. https://doi.org/10.1016/j.cej.2014.12.008.
Figueira, M. M., Ciminelli, V. S. T., de Andrade, M. C., & Linardi, V. R. (1996). Cyanide degradation by an Escherichia coli strain. Can J Microbiol, 42(5), 519–523. https://doi.org/10.1139/m96-070.
Han, Y., Liu, J., Guo, X., & Li, L. (2012). Micro-environment characteristics and microbial communities in activated sludge flocs of different particle size. Bioresour Technol, 124, 252–258. https://doi.org/10.1016/j.biortech.2012.08.008.
Hien, P. G., Oanh, L. T. K., Viet, N. T., & Lettinga, G. (1999). Closed wastewater system in the tapioca industry in Vietnam. Water Sci Technol, 39(5), 89–96. https://doi.org/10.1016/S0273-1223(99)00112-2.
Holt, J. G., Krieg, N. R., Sneath, P. H. A., Staley, J. T., & Williams, S. T. (1994). Bergey’s manual of determinative bacteriology (pp. 74) (9th ed.). United States of America: Williams & Wilkins.
Ibrahim, K. K., Syed, M. A., Shukor, M. Y., & Ahmad, S. Q. (2015). Biological remediation of cyanide: A review. Biotropia Journal, 22(1), 151–163. https://doi.org/10.11598/btb.2015.22.2.393.
Kaewkannetra, P., Imai, T., Garcia-Garcia, F. J., & Chiu, T. Y. (2009). Cyanide removal from cassava mill wastewater using Azotobactor vinelandii TISTR 1094 with mixed microorganisms in activated sludge treatment system. J Hazard Mater, 172(1), 224–228. https://doi.org/10.1016/j.jhazmat.2009.06.162.
Kaewkannetra, P., Chiwes, W., & Chiu, T. Y. (2011). Treatment of cassava mill wastewater and production of electricity through microbial fuel cell technology. Fuel, 90(8), 2746–2750. https://doi.org/10.1016/j.fuel.2011.03.031.
Kargi, F., & Uygur, A. (2002). Nutrient removal performance of a sequencing batch reactor as a function of the sludge age. Enzym Microb Technol, 31(6), 842–847. https://doi.org/10.1016/S0141-0229(02)00209-0.
Kumar, V., Kumar, V., & Bhalla, T. C. (2015). Packed bed reactor for degradation of simulated cyanide-containing wastewater. 3. Biotech, 5, 641–646. https://doi.org/10.1007/s13205-014-0261-6.
Li, L., Zhang, J., Lin, J., & Liu, J. (2015). Biological technologies for the removal of sulfur containing compounds from waste streams: Bioreactors and microbial characteristics. World J Microbiol Biotechnol, 31, 1501–1515. https://doi.org/10.1007/s11274-015-1915-1.
Ma, W., Han, Y., Ma, W., Han, H., Zhu, H., Xu, C., Li, K., & Wang, D. (2017). Enhanced nitrogen removal from coal gasification wastewater by simultaneous nitrification and denitrification (SND) in an oxygen-limited aeration sequencing batch biofilm reactor. Bioresour Technol, 244, 84–91. https://doi.org/10.1016/j.biortech.2017.07.083.
Magri, A., Vanotti, M. B., & Szogi, A. A. (2012). Anammox sludge immobilized in polyvinyl alcohol (PVA) cryogel carriers. Bioresour Technol, 114, 231–240. https://doi.org/10.1016/j.biortech.2012.03.077.
Maier, R. M., Pepper, I. L., & Gerba, C. P. (2009). Environmental microbiology (pp. 45–59) (2nd ed.). Massachusetts: Academic Press.
Maniyam, M. N., Sjahrir, F., Ibrahim, A. L., & Cass, A. E. G. (2015). Enzymatic cyanide degradation by cell-free extract of Rhodococcus UKMP-5M. Journal of Environmental Science and Health, Part A Toxic/ Hazardous Substances and Environmental Engineering, 50(4), 357–364. https://doi.org/10.1080/10934529.2015.987524.
Masłoń, A., & Tomaszek, J. A. (2015). A study on the use of the BioBall® as a biofilm carrier in a sequencing batch reactor. Bioresour Technol, 196, 577–585. https://doi.org/10.1016/j.biortech.2015.08.020.
Mekuto, L., Jackson, V. A., & Ntwampe, S. K. O. (2013). Biodegradation of free cyanide using Bacillus sp. consortium dominated by Bacillus safensis, Lichenformis and Tequilensis strains: A bioprocess supported solely with whey. Journal of Bioremediation & Biodegradation, S18, 004. https://doi.org/10.4172/2155-6199.S18-004.
Metcalf and Eddy. (1991). Wastewater engineering: Treatment Disposal Reuse (pp. 314–720) (3rd ed.). New York: McGraw-Hill.
Meuwly, F., Loviat, F., Ruffieux, P. A., Bernard, A. R., Kadouri, A., & von Stockar, U. (2005). Oxygen supply for CHO cells immobilized on packed bed of Fibra-Cel disks. Biotechnol Bioeng, 93(4), 791–800. https://doi.org/10.1002/bit.20766.
Mpongwana, N., Ntwampe, S. K. O., Omodanisi, E. I., Chidi, B. S., & Razanamahandry, L. C. (2019). Sustainable approach to eradicate the inhibitory effect of free-cyanide on simultaneous nitrification and aerobic denitrification during wastewater treatment. Sustainability, 11(21), 6180. https://doi.org/10.3390/su11216180.
Naveen, D., Majumder, C. B., Mondal, P., & Shubha, D. (2011). Biological treatment of cyanide containing wastewater. Research Journal of Chemical Sciences, 1(7), 15–21.
Nicolella, C., van Loosdrecht, M. C. M., & Heijnen, J. J. (2000). Wastewater treatment with particulate biofilm reactors. J Biotechnol, 80(1), 1–33. https://doi.org/10.1016/S0168-1656(00)00229-7.
Parga, J. R., Shukla, S. S., & Carrillo-Pedroza, F. R. (2003). Destruction of cyanide waste solutions using chlorine dioxide, ozone and titania sol. Waste Manag, 23(2), 183–191. https://doi.org/10.1016/S0956-053X(02)00064-8.
Patil, Y. B., & Paknikar, K. M. (2000a). Biodetoxification of silver-cyanide from electroplating industry wastewater. Lett Appl Microbiol, 30(1), 33–37. https://doi.org/10.1046/j.1472-765x.2000.00648.x.
Patil, Y. B., & Paknikar, K. M. (2000b). Development of a process for biodetoxification of metal cyanides from waste waters. Process Biochem, 35(10), 1139–1151. https://doi.org/10.1016/S0032-9592(00)00150-3.
Peters, D., & Ngai, D. D. (2005). Agro-processing waste assessment and management in Peri-urban Hanoi, Vietnam. J Sustain Agric, 25(1), 69–95. https://doi.org/10.1300/J064v25n01_07.
Petrozzi, S., & Dunn, I. J. (1994). Biological cyanide degradation in aerobic fluidized bed reactors: Treatment of almond seed wastewater. Bioprocess Eng, 11, 29–38. https://doi.org/10.1007/BF00369612.
Potivichayanon, S., & Kitleartpornpairoat, R. (2014). Degradation of cyanide to ammonia and nitrate by mixed culture of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2. In: Proceeding of the European conference on sustainability, energy and the environment (pp. 251–264). United Kingdom: Brighton.
Potivichayanon, S., Pokethitiyook, P., & Kruatrachue, M. (2006). Hydrogen sulfide removal by a novel fixed-film bioscrubber system. Process Biochem, 41(3), 708–715. https://doi.org/10.1016/j.procbio.2005.09.006.
Potivichayanon, S., Supromin, N., & Toensakes, R. (2017). Development of a mixed microbial culture for thiocyanate and metal cyanide degradation. 3. Biotech, 7, 191. https://doi.org/10.1007/s13205-017-0814-6.
Pueyo, N., Rodriguez-Chueca, J., Ovelleiro, J. L., & Ormad, M. P. (2016). Limitations of the removal of cyanide from coking wastewater by treatment with hydrogen peroxide. Water Air Soil Pollut, 227, 222. https://doi.org/10.1007/s11270-016-2915-y.
Ripley, E. A., Redmann, R. E., & Crowder, A. A. (1996). Environmental Effects of Mining. Florida: St. Lucie Press.
Romero Aguilar, M. A., Fdez-Güelfo, L. A., Álvarez-Gallego, C. J., & Romero García, L. I. (2013). Effect of HRT on hydrogen production and organic matter solubilization in acidogenic anaerobic digestion of OFMSW. Chem Eng J, 219, 443–449. https://doi.org/10.1016/j.cej.2012.12.090.
Sangyoka, S., Reungsang, A., & Moonamart, S. (2007). Repeated-batch fermentative for bio-hydrogen production from cassava starch manufacturing wastewater. Pak J Biol Sci, 10(11), 1782–1789. https://doi.org/10.3923/pjbs.2007.1782.1789.
Siller, H., & Winter, J. (1998). Degradation of cyanide in agroindustrial or industrial wastewater in an acidification reactor or in a single-step methane reactor by bacteria enriched from soil and peels of cassava. Appl Microbiol Biotechnol, 50, 384–389. https://doi.org/10.1007/s002530051309.
Sirianuntapiboon, S., & Chuamkaew, C. (2007). Packed cage rotating biological contactor system for treatment of cyanide wastewater. Bioresour Technol, 98(2), 266–272. https://doi.org/10.1016/j.biortech.2006.01.014.
Sirianuntapiboon, S., Chairattanawan, K., & Rarunroeng, M. (2008). Biological removal of cyanide compounds from electroplating wastewater (EPWW) by sequencing batch reactor (SBR) system. J Hazard Mater, 154(1–3), 526–534. https://doi.org/10.1016/j.jhazmat.2007.10.056.
Suh, Y.-J., Park, J. M., & Yang, J.-W. (1994). Biodegradation of cyanide compounds by Pseudomonas fluorescens immobilized on zeolite. Enzym Microb Technol, 16(6), 529–533. https://doi.org/10.1016/0141-0229(94)90025-6.
Supromin, N., Potivichayanon, S., & Toensakes, R. (2015). Degradation of metal cyanide from real electroplating wastewater by mixed culture of SUTS 1 and SUTS 2. 3rd International conference on biological, chemical & environmental sciences (BCES-2015). Kuala Lumpur. Malaysia., 3, 75–80.
Terada, A., Yamamoto, T., Tsuneda, S., & Hirata, A. (2006). Sequencing batch membrane biofilm reactor for simultaneous nitrogen and phosphorus removal: Novel application of membrane-aerated biofilm. Biotechnol Bioeng, 94(4), 730–739. https://doi.org/10.1002/bit.20887.
Third, K. A., Burnett, N., & Cord-Ruwisch, R. (2003). Simultaneous nitrification and denitrification using stored substrate (phb) as the electron donor in an SBR. Biotechnol Bioeng, 83(6), 706–720. https://doi.org/10.1002/bit.10708.
Watanabe, A., Yano, K., Ikebukuro, K., & Karube, I. (1998). Cyanide hydrolysis in a cyanide-degrading bacterium, Pseudomonas stutzeri AK61, by cyanidase. Microbiology, 144(6), 1677–1682. https://doi.org/10.1099/00221287-144-6-1677.
White, D. M., & Schnabel, W. (1998). Treatment of cyanide waste in a sequencing batch biofilm reactor. Water Res, 32(1), 254–257. https://doi.org/10.1016/S0043-1354(97)00167-X.
White, D. M., Pilon, T. A., & Woolard, C. (2000). Biological treatment of cyanide containing wastewater. Water Res, 34(7), 2105–2109. https://doi.org/10.1016/S0043-1354(99)00362-0.
Wilen, B.-M., & Balmer, P. (1998). Short term effects of dissolved oxygen concentration on the turbidity of the supernatant of activated sludge. Water Sci Technol, 38(3), 25–33. https://doi.org/10.1016/S0273-1223(98)00448-X.
Yanase, H., Sakamoto, A., Okamoto, K., Kita, K., & Sato, Y. (2000). Degradation of the metal-cyano complex tetracyanonickelate (II) by Fusarium oxysporium N-10. Appl Microbiol Biotechnol, 53, 328–334. https://doi.org/10.1007/s002530050029.
Zheng, D., Chang, Q., Gao, M., She, Z., Jin, C., Guo, L., Zhao, Y., Wang, S., & Wang, X. (2016). Performance evaluation and microbial community of a sequencing batch biofilm reactor (SBBR) treating mariculture wastewater at different chlortetracycline concentrations. J Environ Manag, 182, 496–504. https://doi.org/10.1016/j.jenvman.2016.08.003.
Zhou, X., Liu, L., Chen, Y., Xu, S., & Chen, J. (2007). Efficient biodegradation of cyanide and ferrocyanide by Na-alginate beads immobilized with fungal cells of Trichoderma koningii. Can J Microbiol, 53(9), 1033–1037. https://doi.org/10.1139/W07-070.
Funding
The authors gratefully thank the Suranaree University of Technology, Nakhon Ratchasima, Thailand, for supporting, funding, and providing the facilities for this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Potivichayanon, S., Toensakes, R., Supromin, N. et al. Removal of High Levels of Cyanide and COD from Cassava Industrial Wastewater by a Fixed-Film Sequencing Batch Reactor. Water Air Soil Pollut 231, 301 (2020). https://doi.org/10.1007/s11270-020-04642-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04642-7