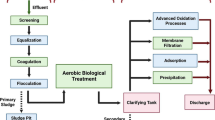
Abstract
This research work evaluates the use of hydrogen peroxide for the removal of cyanide from coking wastewater deriving from the washing of gases in coal combustion furnace. The effect of the presence or absence of suspended solids and organic micropollutants on the efficiency of the treatment is analyzed. Various dosages of hydrogen peroxide (6.5–200 mg/L) were added to both aqueous solution (at pH 10.5) and industrial wastewater (at pH 10.3) samples. The influence of suspended solids in coking wastewater was analyzed by applying a coagulation–flocculation–decantation process before the hydrogen peroxide treatment. The preliminary cyanide removal treatment in aqueous solution showed that the maximum cyanide removal did not exceed 14 % using a mass ratio of hydrogen peroxide to cyanide of 11.6. The maximum cyanide removal obtained in coking wastewater was 47 % with a mass ratio of hydrogen peroxide to cyanide of 12.2 provided that a coagulation–flocculation–decantation pretreatment was applied to remove the suspended solids composed mainly of coal, calcium carbonate, and magnesium carbonate. On the other hand, the cyanide removal treatment in coking wastewater with hydrogen peroxide showed promising results in the removing of different organic micropollutants formed mainly by polycyclic aromatic hydrocarbons and quinolines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cyanide is a pollutant detected in industrial wastewater in mining, metal processing, electroplating, coke processing, the automobile industry, the chemical manufacturing industry, the pharmaceutical industry, the dye and textile industry, the photographic industry, etc. (Kjeldsen 1999; Dash et al. 2009). The total cyanide concentration in industrial wastewater is generally between 0.01 and 10 mg/L (Wild et al. 1994; Mudder et al. 2001). However, wastewater from industries like electroplating may contain even higher cyanide concentrations of 100,000 mg/L (Wild et al. 1994). Cyanides in all their forms, such as free cyanide, cyanide salts, or metal-cyanide complexes, are potentially toxic with free cyanide being the most hazardous form (Boening and Chew 1999; Eisler 2000; Mudder et al. 2001). Cyanides do not accumulate in the body, but because of their ability to bind iron in blood by forming metal-cyanide complexes they can inhibit oxygen transfer to the cells, thereby causing suffocation of animals and humans (Kuyucak and Akcil 2013; Griffiths et al. 2014). Therefore, to protect human health and the quality of the environment, cyanide-polluted effluent must be adequately treated prior to discharge to receiving waterways.
European legislation considers cyanide as a polluting substance, and member states are obliged to establish emission limit values for cyanide emitted from industrial installations (Directive 2010/75/EU). The Spanish legislation (Royal Decree 817/2015) classifies cyanide as a preferential pollutant due to its significant risk to the environment in surface waters in Spain. The environmental quality standard sets out a maximum concentration of 40 μg/L for cyanide in continental surface waters.
Cyanide removal in wastewater can be accomplished using various separation and oxidation processes (Young and Jordan 1995). The efficiency of these processes depends on the type and concentration of the cyanide species present (Young and Jordan 1995; Yazici et al. 2006). Separation processes based on physical, complexation, or adsorption methods generate toxic by-products that must be treated subsequently (Young and Jordan 1995; Mudder et al. 2001). However, oxidation processes are the only treatments capable of removing cyanide and generate less toxic compounds such as ammonia, cyanate, and nitrate. Cyanide oxidation processes are based on biological, catalytic, electrolytic, chemical, and photolytic methods (Young and Jordan 1995; Mudder et al. 2001; Dash et al. 2009). The cyanide removal treatments most commonly applied on an industrial scale are natural degradation and chemical and biological degradation processes. Natural degradation, hydrogen peroxide, and Inco SO2/air processes are known to be widely used processes while other processes find limited application (Kuyucak and Akcil 2013).
Cyanide removal by hydrogen peroxide has been used since the 1980s. The first full-scale hydrogen peroxide treatment was built and operated by Degussa at the Ok Tedi Mining Limited gold mine in Papua New Guinea. The concentration of total cyanide in gold mill tailings was reduced to less than 10 mg/L (Knorre and Griffiths 1984). Treatment with hydrogen peroxide consists of oxidizing cyanide to produce cyanate which subsequently hydrolyses to give ammonia and carbonate (Eqs. 1 and 2) (Knorre and Griffiths 1984; Young and Jordan 1995; Mudder et al. 2001). Normally, about 10–20 % of cyanide is converted to ammonia during treatment (Mudder et al. 2001). If there is an excess of hydrogen peroxide, it can form nitrite and carbonate (Eq. 3) and eventually nitrate (Eq. 4) (Young and Jordan 1995). According to Hartinger (1994), a hydrogen peroxide excess of 100 % of stoichiometric dosage is used in full-scale applications. However, another study considers that the excess of hydrogen peroxide must be 200–450 % (Young and Jordan 1995). The excess hydrogen peroxide added is decomposed to oxygen and water being able to even react with other oxidizable compounds (Eq. 5) (Young and Jordan 1995).
Cyanide treatment by hydrogen peroxide has been investigated previously. Many studies have analyzed the use of homogeneous or heterogeneous catalysts for the removal of cyanide by treatment with hydrogen peroxide. For instance, catalyzed treatment has been investigated in the presence of Ru/MgO (Pak and Chang 1997), cadmium (Lee et al. 2004), activated carbon (Yeddou et al. 2010), copper (Sarla et al. 2004; Kitis et al. 2005a; Yazici et al. 2006; Chen et al. 2014), copper-impregnated pumice (Kitis et al. 2005b), and copper-impregnated activated carbon (Yeddou et al. 2011). The rate and extent of cyanide decomposition by hydrogen peroxide are dependent upon different factors including the pH, temperature, initial cyanide concentration, hydrogen peroxide concentration, absence or presence of catalyst, and type and concentration of catalyst (Lee et al. 2004; Kitis et al. 2005a; Yazici et al. 2006; Yeddou et al. 2010). Sarla et al. (2004) reported that with an initial cyanide concentration of 100 mg/L in an aqueous solution, 90 % of the cyanide was removed in 24 h with 88.2 mM H2O2 at a pH of 10. By contrast, more than 90 % of the cyanide from an aqueous solution with an initial cyanide concentration of 260 mg/L was removed in 20 min with hydrogen peroxide (initial molar ratio [H2O2]/[CN−] = 3) catalyzed by copper-impregnated activated carbon (10 g/L CuO-AC) at a pH of 11 (Yeddou et al. 2011). Other studies have investigated different advanced oxidation processes for the removal of cyanide using hydrogen peroxide in combination with ozone (Monteagudo et al. 2004; Kepa et al. 2008), UV irradiation (Kim et al. 2003; Sarla et al. 2004), and ozone/UV irradiation (Kim et al. 2003; Monteagudo et al. 2004; Mudliar et al. 2009).
Coking wastewater is a complex industrial waste whose composition is multiple and varies from one industry to another. Different studies have applied processes for the removal of cyanide from coking wastewater (Zhang et al. 2010; Jin et al. 2013; Marañón et al. 2008). For instance, a full-scale pre-denitrification process has been used to treat coking wastewater containing cyanides and a considerable amount of cyanides has not nearly been removed biologically (Park et al. 2008). Van-Leeuwen et al. (2003) reported the efficiency of ozonation for the removal of cyanide from coking wastewater. By contrast, cyanide could not be removed almost entirely from coking wastewater by ozonation due to the inadequate oxidation ability of ozone to remove ozonated by-products under given experimental conditions (Chang et al. 2008). However, it is well-known that ozonation has high economic costs due to their operations often require external energy, chemicals, and operators (Mudder et al. 2001; Kim et al. 2003). Whereas many studies have analyzed the use of hydrogen peroxide for the removal of cyanide from aqueous solutions, the aim of this research work is to evaluate the applicability of the oxidizing agent for the removal of cyanide from coke plant wastewater deriving from the washing of gases in coal combustion furnace. The treatment by hydrogen peroxide is previously applied to the oxidation of cyanide from aqueous solutions in order to evaluate the oxidizing power of the agent in the absence of interfering species in the matrix. In addition, the effect of the presence or absence of suspended solids and organic micropollutants on the efficiency of the treatment applied for the removal of cyanide from industrial wastewater is analyzed.
2 Materials and methods
2.1 Samples
Synthetic wastewater samples (SW) and real wastewater samples (RW) were used in this study. SW with a cyanide concentration of 4.3 mg/L were prepared with sodium cyanide (Merck) and MilliQ® water. The adjustment of pH above 10 was carried out by the addition of sodium hydroxide to prevent the liberation of the toxic hydrogen cyanide gas (Yazici et al. 2009).
RW were obtained from a chemical manufacturing plant located in northwestern Spain. The wastewater emissions resulted from the washing of gases in a scrubber. The maximum concentration of emissions of total cyanide from coking wastewater of this chemical manufacturing plant is 2.0 mg/L. The samples were collected in glass bottles and transported to the laboratory for experiments.
2.2 Experimental Procedure
The treatment by hydrogen peroxide was carried out using a H2O2 solution at 30 % w/v (Panreac) as the oxidizing agent. The experiments were carried out with different dosages of hydrogen peroxide added to the SW samples, i.e., 6.5, 25, 50, 75, 100, 150, and 200 mg/L H2O2. The hydrogen peroxide dosages added to the RW samples were as follows: 25, 50, 100, and 200 mg/L H2O2. All samples were agitated with a magnetic stirrer (40 rpm) to provide an aerated environment and perfect mix. The reaction time was 5 min, which ensured total consumption of the dosages of hydrogen peroxide employed. All the experiments were performed at room temperature and ambient light in order to reproduce full-scale operational conditions.
A coagulation–flocculation–decantation process was carried out for the removal of suspended solids in the RW samples. Polyaluminum chloride PAXC18 was added as a coagulant to obtain a concentration of 4 mg/L in the wastewater samples. A dosage of acrylamide copolymer AS74 of 1 mg/L was added as a flocculant agent. This concentration of flocculating agent was chosen after a previous study about optimization of the coagulation–flocculation–decantation process using different dosages of flocculating agent. A jar-test was performed using a SBS six-paddle stirrer. The mixing conditions were 3 min of rapid mixing at 200 rpm to simulate the coagulation process and 15 min at 40 rpm to simulate the flocculation process, followed by 30 min of settling time. This process was combined with the hydrogen peroxide process in order to study the influence of the presence of suspended solids in the removal of cyanides in industrial wastewater.
All the tests were carried out at least by duplicate. Reproducibility of the results of every duplicated test was checked with statistical analysis, where the confidence percentage was <95 %. The results shown in the tables and graphs were obtained as the average of the replicates and the error bar is the standard deviation.
2.3 Analysis of Samples
2.3.1 Analysis of Water and Wastewater
The total cyanide concentration was determined by a titrimetric method using silver nitrate (Panreac) and 5-(4-dimethylaminobenzylidene)rhodanine indicator (Alfa Aesar) after distillation (4500-D Standard Method (Eaton et al. 2005)). The alkalinity was determined by the 2320-B Standard Method (Eaton et al. 2005). The total hardness was measured by the 2340-C Standard Method (Eaton et al. 2005), and the calcium hardness was determined by the 3500-Ca B Standard Method (Eaton et al. 2005). The total suspended solids were determined by the 2540-D Standard Method (Eaton et al. 2005).
The pH was measured by a pH meter (Crison GLP21), the conductivity by a conductivity meter (Crison Basic 30), the ammonia by an electrometer (Crison GLP22) and an ammonia-selective electrode (Hach 51927-00), and the turbidity by a turbidimeter (Hanna LP2000). The total organic carbon was measured by a total organic carbon analyzer (Shimadzu TOC-VCHS) following the 5310-B Standard Method (Eaton et al. 2005). The organic micropollutants analysis was carried out by a GC/MS analysis (EPA Method 625 1984). A gas chromatograph (Varian 3300) connected to a mass spectrometer (Finnigan MAT 800 ITD) was used for identifying the organic micropollutants. The chromatographic conditions employed are shown in Table 1. The samples underwent a liquid–liquid extraction prior to their analysis by GC/MS.
2.3.2 Analysis of Solid Waste
A field emission scanning electron microscope (FESEM) (Carl Zeiss MerlinTM) was used for observation of the surface morphology and identification of the chemical elements in the solid waste samples. The samples were previously dried in an oven at 103 to 105 °C.
3 Results and Discussion
3.1 Characterization of the Coking Wastewater
The characterization of the coke plant wastewater was carried out by means of an analysis of the wastewater and solid waste. Table 2 summarizes the values of the general parameters and the identification of individual micropollutants in the wastewater samples. The solid waste sample corresponds to the suspended solids obtained after carrying out the coagulation–flocculation–decantation process. Table 3 shows the percentage by weight of the detected elements in the composition of the suspended solids, and Fig. 1 shows the FESEM image of the suspended solids sample.
The characterization reveals that the coking wastewater has a high content of inorganic dissolved solids (measured as conductivity), calcium and magnesium ions (measured as total hardness and calcium hardness), and suspended solids. However, Marañón et al. (2008) reported that the main pollutants present in coke wastewater were ammonia, phenols, thiocyanates, and chemical oxygen demand. GC/MS analysis shows several identified organic micropollutans, including polycyclic aromatic hydrocarbons and quinolines. These compounds are among the main organic pollutants found in coke plant wastewater, these being phenols, polycyclic aromatic hydrocarbons, quinolines, indoles, and pyridines (Xu and Ning 2002; Wei et al. 2012; Wu and Zhu 2012; Zhang et al. 2012; Jin et al. 2013; Zhang et al. 2013).
The FESEM elemental analysis shows that the major elements in the suspended solids sample are carbon, oxygen, magnesium, and calcium. Following an analysis of the relative percentages, it can be seen that the suspended solids sample is composed mainly of coal (C), calcium carbonate (CaCO3) and magnesium carbonate (MgCO3), with other minority elements such as sodium, aluminum, silicon, etc. representing a weight percentage of less than 2.32 %.
3.2 Treatment of Synthetic Wastewater
Figure 2 shows the percentage of cyanide removal from synthetic wastewater as a function of different dosages of hydrogen peroxide. Using a hydrogen peroxide dosage of 6.5 mg/L corresponding to the stoichiometric dosage for a theoretical initial concentration of cyanide of 5.0 mg/L, the cyanide removal efficiency does not exceed 4 %. For hydrogen peroxide dosages of less than 50 mg/L, the cyanide removal efficiency is increased until a maximum value of 14 % if the dosage of the oxidizing agent is increased. The dosage of 50 mg/L H2O2 (43 % of excess H2O2) is much lower than that recommended by other studies which suggest using a 100 % excess of the stoichiometric dosage (Hartinger 1994). When using dosages of hydrogen peroxide higher than 50 mg/L (75–200 mg/L), the cyanide removal efficiency is decreased until a value of 8 %. This result suggests that an added hydrogen peroxide excess has a negative effect on the cyanide removal efficiency. This could be due to the decomposition of the hydrogen peroxide to oxygen and water (Mudder et al. 2001). Besides this decomposition reaction, several authors have suggested for the removal of pollutants using different advanced oxidation processes combined with hydrogen peroxide that the hydroxyl radicals formed can be scavenged by an excess of hydrogen peroxide to form much less oxidative hydroperoxide radicals (Liao and Gurol 1995; Nelieu et al. 2000; Esplugas et al. 2002; Ku et al. 2005). Comparing the reaction rate constant for hydroperoxide radical with hydrogen peroxide and hydroxyl radical (k = 2.7 × 107 M−1 s−1) and the reaction rate constant for hydroxyl radical with cyanide (k = 4.5 × 109 M−1 s−1) (Gottschalk et al. 2000), it is possible to consider that an excess of hydrogen peroxide might react with the hydroxyl radicals competitively to form hydroperoxide radicals. Other studies have suggested that the cyanide removal increases when increasing the dosages of hydrogen peroxide both in aqueous solution (Yazici et al. 2006) and in industrial wastewater (Kitis et al. 2005a). The dosage of hydrogen peroxide that leads to the maximum cyanide removal in synthetic wastewater is 50 mg/L, representing a mass ratio of H2O2 to CN− of 11.6.
3.3 Treatment of Real Wastewater
Table 4 shows the results of the removal of cyanide in the presence of suspended solids in the real wastewater and in the absence of suspended solids after the real wastewater is pre-treated by the coagulation–flocculation–decantation process. The cyanide removal efficiency by hydrogen peroxide in real wastewater does not exceed 8.4 % for a hydrogen peroxide dosage of 25 mg/L. However, the cyanide removal efficiency is tripled to a value of 30.1 % when removing the suspended solids for the same concentration of hydrogen peroxide, according to the results shown in Table 4. This is due to the partial consumption of the hydrogen peroxide to react with suspended matter composed mainly of coal (Table 3). It has been widely assumed the catalytic decomposition of H2O2 by activated carbon (AC) involves the exchange of a surface hydroxyl group on the AC surface with a hydrogen peroxide anion to generate peroxide on the surface. The AC is regenerated by the decomposition of H2O2 to oxygen (Huang et al. 2003; Georgi and Kopinke 2005; Khorramfar et al. 2011). Besides this decomposition reaction, several authors have suggested that the H2O2 can be activated on the AC surface to generate hydroxyl and hydroperoxide radicals and oxidized AC (Eqs. 6 and 7). These radical species have higher oxidation capacity than hydrogen peroxide (Georgi and Kopinke 2005; Khorramfar et al. 2011). In particular, Yeddou et al. (2010) showed that the AC of plant origin had a beneficial effect for cyanide removal in aqueous solution by treatment with hydrogen peroxide.
Figure 3 illustrates the percentage of cyanide removal in the absence of suspended solids in real wastewater as a function of different initial concentrations of cyanide and different dosages of hydrogen peroxide. For an initial concentration of cyanide of 4.1 mg/L, the maximum cyanide removal efficiency of 47 % is achieved with a dosage of hydrogen peroxide of 50 mg/L (43 % of excess H2O2), if the wastewater is pre-treated by the coagulation–flocculation–decantation process. The dosage of H2O2 used to obtain the maximum efficiency of cyanide removal in real wastewater is the same as the dosage of the oxidizing agent added in aqueous solution to achieve maximum cyanide removal (Fig. 2). This result is comparable with that obtained by Monteagudo et al. (2004) in removing cyanide (initial cyanide concentration of 3 mg/L) in thermoelectric power station wastewater. The cyanide removal efficiency was about 60 % with 205.8 mM H2O2 (more than 6000 % of excess H2O2) at a pH of 9.5 in 20 min. Sarla et al. (2004) obtained a 60 % cyanide reduction (with an initial cyanide concentration of 100 mg/L) in aqueous solution with 44.12 mM H2O2 (more than 1300 % excess of H2O2) at a pH of 10 in 4 hours. Using dosages of hydrogen peroxide higher than 50 mg/L (100 or 200 mg/L), cyanides are not removed and are even generated. This may indicate the presence of other cyanide precursor organic micropollutants in the matrix (Table 2) that react with hydrogen peroxide to form this compound following partial oxidation. As occurs in synthetic wastewater (Fig. 2), the increase in the dosage of hydrogen peroxide does not produce an enhancing effect in the removal of cyanide. Centi et al. (1989) showed the dehydration of oximes and similar compounds such as 2-methylbenzonitrile by ammonia (Eq. 2) and oxygen (Eq. 5) to give nitriles. Nekrasov et al. (1989) studied the formation of cyanide by the nitriles formed in the above reaction. Cyanide can be formed from other organic micropollutants that have the same functional group such as 1-naphtalenecarbonitrile. For an initial concentration of cyanide of 17.1 mg/L, cyanides are not removed for any dosages of added hydrogen peroxide. A negative cyanide removal efficiency (−31 %) is obtained using a 50 mg/L dosage of hydrogen peroxide. When using dosages of hydrogen peroxide higher than 50 mg/L, the cyanide removal efficiency is less negative in comparison with cyanide removal tests of low initial concentration. Contrary to what has been suggested (Kitis et al. 2005a; Yazici et al. 2006), cyanide removal is not improved by increasing the dosage of hydrogen peroxide. The conditions that lead to the maximum cyanide removal are a dosage of hydrogen peroxide of 50 mg/L added to real wastewater in the absence of suspended solids, representing a mass ratio of H2O2 to CN− of 12.2.
After evaluating the applicability of hydrogen peroxide for the removal of cyanide, the real wastewater was subjected to a liquid–liquid extraction and a GC/MS analysis in order to determine the removal of the initial organic micropollutants (Table 2). The percentage of removal of the identified micropollutants in the real wastewater is presented in Table 5. It was found that the treatment with hydrogen peroxide is efficient for the total removal of the organic micropollutants. The maximum removal of some organic micropollutants was obtained, such as benzo[a]pyrene, naphtho[1,2-b]phenanthrene, and 1- naphthalenecarbonitrile. In addition, the partial removal of most of them (naphthalene, fluoranthene, anthracene, 2-methylbenzonitrile, etc.) was observed after treatment with hydrogen peroxide. Research reported by Chu et al. (2012) showed results similar to these for removing the organic compounds in coking wastewater. In particular, several organic compounds, including bifuran, quinoline, resorcinol, and benzofuranol, were removed during the advanced Fenton oxidation process using iron powder and hydrogen peroxide.
In the combined treatment, a white precipitate was identified in real wastewater after the addition of hydrogen peroxide. The white precipitate was characterized by means of field emission scanning electron microscopy. Table 6 shows the percentage by weight of the elements detected in the composition of the white precipitate, and Fig. 4 shows the FESEM image of the white precipitate sample. FESEM elemental analysis shows that the major elements of the white precipitate sample are carbon, oxygen, and calcium. Following an analysis of the relative percentages, it can be said that the white precipitate sample is composed mainly of calcium carbonate. The detection of white precipitate is due to the crystallization of CaCO3 in the presence of H2O and O2 from the decomposition of H2O2 (Yan et al. 2012).
4 Conclusions
The main conclusions drawn from this research work are summarized as follows:
-
The maximum cyanide removal in synthetic wastewater at pH 10 does not exceed 14 % using a mass ratio of hydrogen peroxide to cyanide of 11.6. Using higher mass ratios of hydrogen peroxide to cyanide, the cyanide removal efficiency is not improved due to the decomposition of the reactive to oxygen and water.
-
Coal, calcium carbonate, and magnesium carbonate, the main constituents of suspended solids in coking wastewater, decrease the cyanide removal efficiency by treatment with hydrogen peroxide due to partial consumption of the oxidizing agent that reacts with suspended matter.
-
The conditions that lead to the maximum cyanide removal of 47 % in coking wastewater are a mass ratio of hydrogen peroxide to cyanide of 12.2 used in industrial wastewater at pH 10 in the absence of suspended solids. Using higher mass ratios of hydrogen peroxide to cyanide, cyanides are not removed and can even be generated due to the presence of other cyanide precursor organic micropollutants in the matrix that react with hydrogen peroxide to form cyanide following partial oxidation.
-
The cyanide removal treatment in coking wastewater with hydrogen peroxide is efficient for removing the organic micropollutants formed mainly by polycyclic aromatic hydrocarbons and quinolines. Organic micropollutants such as naphthalene, acenaphthylene, anthracene, and 2-methylbenzonitrile are partially removed.
References
Boening, D. W., & Chew, C. M. (1999). A critical review: general toxicity and environmental fate of three aqueous cyanide ions and associated ligands. Water, Air, and Soil Pollution, 109(1), 67–79.
Centi, G., Pinelli, D., & Trifiro, F. (1989). Heterogeneous catalytic ammoxidation of o-xylene to phthalimide: analysis of the reaction network. Gazzetta Chimica Italiana, 119, 133–137.
Chang, E. E., Hsing, H. J., Chiang, P. C., Chen, M. Y., & Shyng, J. Y. (2008). The chemical and biological characteristics of coke-oven wastewater by ozonation. Journal of Hazardous Materials, 156(1–3), 560–567.
Chen, F., Zhao, X., Liu, H., & Qu, J. (2014). Reaction of Cu(CN)3 2− with H2O2 in water under alkaline conditions: cyanide oxidation, Cu+/Cu2+ catalysis and H2O2 decomposition. Applied Catalysis B: Environmental, 158–159, 85–90.
Chu, L., Wang, J., Dong, J., Liu, H., & Sun, X. (2012). Treatment of coking wastewater by an advanced Fenton oxidation process using iron powder and hydrogen peroxide. Chemosphere, 86(4), 409–414.
Dash, R. R., Gaur, A., & Balomajumder, C. (2009). Cyanide in industrial wastewaters and its removal: a review on biotreatment. Journal of Hazardous Materials, 163(1), 1–11.
Directive 2010/75/EU of 24 of November of 2010 laid down industrial emissions (integrated pollution prevention and control). Official Journal of the European Union L 334:17 of December of 2010.
Eaton, A. D., Clesceri, L. S., Rice, E. W., Greenberg, A. E., & Franson, M. A. H. (2005). Standard methods for the examination of water and wastewater. Washington: American Public Health Association.
Eisler, R. (2000). Handbook of chemical risk assessment: health hazards to humans, plants and animals. Boca Raton: Lewis Publishers.
EPA Method 625 (1984). Methods for organic chemical analysis of municipal and industrial wastewater–base/neutrals and acids.
Esplugas, S., Gimenez, J., Contreras, S., Pascual, E., & Rodriguez, M. (2002). Comparison of different advanced oxidation processes for phenol degradation. Water Research, 36(4), 1034–1042.
Georgi, A., & Kopinke, F. (2005). Interaction of adsorption and catalytic reactions in water decontamination processes Part I. Oxidation of organic contaminants with hydrogen peroxide catalyzed by activated carbon. Applied Catalysis B: Environmental, 58(1–2), 9–18.
Gottschalk, C., Libra, J. A., & Saupe, A. (2000). Ozonation of water and waste water: a practical guide to understanding ozone and its applications. Germany: Wiley-VCH.
Griffiths, S. R., Donato, D. B., Coulson, G., & Lumsden, L. F. (2014). High levels of activity of bats at gold mining water bodies: implications for compliance with the International Cyanide Management Code. Environmental Science and Pollution Research, 21(12), 7263–7275.
Hartinger, L. (1994). Handbook of effluent treatment and recycling for the metal finishing industry. Warrington: Finishing Publications.
Huang, H., Lu, M., Chen, J., & Lee, C. (2003). Catalytic decomposition of hydrogen peroxide and 4-chlorophenol in the presence of modified activated carbons. Chemosphere, 51(9), 935–943.
Jin, X., Li, E., Lu, S., Qiu, Z., & Sui, Q. (2013). Coking wastewater treatment for industrial reuse purpose: combining biological processes with ultrafiltration, nanofiltration and reverse osmosis. Journal of Environmental Sciences, 25(8), 1565–1574.
Kepa, U., Stanczyk-Mazanek, E., & Stepniak, L. (2008). The use of the advanced oxidation process in the ozone + hydrogen peroxide system for the removal of cyanide from water. Desalination, 223(1–3), 187–193.
Khorramfar, S., Mahmoodi, N., Arami, M., & Bahrami, H. (2011). Oxidation of dyes from colored wastewater using activated carbon/hydrogen peroxide. Desalination, 279(1–3), 183–189.
Kim, Y. J., Qureshi, T. I., & Min, K. S. (2003). Application of advanced oxidation processes for the treatment of cyanide containing effluent. Environmental Technology, 24(10), 1269–1276.
Kitis, M., Akcil, A., Karakaya, E., & Yigit, N. O. (2005a). Destruction of cyanide by hydrogen peroxide in tailings slurries from low bearing sulphidic gold ores. Minerals Engineering, 18(3), 353–362.
Kitis, M., Karakaya, E., Yigit, N. O., Civelekoglu, G., & Akcil, A. (2005b). Heterogeneous catalytic degradation of cyanide using copper-impregnated pumice and hydrogen peroxide. Water Research, 39(8), 1652–1662.
Kjeldsen, P. (1999). Behaviour of cyanides in soil and groundwater: a review. Water, Air, and Soil Pollution, 115(1), 279–307.
Knorre, H., & Griffiths, A. (1984). Cyanide detoxification with hydrogen peroxide using the Degussa process. Tucson: Proceedings of the Cyanide and the Environment Conference.
Ku, Y., Tu, Y. H., & Ma, C. M. (2005). Effect of hydrogen peroxide on the decomposition of monochlorophenols by sonolysis in aqueous solution. Water Research, 39(6), 1093–1098.
Kuyucak, N., & Akcil, A. (2013). Cyanide and removal options from effluents in gold mining and metallurgical processes. Minerals Engineering, 50–51, 13–29.
Lee, T., Kwon, Y., & Kim, D. (2004). Oxidative treatment of cyanide in wastewater using hydrogen peroxide and homogeneous catalyst. Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances and Environmental Engineering, 39(3), 787–801.
Liao, C. H., & Gurol, M. D. (1995). Chemical oxidation by photolytic decomposition of hydrogen peroxide. Environmental Science and Technology, 29(12), 3007–3014.
Marañón, E., Vázquez, I., Rodríguez, J., Castrillón, L., & Fernández, Y. (2008). Coke wastewater treatment by a three-step activated sludge system. Water, Air, and Soil Pollution, 192(1), 155–164.
Monteagudo, J. M., Rodríguez, L., & Villaseñor, J. (2004). Advanced oxidation processes for destruction of cyanide from thermoelectric power station waste waters. Journal of Chemical Technology and Biotechnology, 79(2), 117–125.
Mudder, T., Botz, M., & Smith, A. (2001). Chemistry and treatment of cyanidation wastes. London: Mining Journal Books Ltd.
Mudliar, R., Umare, S. S., Ramteke, D. S., & Wate, S. R. (2009). Energy efficient—advanced oxidation process for treatment of cyanide containing automobile industry wastewater. Journal of Hazardous Materials, 164(2–3), 1474–1479.
Nekrasov, L. N., Peregudova, S. M., Yur’eva, L. P., Kravtsov, D. N., Uralets, I. A., & Zaitseva, N. N. (1989). Study of redox reactions of bis(arene)chromium complexes by use of the rotating disk and the rotating ring-disk electrodes: V. Cathodic processes involving nitriles; cyano derivatives of benzene and dibenzenechromium. Journal of Organometallic Chemistry, 365(3), 269–284.
Nelieu, S., Kerhoas, L., & Einhorn, J. (2000). Degradation of atrazine into ammeline by combined ozone/hydrogen peroxide treatment in water. Environmental Science and Technology, 34(3), 430–437.
Pak, D., & Chang, W. (1997). Oxidation of aqueous cyanide solution using hydrogen peroxide in the presence of heterogeneous catalyst. Environmental Technology, 18(5), 557–561.
Park, D., Kim, Y. M., Lee, D. S., & Park, J. M. (2008). Chemical treatment for treating cyanides-containing effluent from biological cokes wastewater treatment process. Chemical Engineering Journal, 143(1–3), 141–146.
Royal Decree 817/2015 of 11 of September of 2015, laid down the criteria for monitoring and evaluating the status of continental surface waters and the Environmental Quality Standards. Bulletin Official of State, 219 (I), 12.
Sarla, M., Pandit, M., Tyagi, D. K., & Kapoor, J. C. (2004). Oxidation of cyanide in aqueous solution by chemical and photochemical process. Journal of Hazardous Materials, 116(1–2), 49–56.
Van-Leeuwen, J., Badriyha, B., & Vaczi, S. (2003). Investigation into ozonation of coal coking processing wastewater for cyanide, thiocyanate and organic removal. Ozone Science and Engineering, 25(4), 273–283.
Wei, X., Zhang, Z., Fan, Q., Yuan, X., & Guo, D. (2012). The effect of treatment stages on the coking wastewater hazardous compounds and their toxicity. Journal of Hazardous Materials, 239–240, 135–141.
Wild, S. R., Rudd, T., & Neller, A. (1994). Fate and effects of cyanide during wastewater treatment processes. Science of the Total Environment, 56(2), 93–107.
Wu, Z., & Zhu, L. (2012). Removal of polycyclic aromatic hydrocarbons and phenols from coking wastewater by simultaneously synthesized organobentonite in a one-step process. Journal of Environmental Sciences, 24(2), 248–253.
Xu, S., & Ning, P. (2002). Analysis of organic pollutants from the wastewater of coking plant by GC/MS. Yunnan Chemical Technology, 29(5), 32–34.
Yan, F., Guo, C., Zhang, X., & Yuan, G. (2012). Hydrogen peroxide as a crystal growth modifier of CaCO3. CrystEngComm, 14(6), 2046–2052.
Yazici, E.Y., Deveci, H., Alp, Í., Uslu, T., & Celep, O. (2006). Factors affecting decomposition of cyanide by hydrogen peroxide. Proceedings of the 23rd International Mineral Processing Congress, Istanbul.
Yazici, E. Y., Deveci, H., & Alp, Í. (2009). Treatment of cyanide effluents by oxidation and adsorption in batch and column studies. Journal of Hazardous Materials, 166(2–3), 1362–1366.
Yeddou, A. R., Nadjemi, B., Halet, F., Ould-Dris, A., & Capart, R. (2010). Removal of cyanide in aqueous solution by oxidation with hydrogen peroxide in presence of activated carbon prepared from olive stones. Minerals Engineering, 23(1), 32–39.
Yeddou, A. R., Nadjemi, B., Halet, F., Ould-Dris, A., & Belkouch, J. (2011). Removal of cyanide in aqueous solution by oxidation with hydrogen peroxide in presence of copper-impregnated carbon. Minerals Engineering, 24(8), 788–793.
Young, C.A., & Jordan, T.S. (1995). Cyanide remediation: current and past technologies. Proceedings of the 10th Annual Conference on Hazardous Waste Research, Kansas.
Zhang, W., Liu, W., Lv, Y., Li, B., & Ying, W. (2010). Enhanced carbon adsorption treatment for removing cyanide from coking plant effluent. Journal of Hazardous Materials, 184(1–3), 135–140.
Zhang, W., Wei, C., Chai, X., He, J., Cai, Y., Ren, M., Yan, B., Peng, P., & Fu, J. (2012). The behaviors and fate of polycyclic aromatic hydrocarbons (PAHs) in a coking wastewater treatment plant. Chemosphere, 88(2), 174–182.
Zhang, W., Wei, C., Yan, B., Feng, C., Zhao, G., Lin, C., Yuan, M., Wu, C., Ren, Y., & Hu, Y. (2013). Identification and removal of polycyclic aromatic hydrocarbons in wastewater treatment processes from coke production plants. Environmental Science and Pollution Research, 20(9), 6418–6432.
Acknowledgments
This work was funded by the DGA-FSE Research Team T33 and the University of Zaragoza (Project JIUZ2014-TEC-11).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pueyo, N., Rodríguez-Chueca, J., Ovelleiro, J.L. et al. Limitations of the Removal of Cyanide from Coking Wastewater by Treatment with Hydrogen Peroxide. Water Air Soil Pollut 227, 222 (2016). https://doi.org/10.1007/s11270-016-2915-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2915-y