Abstract
The degradation capacity of a mixed culture of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2 for thiocyanate and metal cyanide, in the form of zinc and cadmium, has been determined. The growth of a mixed culture of SUTS 1 and SUTS 2 in cyanide complexes and the cyanide removal efficiency of a fixed-film bio-column system were studied. The results showed that the mixed culture of bacteria can survive and grow in broth media containing thiocyanate and metal cyanide complexes with a maximum cell of 1.03 × 108 CFU/mL on day 3. In addition, the optimal conditions of the fixed-film bio-column system were continuously tested for 24 h, and it was found that this system had the highest removal efficiency at a flow rate of 10 mL/min and 21 min of empty bed retention time, with decreasing thiocyanate, zinc, and cadmium from 85, 0.44, and 0.044 to 65, 0.21, and 0.038 mg/L, respectively; this is in contrast to cyanide, which was not found within 12 h. Next, the conditions were maintained for 30 days, and it was found that the system had removed more than 50% of cyanide complexes, except cadmium. The complex residues were 29.96, 0.16, 0.204, and 0.085 mg/L of thiocyanate, cyanide, zinc, and cadmium, respectively. In addition, the growth of the SUTS 1 and SUTS 2 mixed culture increased. The by-product compounds sulfate and nitrate were found throughout the experiment, whereas bicarbonate and ammonia were found only on certain days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanide is widely used in several industries, such as mining, metallurgy, painting, paper, and cassava (Medwith and Lefelhocz 1981; Melcer and Nutt 1988; Aronstein et al. 1994; Liu et al. 1996; EPA 1997; Patil and Paknikar 2000). As a result of the activities of these industries, aquatic environmental pollution in the form of cyanide compounds, thiocyanates, phenols, and ammonia are discharged from industrial processes at almost 10–150 mg/L. Thiocyanate concentrations in goal mining wastewater have been normally found from 100 mg/L. In addition to thiocyanate, heavy metals in the form of zinc and cadmium from mining effluents were also reported in the range of 0.01–0.1 and 0.005–1.0 mg/L, respectively (Mudder et al. 2001; Dzombak et al. 2006). Furthermore, cyanide can react with metals and heavy metals to form metal cyanide complexes, including Cu(CN) −2 , Ni(CN) 2−4 , Ag(CN) −2 , Zn(CN) 2−4 and Fe(CN) 4−6 , which differ considerably in toxicity and environmental persistence. The presence of free and metal cyanide complexes in industrial wastewater is an important environmental problem with acute toxicity affecting aquatic organisms and human health. The metal complexes in the form of zinc cyanide (Zn(CN)2) and cadmium cyanide (Cd(CN)2) are in the range of 0.02–0.3 mg/L; it can be harmful to aquatic organisms (Ingles and Scott 1987; Moran 1998).
Free cyanide also reacts with forms of sulfur in mining effluents, such as sulfur, thiosulfates, or sulfide ions, to produce thiocyanates. Thiocyanate can react with various metals, such as silver (Ag), mercury (Hg), lead (Pb), copper (Cu), and zinc (Zn), to form metal-thiocyanate complexes and turns into a salt that does not dissolve in water (Staden et al. 1997; Moran 2017). Plumlee et al. (1995) reported that relatively high concentrations of thiocyanate may persist in the presence of acidic solutions, which causes greater toxicity.
Thiocyanate can affect many organisms and accumulate into the environment in water and soil. Thiocyanate concentration in the range between 90 and 200 mg/L is toxic to aquatic organisms, especially in fish (Ingles and Scott 1987). Furthermore, it is toxic to many higher organisms at relatively low concentrations (1–2 mM), because it has strong tendencies to bind to proteins and acts as a non-competitive inhibitor (Wood et al. 1998). It is considered a health hazard and is acutely toxic to humans via entry through inhalation, ingestion, or absorption, especially can also attack the central nervous system of humans which is toxic include inhibition of halide transport to the thyroid gland (Boening and Chew 1997; Ahn et al. 2004; Ryu et al. 2014). The rate of skin absorption is enhanced when the skin is cut, abraded, or moistened. Thus, most countries require complete removal of these pollutants from effluent before disposal. In Thailand, the statutory limit for disposal of wastewater containing cyanide (as HCN), zinc, and cadmium into bodies of water is 0.2, 5.0, and 0.03 mg/L, respectively (Notification the Ministry of Science, Technology and Environment 1996). Although the disposal of thiocyanate is not defined by industrial standards, it should be removed before discharge into the environment, in addition to the removal of cyanide and heavy metals.
Cyanide can be degraded or detoxified through several methods; the biodegradation method is the process that is usually used to treat cyanide-containing substances by means of cyanide-oxidizing bacteria that break down and transform hazardous materials into simple non-toxic substances or harmless compounds. The biodegradation of metal cyanide complex anions is carried out by living cells and involves the metabolism and/or transformation of cyanide complexes into less toxic products (Dursun and Aksu 2000; Bose et al. 2002; Chakraborty and Veeramani 2006; Jeong and Chung 2006; Sirianuntapiboon and Chuamkaew 2007; Potivichayanon and Kitleartpornpairoat 2014; Supromin et al. 2015). Some microorganisms can adapt to grow in the presence of metal cyanide complexes by synthesizing enzymes that degrade cyanide complexes and utilizing cyanide as a source of nitrogen for growth. Although there are several reports on the treatment of cyanide compounds, the treatment for both thiocyanate and cyanide metals has rarely been reported. Most research has separately studied the treatment of each cyanide compound, including separate cyanide, thiocyanate, or metal cyanide degradation studies (Silva-Avalos et al. 1990; Hung and Pavlostathis 1997; Barclay et al. 1998; Patil and Paknikar 2000; Jeong and Chung 2006; Dash et al. 2009; Gurbuz et al. 2009; Potivichayanon and Kitleartpornpairoat 2014; Supromin et al. 2015). In addition, immobilized cell technology is promoted and used for the removal of pollutants that contaminate the environment. Immobilized microbial cell systems could also provide additional advantages over freely suspended cells (Kacer et al. 2002). For these reasons, the objective was to study the growth and removal efficiency of a mixed culture of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2 in the degradation of thiocyanate and metal cyanide complexes and develop a fixed-film bio-column system to determine the optimum flow rate for wastewater treatment.
Materials and methods
Microorganisms
The two different types of bacteria used were Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2; both were isolated from a wastewater treatment system in the cassava starch industry. The mixed cultures showed a high efficiency for cyanide degradation (Potivichayanon and Kitleartpornpairoat 2014). In this study, bacterial cells were cultured in a buffer medium containing 2.7 g KH2PO4, 3.5 g K2HPO4, 10 mL of a mineral salt solution (the following composition: 300 mg FeSO4·7H2O, 180 mg MgCl2·6H2O, 130 mg Co(NO3)2·6H2O, 40 mg CaCl2, 40 mg ZnSO4 and 20 mg MoO3 in 1 L deionized water), 100 mg/L of potassium thiocyanate (KSCN), and metal cyanide in the form of 0.05 mg/L zinc cyanide (Zn(CN)2) and cadmium cyanide (Cd(CN)2) by mixing 0.064 mg/L cadmium sulfate (3CdSO4·8H2O) and 0.04 mg/L potassium cyanide (KCN). The inoculum of 10 mL of mixed culture was added into an Erlenmeyer flask containing 90 mL of buffer medium and the cyanide complexes and incubated at room temperature at 150 rpm on a rotary shaker. The growth of the mixed culture was studied for 7 days by the colony counting technique on buffer medium agar and incubated at 30 °C. The exponential phase of bacterial growth was investigated to develop a fixed-film bio-column system for the treatment of thiocyanate and metal cyanide.
Immobilization of mixed culture bacteria
The mixed culture of SUTS 1 and SUTS 2 grown in the exponential phase was immobilized on packing materials [Polypropylene pall (PP) ring]. The diameter of the polypropylene pall ring is 1 inch (Fig. 1). The PP rings were sterilized with ultraviolet light (UV) and transferred to the Erlenmeyer flask containing buffer medium. After that, the mixed culture bacteria were added to the flask at a ratio of 10:100 v/v and shaken at 100 rpm on a rotary shaker at room temperature. Processed immobilized cells were prepared for 15 days for short-term study and 30 days for long-term study. Afterwards, 2 PP rings were randomly sampled to evaluate the increase in dry weight after cellular immobilization, and then, the immobilized PP rings were packed into a column.
Experimental design and operating conditions
The experiments consisted of a bio-column that was 3 cm in diameter and 50 cm in height and a synthetic wastewater tank. The immobilized PP rings were packed within the bio-column at a height of 30 cm. The synthetic wastewater containing thiocyanate and metal cyanide was prepared according to the conditions shown in Table 1 and recirculated throughout the bio-column by a peristaltic pump (Fig. 1).
The experimental system was divided into two steps: (1) A short-term study was conducted to find the optimal rate for running the long-term system; the wastewater flow rate was varied from 10 to 30 mL/min. The system continuously ran for a period of 24 h, and samples were collected for analysis at 0, 6, 12, and 24 h. (2) A long-term study was conducted, and the system was set to the optimal conditions and continuously operated for 30 days.
Analytical methods
All experiments were carried out in duplicate, and the effluent was analyzed in the forms of thiocyanate (SCN−), cyanide (CN−), cadmium (Cd), Zinc (Zn), ammonia (NH3), sulfate (SO4 2−), and bicarbonate (HCO3 −), according to the standard methods for the examination of water and wastewater (APHA, AWWA, WPCF 2005) and nitrate (NO3 −) (APHA, AWWA, WPCF 1998). The growth of the mixed culture was studied using the colony counting technique. All chemical ingredients were analytical grade and purchased from Merck (Darmstadt, Germany), Sigma-Aldrich (St. Louis, USA), or Ajax (Auckland, New Zealand).
Residual thiocyanate and cyanide were calculated using the following equations:
where a 1 = Absorbance of sample solution, and m = Slope and b = Intercept.
where: A = mL of standard AgNO3 for the sample, and B = mL of standard AgNO3 for the blank.
The performance of system was calculated in terms of removal efficiency, RE (%) by the following equation:
where C i is the influent concentration (mg/L), and C f is the effluent concentration of the treated compounds (mg/L).
Results and discussion
Growth of mixed culture bacteria
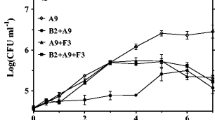
The maximum growth rate of mixed culture bacteria was obtained on day 3 of the incubation, which corresponded to approximately 1.03 × 108 CFU/mL (Fig. 2). When considering the maximum growth rate of each bacterium, Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2 grew to 8.70 × 107 and 1.70 × 107 CFU/mL on day 3 and day 1, respectively. However, the maximum growth rate of the mixed culture was selected, because they expressed the highest growth on thiocyanate and metal cyanide complexes. In addition, the growth of this mixed culture obtained the exponential phase of growth in copper and zinc cyanide condition (Supromin et al. 2015). It might be due to a synergistic effect from biodegradation process of mixed culture; for example, SUTS 1 and SUTS 2 may be capable of respiration in the presence of some product such as nitrate and use nitrate as an alternate electron acceptor for their growth (Bergey and John 1994). Therefore, microbial utilization of cyanide complexes has been investigated, and it was found that thiocyanate could be degraded by many microorganism species, such as Arthrobacter, Bacillus, Escherichia, Klebsiella, Methylobacterium, Pseudomonas, and Thiobacillus (Boucabeille et al. 1994; Ebbs 2004; Ahn et al. 2005). Moreover, this bacterium, Agrobacterium sp., is the first bacterium studied that was able to degrade cyanide complexes in the form of thiocyanate, zinc cyanide, and cadmium cyanide.
Short-term mixed culture microbial development of thiocyanate and metal cyanide degradation
Thiocyanate and metal cyanide degradation
The biodegradation rate of the bio-column system was significantly dependent on the flow rate. The results demonstrated that the system can remove thiocyanate throughout the experiments, with an efficiency of approximately 23.23 and 13.21% at flow rates of 10 and 30 mL/min, respectively. Moreover, at a flow rate of 10 mL/min, the removal efficiency increased every 6 h, because this flow rate had an empty bed retention time (EBRT) of approximately 21 min, indicating that the mixed culture bacteria in the system had a suitable duration time for contact with pollutants to degrade thiocyanate. In contrast, at flow rate of 30 mL/min, the system showed a lower efficiency. Therefore, the sufficient time period for treatment promotes an enhanced removal of cyanide compounds in the system, which the microbes are able to utilize for sources of carbon, nitrogen, and/or sulfur (Dzombak et al. 2006). Karavaiko et al. (2000) reported that the nitrogen utilization efficiency by Pseudomonas putida strain 21, Pseudomonas stutzeri strain 18, and Pseudomonas sp. strain 5 was decreased when wastewater flow rate increased. It is necessary to have an appropriate contact time with microorganisms to promote degradation. Furthermore, degradation also depends on the design of the treatment system, the concentration of thiocyanate, and type of microorganism. However, Ahn et al. (2005) reported that the initial thiocyanate concentration of 250 mg/L could be degraded by Klebsiella sp. in 7.5 days. In addition, Lim et al. (2008) reported that the degradation of thiocyanate could be completed after 2.8 days of incubation at an initial KSCN concentration of 838 mg/L.
In addition to thiocyanate, cyanide was completely degraded after the first 12 h, which is more than 99.99% efficiency. The results of metal removal efficiency in the form of zinc and cadmium were approximately 52.29 and 14.26% at 24 h. However, when increasing the wastewater flow rate to 30 mL/min, it was demonstrated that metal cyanide was able to be continuously degraded, but its efficiency was lower than 10 mL/min (Table 2). An interesting result of this study was that the mixed culture in the bio-column system was able to remove zinc better than cadmium. It may be due to zinc being an essential nutrient for microorganisms. Zinc is a component of a variety of enzymes and DNA-binding proteins to supporting cellular growth (Nies 1999). The resistance mechanisms for zinc toxicity occur during intracellular accumulation of bacteria and are transported by members of a variety of protein families. For example, Pseudomonas sp. was resistant to zinc owing to the accumulation of this metal in the cell (Mago and Srivastava 1994). The system for detoxification in microbes may be through zinc efflux mechanisms or by reducing intracellular uptake (Nies 1999; Ahuja et al. 2001). Furthermore, the previous study found that the mixed culture of SUTS 1 and SUTS 2 was able to degrade zinc cyanide rapidly within 24 h (Supromin et al. 2015).
However, cadmium is more toxic to microorganisms than zinc (Ragan and Mast 1990), but SUTS 1 and SUTS 2 are still resistant in wastewater containing cadmium compounds, and they can grow continuously, as was demonstrated throughout the experiment (Fig. 3).
By-products from thiocyanate and metal cyanide degradation
The biotransformation of thiocyanate and metal cyanide has been investigated (Table 2). In this case, thiocyanate and metal cyanide were degraded and transformed into bicarbonate, ammonia, sulfate, and nitrate. At a flow rate of 10 mL/min, sulfate and nitrate increased throughout and showed the highest concentrations of 60.60 and 0.06 mg/L at 24 h, respectively. This interesting result demonstrates that by-product concentrations increased when mixed culture bacteria have an increased rate of thiocyanate and metal cyanide degradation. In this case, bicarbonate reached its highest concentration at 6 h (75 mg CaCO3/L), and thereafter, the bicarbonate concentration decreased. At that time, when increasing the flow rate to 30 mL/min, sulfate and nitrate increased throughout the study period and exhibited a higher concentration than the culture with a flow rate of 10 mL/min. Bicarbonate and ammonia were not detected during this study. It is possible that bicarbonate was not detected in the bio-column system owing to the transformation of the compound into carbon dioxide, ammonia, or nitrogen gas (Whitlock 1990; Hung and Pavlostathis 1997; Akcil 2003). In addition, ammonia was not detected in this bio-column system, it might be due to the ammonia which was oxidized rapidly and transformed into a less toxic form of the nitrate (Chapatwala et al. 1998; Sirianuntapiboon and Chuamkaew 2007) or the ammonia was utilized directly by microbes for their growth (Hung and Pavlostathis 1999; Sorokin et al. 2001). In addition, the previous study found that the mixed culture of SUTS 1 and SUTS 2 was able to degrade cyanide and transform into ammonia and nitrate (Potivichayanon and Kitleartpornpairoat 2014). It was also found that nitrate increased with a decrease in ammonia concentration or was not detected.
In this fixed-film bio-column system, both the mixed culture bacteria were immobilized with polypropylene ball ring (PP ring) or were suspended in an aqueous phase. Thus, mixed culture bacteria in the bio-column system had a high efficiency for removal of cyanide compounds in the form of thiocyanate and metal cyanide. Other studies have found that P. putida immobilized cell degraded sodium cyanide (NaCN) more efficiently than non-immobilized cells (Chapatwala et al. 1998). This is similar to data suggesting that higher concentrations of cyanide (up to 120 mM) can be degraded by immobilized cells than non-immobilized cells of P. putida, which could degrade a maximum of 4 mM (Babu et al. 1992), but the maximum thiocyanate degradation rate of a biofilm reactor system is much higher than those obtained from suspended reactors (Jeong and Chung 2006). The mixed culture immobilized on the PP ring was investigated by random sampling at the top, middle, and bottom positions of a bio-column. The results show that the mixed culture on a PP ring was 4.95 × 105, 2.16 × 106 and 1.37 × 106 CFU/mL, respectively. Therefore, the persistence of a mixed culture either immobilized or suspended showed that the SUTS 1 and SUTS 2 can efficiently grow using cyanide compounds as a source of nutrients and can lead to the development of a biodegradation system for long-term treatment of thiocyanate and metal cyanide by the fixed-film bio-column system.
Long-term fixed-film bio-column system
From the short-term study, the optimal flow rate of 10 mL/min with EBRT for 21 min was applied to the long-term study. Thiocyanate and metal cyanides were degraded within the first 24 h. Nevertheless, thiocyanate degradation occurred quite regularly over the last 13–24 days, in which thiocyanate degraded from 72.04 mg/L down to approximately 44.19 mg/L, and the efficiency was approximately 38.66% (Fig. 4). Afterwards, the efficiency for thiocyanate degradation increased from day 25 to 30. At day 30 of the experiment, the system exhibited the highest thiocyanate degradation efficiency (58.41%) with residual thiocyanate at 29.96 mg/L. It might be due to the mixed culture of SUTS 1 and SUTS 2 adapted and began to tolerate the high thiocyanate concentrations throughout the 30 days, as shown in Fig. 4 and Table 3. Other studies have reported that thiocyanate utilization occurred mainly during the exponential phase of growth for mixed culture and pure bacterial strains (Souza-Fagundes et al. 2004). A similar result was previously reported for Arthrobacter species (Betts et al. 1979) using thiocyanate as energy and a sulfur and/or nitrogen source for growth (Chapatwala et al. 1998; Sorokin et al. 2001). Sulfate, the by-product, was observed for 30 days, and it stabilized in the range of 30–50 mg/L during days 3–24 and then increased to 61–100 mg/L during days 25–30 of the study, which was consistent with the degradation of thiocyanate (Fig. 4). This is consistent with other studies which reported that sulfate is the end-product of thiocyanate degradation by microbes as shown in the following equation (Akcil and Mudder 2003; Lim et al. 2008; Grigor’eva et al. 2009; Huddy et al. 2015):
The fixed-film bio-column treatment system rapidly removed zinc within the first 24 h (Fig. 5). The removal efficiency of zinc was 62.80%, with zinc at an initial concentration of 0.561 mg/L. The ability of the bio-column system to remove zinc began 22–30 days after culture, and the maximum removal efficiency of zinc was 68.60% on day 27. For cadmium removal, which started in the form of cadmium cyanide at an initial concentration of 0.105 mg/L, the results illustrated that the bio-column system was capable of cadmium removal within the first 24 h, similar to zinc, and showed a maximum removal efficiency of 27.17% on day 2. Afterwards, the removal efficiency of cadmium decreased for 30 days. There was a negligible trend of removal when compared to zinc removal, similar to the short-term study. During cyanide treatment, the system was less effective at degrading metal cyanide complexes such as zinc cyanide and cadmium cyanide for use as a carbon source compared with the free cyanide form (CN−). It was also found that cyanide can be rapidly degraded within 2 days (Fig. 6). During the initial period of running the system, the effective treatment may not have been continuous owing to the presence of several compounds. After day 9 of the experiment, cyanide degradation became more steady. The cyanide degradation rate reached its maximum on day 17 (80.56%) from an initial concentration of 0.76 mg/L and degraded to 0.15 mg/L. It still contained residual cyanide of approximately 0.17 mg/L on days 9–30; thus, the efficiency of cyanide degradation was approximately 77.85%.
According to other studies, cyanide was degraded by microorganisms, with the maximum rate of degradation in the exponential growth phase (the first 6–24 h), and they were able to utilize cyanide compounds and transform them into non-toxic substances, such as ammonia, nitrite, or nitrate (Chakraborty and Veeramani 2006; Sirianuntapiboon and Chuamkaew 2007; Potivichayanon and Kitleartpornpairoat, 2014). There are a wide variety of microorganisms, and each type of bacteria has the ability to remove heavy metals in different ways. For example, several species of Pseudomonas were able to remove metals and reduce toxicity to tolerate cadmium and/or zinc (Malik and Jaiswal 2000; Raja et al. 2006; Ozgur and Aysel 2012). Sannasi et al. (2009) showed that Pseudomonas sp., Serratia sp., Flavobacterium sp., Chryseomonas sp., Xanthomonas sp., Agrobacterium sp., Bacillus sp., Arthrobacter sp., and Micrococcus sp. can remove heavy metals in the form of Cd(II), Cr(VI), Cu(II), Ni(II), and Pb(II). The previous study also found that mixed culture of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2 in batch experiment can remove copper and zinc cyanide (Supromin et al. 2015). In this work, the mixed culture of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2 on a fixed-film and in suspension could eliminate zinc and cadmium in the form of Zn (CN)2 and Cd (CN)2. It was found that the elimination of zinc increased for 30 days, but cadmium was removed only moderately. Although cadmium accumulated in the system, the mixed culture bacteria were able to multiply (Fig. 7; Table 3). This indicated that the bacteria are resistant to the toxicity of cadmium. Another study also reported that Pseudomonas aeruginosa B237 and Cupriavidus taiwanensis E324 were tolerant of both cadmium and zinc, while Tsukamurella paurometabola A155 were tolerant of zinc only; in addition, more Zn2+ and Cd2+ absorbed on to cell walls than inside the cells (Limcharoensuk et al. 2015). This is possible, because the anionic functional groups on the surface of the cell wall are involved in extracellular accumulation and/or sequestration of Zn2+ and Cd2+ ions (Lima et al. 2006; Vijayaraghavan and Yun 2008). Gurbuz et al. (2009) noted that the uptake of metals (Zn, Cu, Cd, and Al) decreased with an increase in the complexity of the solution. In addition, cyanide degradation was affected by other metals in the solution, which was similar to this study where the complexity of thiocyanate, zinc, and cadmium cyanide was evident. The interval of maximum efficiency demonstrated that the fixed-film bio-column system can treat cyanide rather than zinc, thiocyanate, and cadmium. These efficiencies were approximately 80.56, 68.60, 58.41, and 27.17 for cyanide, zinc, thiocyanate, and cadmium, respectively. However, it is interesting that when more than one type of cyanide was treated, these compounds had a direct effect on biological treatment. Certain substances may have an inhibitory effect on the biodegradation of other substances. Shivaraman et al. (1985) reported that the presence of phenol and cyanide had a negative influence on the degradation of thiocyanate; the studies of Paruchuri et al. (1990) illustrated that the concentration of cyanide above 2.5 mg/L required a longer time to complete the degradation of thiocyanate, and complete inhibition occurred at 10 mg/L. The results also found that bicarbonate was 54.50 mg CaCO3/L after the first 24 h and had a maximum concentration on day 2 of 92.00 mg CaCO3/L. Bicarbonate may be produced from thiocyanate degradation and/or metal cyanide degradation, as shown in Eq. 5, so it is related to the degradation of thiocyanate and cyanide, especially zinc and cadmium cyanide, which rapidly degraded on day 3, but no bicarbonate was detected afterwards (data not shown). Moreover, ammonia in this study was not detected, except on days 19–21, when it reached a concentration of 0.14 mg/L. This may be due to the ammonia oxidizing and transforming into its nitrate form (Eqs. 6, 7) (Akcil and Mudder 2003):
Furthermore, the mixed culture of SUTS 1 and SUTS 2 was able to use ammonia as a source of nitrogen and/or carbon. This system could detect nitrate for 30 days (Fig. 6). The value of nitrate was 0.40–0.50 mg/L for the first 20 days. Maximum nitrate was up to 0.61 mg/L on day 5 of the study. The nitrate concentration depended on the cyanide degradation rate by mixed culture bacteria. However, the concentration of ammonia and nitrate at 3 and 1.5 g/L, respectively, slightly inhibited thiocyanate degradation (Kwon et al. 2002). In contrast, it demonstrated that the concentrations of ammonia and nitrate in this study did not cause or inhibit the degradation of thiocyanate. In addition, it was observed from increasing the treatment efficiency. Consequently, the fixed-film bio-column system for the integrated degradation of thiocyanate and metal cyanide by immobilized of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2 is an efficient means of detoxification, which is technically feasible and is highly effective, especially in the elimination of cyanide, zinc, and thiocyanate, and has a high removal efficiency of greater than 50%.
Conclusions
The mixed culture of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2 in a fixed-film bio-column system revealed the optimal removal efficiency for cyanide complexes in the form of thiocyanate, zinc cyanide, and cadmium cyanide. The wastewater flow rate for the fixed-film bio-column system of 10 mL/min had the highest removal efficiency for metal cyanide treatment compared to a flow rate of 30 mL/min, except for the removal efficiency of cadmium. It can be concluded that the efficiency of cyanide complex degradation is high during a short period of time, whereas a longer period of time showed the accumulation of by-products and may have some effect on the removal efficiency. Therefore, wastewater treatment systems should be employed over the long term to study its effect and optimal efficiency.
References
Ahn JH, Kim J, Lim J, Hwang SH (2004) Biokinetic evaluation and modeling of continuous thiocyanate biodegradation by Klebsiella sp. Biotechnol Progr 20(4):1069–1075. doi:10.1021/bp049967n
Ahn JH, Lee S, Hwang S (2005) Growth kinetic parameter estimation of Klebsiella sp. utilizing thiocyanate. Process Biochem 40(3–4):1363–1366. doi:10.1016/j.procbio.2004.06.004
Ahuja P, Mohapatra H, Saxena RK, Gupta R (2001) Reduced uptake as a mechanism of zinc tolerance in Oscillatoria anguistissima. Curr Microbiol 43(5):305–310. doi:10.1007/s002840010307
Akcil A (2003) Destruction of cyanide in gold mill effluents: biological versus chemical treatments. Biotechnol Adv 21(6):501–511. doi:10.1016/S0734-9750(03)00099-5
Akcil A, Mudder T (2003) Microbial destruction of cyanide wastes in gold mining: process review. Biotechnol Lett 25(6):445–450. doi:10.1023/A:1022608213814
APHA, Awwa, WPCF (1998) Standards method for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
APHA, Awwa, WPCF (2005) Standards method for the examination of water and wastewater, 21st edn. American Public Health Association, Washington
Aronstein BN, Maka A, Srivastava VJ (1994) Chemical and biological removal of cyanides from aqueous and soil-containing systems. Appl Microbiol Biotechnol 41(6):700–707. doi:10.1007/BF00167288
Babu GRV, Wolfram JH, Chapatwala KD (1992) Conversion of sodium cyanide to carbon dioxide and ammonia by immobilized cells of Pseudomonas putida. J Ind Microbiol 9(3):235–238. doi:10.1007/BF01569629
Barclay M, Hart A, Knowles CJ, Meeussen JCL, Tett VA (1998) Biodegradation of metal cyanides by mixed and pure cultures of fungi. Enzym Microb Technol 22(4):223–231. doi:10.1016/S0141-0229(97)00171-3
Bergey DH, John GH (1994) Bergey’s manual of determinative bacteriology, 9th edn. Williams & Wilkins, Baltimore
Betts PM, Rinder DF, Fleeker JR (1979) Thiocyanate utilization by an Arthrobacter. Can J Microbiol 25(11):1277–1282. doi:10.1139/m79-201
Boening DW, Chew CM (1997) A critical review: general toxicity and environmental fate of three aqueous cyanide ions and associated ligands. Water Air Soil Pollut 109(1):67–79. doi:10.1023/A:1005005117439
Bose P, Bose MA, Kumar S (2002) Critical evaluation of treatment strategies involving adsorption and chelation for wastewater containing copper, zinc and cyanide. Adv Environ Res 7(1):179–195. doi:10.1016/S1093-0191(01)00125-3
Boucabeille C, Bories A, Ollivier P, Michel G (1994) Microbial degradation of metal complexed cyanides and thiocyanate from mining wastewaters. Environ Pollut 84(1):59–67. doi:10.1016/0269-7491(94)90071-X
Chakraborty S, Veeramani H (2006) Effect of HRT and recycle ratio on removal of cyanide, phenol, thiocyanate and ammonia in an anaerobic–anoxic–aerobic continuous system. Process Biochem 41(1):96–105. doi:10.1016/j.procbio.2005.03.067
Chapatwala KD, Babu GR, Vijaya OK, Kumar KP, Wolfram JH (1998) Biodegradation of cyanides, cyanates and thiocyanates to ammonia and carbon dioxide by immobilized cells of Pseudomonas putida. J Ind Microbiol Biotechnol 20(1):28–33. doi:10.1038/sj.jim.2900469
Dash RR, Balomajumder C, Kumar A (2009) Removal of metal cyanides from aqueous solutions by suspended and immobilized cells of Rhizopus oryzae (MTCC 2541). Eng Life Sci 9(1):53–59. doi:10.1002/elsc.200700024
Dursun AY, Aksu Z (2000) Biodegradation kinetics of ferrous (II) cyanide complex ions by immobilized Pseudomonas fluorescens in a packed bed column reactor. Process Biochem 35(6):615–622. doi:10.1016/S0032-9592(99)00110-7
Dzombak DA, Ghosh RS, Wong-Chong GM (2006) Cyanide in water and soil: chemistry, risk, and management. CRC Press, United States, pp 93–121
Ebbs S (2004) Biological degradation of cyanide compounds. Curr Opin Biotechnol 15(3):231–236. doi:10.1016/j.copbio.2004.03.006
EPA (1997) Methylene bis (Thiocyanate). United States Environmental Protection Agency, EPA-738-F-97-005
Grigor’eva NV, Smirnova YV, Dulov LE (2009) Thiocyanate decomposition under aerobic and oxygen-free conditions by the aboriginal bacterial community isolated from the waste water of a metallurgical works. Microbiology 78(4):402–406. doi:10.1134/S002626170904002X
Gurbuz F, Ciftci H, Akcil A (2009) Biodegradation of cyanide containing effluents by Scenedesmus obliquus. J Hazard Mater 162(1):74–79. doi:10.1016/j.jhazmat.2008.05.008
Huddy RJ, van Zyl AW, van Hille RP, Harrison STL (2015) Characterisation of the complex microbial community associated with the ASTER™ thiocyanate biodegradation system. Miner Eng 76:65–71. doi:10.1016/j.mineng.2014.12.011
Hung CH, Pavlostathis SG (1997) Aerobic biodegradation of thiocyanate. Water Res 31(11):2761–2770. doi:10.1016/S0043-1354(97)00141-3
Hung CH, Pavlostathis SG (1999) Kinetics and modeling of autotrophic thiocyanate biodegradation. Biotechnol Bioeng 62(1):1–11
Ingles J, Scott JS (1987) State of the art processes for the treatment of gold mill effluents: Industrial programs branch. Environment Canada, Ottawa
Jeong YS, Chung JS (2006) Biodegradation of thiocyanate in biofilm reactor using fluidized-carriers. Process Biochem 41(3):701–707. doi:10.1016/j.procbio.2005.09.004
Kacer Y, Arpa C, Tan S, Denizli A, Genc O, Arica MY (2002) Biosorption of Hg (II) and Cd (II) from aqueous solutions: comparison of biosorptive capacity of alginate and immobilized live and heat inactivated Phanerochaete chrysosporium. Process Biochem 37(6):601–610. doi:10.1016/S0032-9592(01)00248-5
Karavaiko GI, Kondrat’eva TF, Savari EE, Grigor’eva NV, Avakyan ZA (2000) Microbial degradation of cyanide and thiocyanate. Microbiology 69(2):167–173. doi:10.1007/BF02756193
Kwon HK, Woo SH, Park JM (2002) Thiocyanate degradation by Acremonium strictum and inhibition by secondary toxicants. Biotechnol Lett 24(16):1347–1351. doi:10.1023/A:1019825404825
Lim J, Lee S, Kim SD, Hwang S (2008) Biochemical indication of microbial mass changes using ATP and DNA measurement in biological treatment of thiocyanate. Appl Microbiol Biotechnol 80(3):525–530. doi:10.1007/s00253-008-1601-4
Lima AIG, Corticeiro SC, de Figueira EM (2006) Glutathione-mediated cadmium sequestration in Rhizobium leguminosarum. Enzym Microb Technol 39(4):763–769. doi:10.1016/j.enzmictec.2005.12.009
Limcharoensuk T, Sooksawat N, Sumarnrote A, Awutpet T, Kruatrachue M, Pokethitiyook P, Auesukaree C (2015) Bioaccumulation and biosorption of Cd2+ and Zn2+ by bacteria isolated from a zinc mine in Thailand. Ecotoxicol Environ Saf 122:322–330. doi:10.1016/j.ecoenv.2015.08.013
Liu J, Baozhen W, Li W, Chengji J, Cao X, Wang L (1996) Removal of nitrogen from coal gasification and coke plant wastewaters in A/O submerged biofilm-activated sludge (SBF-AS) hybrid system. Water Sci Technol 34(10):17–24. doi:10.1016/S0273-1223(96)00692-0
Mago R, Srivastava S (1994) Uptake of zinc in Pseudomonas sp. Strain UDG26. Appl Environ Microbiol 60(7):2367–2370
Malik A, Jaiswal R (2000) Metal resistance in Pseudomonas strains isolated from soil treated with industrial wastewater. World J Microbiol Biotechnol 16(2):177–182. doi:10.1023/A:1008905902282
Medwith BW, Lefelhocz JF (1981) Single-stage biological treatment of coke plant wastewater with a hybrid suspended growth fixed film reactor. In: Proceeding Ind. Waste Conf.; (United States); annual Purdue industrial waste conference, Lafayette, IN, USA, pp 68–76
Melcer H, Nutt SG (1988) Nitrogen control of complex industrial wastewaters. J Environ Eng ASCE 114(1):166–178. doi:10.1061/(ASCE)0733-9372
Moran RE (1998) Cyanide uncertainties: observations on the chemistry, toxicity and analysis mining-related waters. Mineral policy center issue paper No.1, Washington
Moran RE (2017) Cyanide in mining: some observations on the chemistry, toxicity and analysis mining-related waters. https://www.earthworksaction.org/files/publications/morancyanidepaper.pdf. Accessed 27 March 2017
Mudder TI, Botz MM, Smith A (2001) Chemistry and treatment of cyanidation wastes, 2nd edn. Mining Journal Books Limited, London
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51(6):730–750
Notification the Ministry of Science, Technology and Environment (1996) No. 3, B.E. 2539 issued under the Enhancement and Conservation of the National Environment Quality Act B.E. 2535-1992, published in the Royal Government Gazette, Vol. 113 Part 13 D, dated February 13, B.E. 2539–1996
Ozgur C, Aysel U (2012) Bio-monitoring of heavy metal resistance in Pseudomonas and Pseudomonas related genus. J biol Environ Sci 6(18):233–242
Paruchuri YL, Shivaraman N, Kumaran P (1990) Microbial transformation of thiocyanate. Environ Pollut 68(1–2):15–28. doi:10.1016/0269-7491(90)90011-Z
Patil YB, Paknikar KM (2000) Development of a process for biodetoxification of metal cyanides from waste waters. Process Biochem 35(10):1139–1151. doi:10.1016/S0032-9592(00)00150-3
Plumlee GS, Smith KS, Mosier EL, Ficklin WH, Montour M, Briggs PH, Meier AL (1995) Geochemical processes controlling acid-drainage generation and cyanide degradation at Summitville. In: Proc, Summitville Forum, Colo. Geological survey special publication 38:23–34
Potivichayanon S, Kitleartpornpairoat R (2014) Degradation of cyanide to ammonia and nitrate by mixed culture of Agrobacterium tumefaciens SUTS 1 and Pseudomonas monteilii SUTS 2. In: Proceeding of the European Conference on Sustainability, energy and the environment. Brighton, United Kingdom. pp 251–264
Ragan HA, Mast TJ (1990) Cadmium inhalation and male reproductive toxicity. Rev Environ Contam Toxicol 114:1–22. doi:10.1007/978-1-4612-3368-8_1
Raja CE, Anbazhagan K, Selvam GS (2006) Isolation and characterization of a metal-resistant Pseudomonas Aeruginosa Strain. World J Microbiol Biotechnol 22(6):577–585. doi:10.1007/s11274-005-9074-4
Ryu BG, Kim J, Yoo G, Lim JT, Kim W, Han JI, Yang JW (2014) Microalgae-mediated simultaneous treatment of toxic thiocyanate and production of biodiesel. Bioresource Technol 158:166–173. doi:10.1016/j.biortech.2014.01.128
Sannasi P, Kader J, Othman O, Salmijah S (2009) Physical growth and biomass characterization of bacterial cells exposed to Cd(II), Cr(VI), Cu(II), Ni(II) and Pb(II). J Environ Res Develop 4(1):8–18
Shivaraman N, Kumaran P, Pandey RA, Chatterjee SK, Chowdhary KR, Parhad NM (1985) Microbial degradation of thiocyanate, phenol and cyanide in a completely mixed aeration system. Environ Pollut Ser A 39(2):141–150. doi:10.1016/0143-1471(85)90012-1
Silva-Avalos J, Richmond MG, Nagappan O, Kunz DA (1990) Degradation of the metal-cyano complex tetracyanonickelate (II) by cyanide-utilizing bacterial isolates. Appl Environ Microbiol 56(12):3664–3670
Sirianuntapiboon S, Chuamkaew C (2007) Packed cage rotating biological contactor system for treatment of cyanide wastewater. Bioresour Technol 98(2):266–272. doi:10.1016/j.biortech.2006.01.014
Sorokin DY, Tourova TP, Lysenko AM, Kuenen JG (2001) Microbial thiocyanate utilization under highly alkaline conditions. Appl Environ Microbiol 67(2):528–538. doi:10.1128/AEM.67.2.528-538.2001
Souza-Fagundes EM, Rosa LH, Gomes NCM, Santos MH, Pimentel PF (2004) Thiocyanate degradation by pure and mixed cultures of microorganisms. Braz J Microbiol 35(4):333–336. doi:10.1590/S1517-83822004000300012
Staden JF, Saling C, Malan D, Taljaard RE (1997) Non-linearity with metal ligand complex reactions in flow injection system. Metal-thiocyanate reactions. Analytica Chimica Acta 350:37–50. doi:10.1016/S0003-2670(97)00173-6
Supromin N, Potivichayanon S, Toensakes R (2015) Degradation of metal cyanide from real electroplating wastewater by mixed culture of SUTS 1 and SUTS 2. 3rd International conference on biological, chemical & environmental sciences (BCES-2015). Kuala lumper, Malaysia 3:75–80
Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26(3):266–291. doi:10.1016/j.biotechadv.2008.02.002
Whitlock JL (1990) Biological detoxification of precious metal processing wastewaters. Geomicrobiol J 8(3–4):241–249. doi:10.1080/01490459009377896
Wood PA, Kelly PD, McDonald RI, Jordan LS, Morgan DT, Khan S, Murrell JC, Borodina E (1998) A novel pink pigmented facultative methylotroph, Methylobacterium thiocyanatum sp. nov., capable of growth of thiocyanate or cyanate as sole nitrogen sources. Arch Microbiol 169:148–158
Acknowledgements
This research was supported and funded by Suranaree University of Technology, Nakhon Ratchasima, Thailand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Potivichayanon, S., Supromin, N. & Toensakes, R. Development of a mixed microbial culture for thiocyanate and metal cyanide degradation. 3 Biotech 7, 191 (2017). https://doi.org/10.1007/s13205-017-0814-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0814-6