Abstract
The persistent organic pollutants decachlorobiphenyl (DCB) are a group of synthetic substances of high risk to human and environmental health. This study was aimed to evaluate the potential removal of DCB by earthworm Eisenia fetida and its symbiotic bacteria in a vermicomposting system for a period of 72 days using a complete randomized design. The results showed that the vermicomposting system was able to significantly remove high concentrations decachlorobiphenyl (DCB) from the polluted substrate. The addition of a concentration of 1000 mg L−1 during vermicomposting were a removal of 230.28 mg L−1 DCB and the results obtained from adding a concentration of 1500 mg L−1 DCB were 424.11 mg L−1. The earthworms bioaccumulated less than 5 mg L−1 of DCB without an apparent toxic effect. The earthworm weight decreased during vermicomposting and DCB concentration compared to the control (non-polluting); however, earthworms survived until the end of experiment. Phylogenetic analysis of 16S rDNA gene sequences of Eisenia fetida gut strains grown in the presence of 1500 mg L−1 DCB were identified as Bacillus, Paenibacillus, Pseudomonas, Solibacillus, and Staphyloccocus at zero time (0-days). At 7 days of culture, the genera, Acinetobacter, Bacillus, Enterobacter, Klebsiella, and Staphylococcus were identified, and at 72 days, the symbiotic bacteria isolated were classified into the genera, Bacillus, Enterobacter, Klebsiella, and Staphylococcus. The strains Pseudomonas extremaustralis ADA-5 and Staphylococcus sciuri ADA-12 showed higher potential of removal from the DCB (219.7 and 162.74 mg L−1, respectively) at an initial concentration of 1500 mg L−1. Both vermicomposting system and degrading bacteria from Eisenia fetida worms are useful to remove high concentrations of decachlorobiphenyl from contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polychlorinated biphenyls (PCBs) are compounds of toxic and highly persistent organic mixtures that comprise 209 congeners differing in the number and position of chlorine atoms bound to the two coupled-biphenyl rings (Polak et al. 2016). Within these compounds stands out the decachlorobiphenyl (DCB). Decachlorobiphenyl is completely chlorinated congener in the PCB class (2,2-3,3-4,4-5,5-6,6-DCB), it is also characterized by their lipophilicity and persistence and their biomagnification in food chains (Han et al. 2009). DCB is found worldwide, including some very remote areas, for example, in the arctic, and even in breast milk or in human populations (She et al. 2007; Perez-Gonzalez et al. 2017). The congeners of PCB exhibit different coplanar levels according to the degree of substitution of chlorines, the PCB’s mono-ortho-coplanares are easy to remove; however, the congeners with substitutions of cloros di-ortho in the DCB correspond to non-coplanar congeners and exhibit toxicological potential different from coplanar congeners, also increasing their elimination difficulty (Alonso et al. 2008; Han et al. 2009). This compound is still considered as a risk to both wildlife and humans. Health effects associated with exposure to DCB include carcinogenicity, genotoxicity, neurotoxicity, and reproductive toxicity (Qiu et al. 2016). Due to their high thermodynamic stability, metabolic degradation is generally very slow (Costabeber et al. 2006). Different methods like chemical and physical have been used for the destruction of PCBs in the environment (Zhao et al. 2012; Huang et al. 2014). The incineration has been used and approved as the standard method for the destruction of the DCB, in soil and sediment (Hatamian-Zarmi et al. 2009). However, this method has disadvantages that limit their full-scale applications and the contaminants can decompose partially (Tharakan et al. 2006).
Earthworms participate in the decomposition, transformation, and mineralization of organic matter by way of processes that take place in their digestive system (Šmídová and Hofman 2014). Earthworms can speed up the removal of contaminants from soil. The presence of earthworms in contaminated soil indicates that they can survive a wide range of different organic contaminants (Hernández-Castellanos et al. 2013; Rodriguez-Campos et al. 2014). For this reason, these have been employed as alternatives for bioremediation of PCBs due to the fact that they can tolerate a toxic chemical environment (Yadav and Garg 2011; Lin et al. 2016). However, little is known about the removal of the highly recalcitrant compound decachlorobiphenyl using vermicomposting and endosymbiotic bacteria of E. foetida as a suitable system due to the fact that these studies have used less chlorinated compounds such as Aroclor.
The microbial degradation has been reported as one of the most effective methods for removing chemical contaminants from the environment (Field and Sierra-Alvarez 2008; Mrozik and Piotrowska-Seget 2010; Rodriguez-Campos et al. 2014; Garrido-Sanz et al. 2018; Horváthová et al. 2018). Several species of bacteria have been secluded from the intestinal tract of the earthworm Eisenia fetida with high ability to biodegrade various types of toxic chemical contaminants (Hatamian-Zarmi et al. 2009; Asgharnia et al. 2014). Lin et al. (2016) identified the bacteria associated with earthworms; the bacteria found were Alcaligenes, Pseudomonas, and Sphingomonas which are known to degrade hydrocarbons and other organic compounds. However, little is known about DCB degrading bacteria associated with Eisenia fetida in vermicomposting process. Therefore, the aims of this study were as follows: (1) to evaluate the efficiency of a vermicomposting system using the earthworm Eisenia fetida for the removal of high concentrations of decachlorobiphenyl and (2) to determine the ability of the symbiotic bacteria isolated from earthworm intestine to degrade high concentrations of decachlorobiphenyl.
2 Materials and Methods
2.1 Reagents and Materials
Decachlorobiphenyl was 99.1% pure (Sigma-Aldrich®, USA). The substrate was formulated with rabbit manure and peat moss. Rabbit manure was collected from a rabbit farm located in Tuxtla Gutiérrez, Chiapas (Mexico), and the peat moss was a commercialized product acquired from Promix® Canadian Sphagnum (Quebec, Canada). Both substrates were milled to particle diameter (0.144 mm) and used as support for the earthworms.
2.2 Earthworm Used
Earthworms (Eisenia fetida) were cultivated in support based on rabbit manure and peat moss for 6 months for adaptation and growth. After, adult earthworms with developed clitellum (sexually mature) and with an average weight of 0.5 ± 0.1 g were selected for the experiment and protected from sunlight to avoid premature death.
2.3 Physicochemical Analyses from Vermicompost
Physicochemical characterization from vermicompost contaminated with a high concentration of DCB (1500 mg L−1) and without the contaminant (negative control) during 0, 7 and 72 days of vermicomposting was analyzed according to the Association of Official Analytic Chemist (AOAC 1996). The pH and electric conductivity (EC) were measured using a digital pH meter Mettler Toledo® Model S220 (New York, USA) in 1:10 (weight/volume) aqueous solution. Total nitrogen was measured by Kjeldhal method (Bremner 1996). The organic carbon content and C/N ratio and were analyzed according to AOAC methods (1996).
2.4 Experimental Design
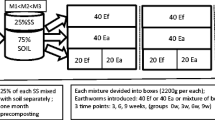
The removal of decachlorobiphenyl (DCB) was evaluated using three concentrations at 0, 1000 and 1500 mg L−1 during 0, 7, 14, 28, 56, and 72 days, which generated 18 different combinations. Three replicas were used per treatment (concentration × sample day) and these were arranged in a completely randomized design, with a total of 54 experimental units that were evaluated. Each experimental unit consisted of a 1-L flask containing 30 g of total substrate of peat moss and rabbit manure 85:15 (weight/weight) sterilized three times for 30 min with an interval of a day with pressurized steam at 121 °C in autoclave. The total substrate was mixed and adjusted to a moisture of 75% by adding distilled sterilized water. Each experimental unit was inoculated with three adult earthworms as recommended by Villalobos-Maldonado et al. (2015). The flasks were stoppered with cloth to avoid earthworms from leaving and to establish aerobic conditions; the flasks were incubated at 25 °C, and earthworm behavior was monitored at the beginning of and over the 72-day period, moisture was monitored every 7 days and adjusted to 75% with distilled sterilized water. The average weight of earthworms and DCB concentration were evaluated.
2.5 Quantification of Decachlorobiphenyl in the Earthworms and Vermicompost
Earthworms were separated carefully from each experimental flask and their weight was recorded. The earthworms were placed in sterile glass vials for 24 h to remove traces of organic matter. After, earthworms were triturated to a fine powder with liquid nitrogen and adding anhydrous sodium sulfate to 1.5 times the weight of the worm. In the case of vermicompost, it was dried for 24 h at 50 °C and 10 g of it was weighed and transferred to 50-mL Falcon tubes. Samples corresponding to the earthworms or vermicompost were analyzed separately, as follows: 20 mL of pentane HPLC grade (Sigma-Aldrich®, USA) were added to the sample and, mixed on a vortex for 5 min, and subsequently sonicated for 40 min and centrifuged at 4000 rpm for 15 min. Next, the supernatant was placed in a 50-mL Falcon tube, and the procedure was repeated twice until the concentrate attained a volume of 1 mL. Finally, the accumulated supernatant was allowed to evaporate for 24 h using a chamber of evaporation. Then, the dry sample was suspended in 5 mL pentane HPLC grade. The decachlorobiphenyl in the sample (Earthworms or Vermicompost) was determined in an Agilent Technologies 7890 chromatograph coupled with a mass spectrometry MSD VL 5975 C (Wilmington, USA) using the 8270D method (USEPA 2007). The equipment operating conditions were as follows: capillary column PE-XLB measures 30 m × 0.25 mm × 0.25 μm, helium carrier gas at a pressure of 16 psig, injection temperature 110 °C, detector temperature 150 °C, quantified initial temperature 110 °C × 0.5 min, temperature program of 110 to 300 °C at 15 °C min−1 and 300 to 320 °C for 5 min. Flow 1.4 mL min−1 and final temperature 320 °C (Villalobos-Maldonado et al. 2015).
2.6 Diversity and DCB Removal Potential of E. fetida Gut Bacteria from Vermicomposting System
Based on removal kinetics of 1500 mg L−1 DCB (Fig. 1); days 7 and 72 were selected to study the bacterial diversity of E. fetida gut in vermicomposting system. For this initial process, the intestinal content of three sexually mature earthworms (approximately 1.0 g) were placed in 9.0 mL of Brain Heart Infusion (BHI) broth obtaining a base solution. Then serial dilutions were done 10−1 to 10−6. Then, 5 mL of each bacterial dilution was striated on BHI medium (Hyun-Jung et al. 2004). Plates were incubated at 30 °C for 5 days. Pure cultures were preserved at 4 °C in 65% glycerol-BHI broth for temporary storage. Total genomic DNA of each strain was extracted using the DNA Isolation Kit for Cells and Tissues (ROCHE®, Basel, Switzerland) according to the manufacturer specifications. After, PCR was performed with bacterial universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-AAGGAGGTGATCCAGCC-3′) for 16S rDNA, which amplify products of approximately 1500 bases (Weisburg et al. 1991). The PCR-16S rDNA products were digested with the RsaI restriction enzyme (Thermo Scientific®) using the Amplified rDNA Restriction Analysis (ARDRA) technique and then the genomic profiles were analyzed and the Shannon-Weaver index related to the diversity and abundance of the bacterial species associated with the earthworm E. fetida were determined (Pereira et al. 2009). PCR products of the strains selected by ARDRA were purified using the PCR Product Purification System Kit from Roche® and sequenced (Macrogen®, Korea). All sequences were compared using BLAST (Altschul et al. 1990) and were aligned by the CLUSTAL X (2.0) software (Larkin et al. 2007). Phylogenetic and molecular evolutionary analyses were done with MEGA v5.2 software. The phylogenetic tree was constructed by Neighbor-Joining model (Tamura et al. 2011). 16S rDNA gene sequences were deposited in the GenBank database under the accession numbers KY110406 to KY110420.
Representative strain of each bacterial genus, identified by 16S rDNA gene sequence analysis, was used to determine DCB removal potential. Flasks containing 25 mL of BHI broth were amended with 1500 mg L−1 of DCB. Each of the strains were individually subject to a DCB containing medium during 120 h, the time corresponding to the greatest removal potential observed in vermicomposting process (T-7 days). Samples of removed DCB were extracted at the final time (120 h), and simultaneously, extraction of control flasks (uninoculated broth with DCB). To measure the amount of DCB remaining in the medium at final time, 5 mL of broth were taken and added with pentane in a 1:1 ratio (volume/volume). The mixture was maintained for 20 min in vigorous shaking and 30 min under a sonicator. The supernatant obtained on organic phase was separated and concentrated in a solution of 1 mL, the process was repeated three times. The remaining 20 mL were used to separate biomass produced by each one of the evaluated bacteria. Samples were centrifuged at 4000 rpm for 5 min to form a biomass pellet and then were treated with the cellular breakdown according to the procedure recommended by Valenzuela-Encinas et al. (2009) with modifications that consist on adding 15 mL of pentane and sonicating for 30 min after heat shock. Afterwards, the supernatant obtained on organic phase was separated and concentrated in a solution of 1 mL, this method was repeated three times. Samples from broth and cellular biomass were injected into a gas chromatograph coupled to mass spectroscopy and then DCB concentration was determined.
2.7 Statistical Analysis
The variables, average earthworm weight and, DCB concentration were subjected to a one-way analysis of variance (ANOVA) using the Statgraphics Plus v.XV.5 for windows (1999), and mean difference significance was tested with the Tukey test (P < 0.05) and also by the t student’s statistics.
3 Results
3.1 Characteristics of Vermicompost Contaminated with Decachlorobiphenyl
The physicochemical characteristics of the uncontaminated vermicompost and contaminated with 1500 mg L−1 decachlorobiphenyl at 0, 7, and 72 days are shown in Table 1. The initial alkaline pH decreases significantly (P < 0.05) through the vermicomposting process. The electrical conductivity (EC), which is an indirect measure of salinity, decreased drastically after 7 days in the vermicomposting system with 1500 mg L−1 DCB, and remained stable until 72 days, while the EC had no difference significant statistics in vermicompost without contaminants. The organic carbon content increased significantly (P < 0.05) along the composting process, while the total nitrogen showed a significant increase (P < 0.05) up after 7 days and remained stable until 72 days. The C/N relation increased significantly (P < 0.05) with respect to the time of vermicomposting, indicating the increase of organic nutrients in the system.
3.2 Weight of Earthworms during Vermicomposting
In the vermicomposting system amended with DCB, the E. fetida earthworms showed a significant decrease in weight (P < 0.05) during sampling days (Table 2). During the first 14 days, the earthworms registered the greatest weight in the control treatment (without DCB). However, the weight of earthworms at a concentration of DCB 1000 mg L−1 decreased significantly during the 72-day period (P < 0.05). The weight of the earthworms under the influence of the contaminant at 1500 mg L−1 showed a similar behavior. At the end of the experiment (72 days), the earthworms that grew at a concentration of 1500 mg L−1 of DCB had a higher weight compared to the other treatments evaluated (P < 0.05).
3.3 Removal of Decachlorobiphenyl in a Vermicomposting System
The decrease of DCB in the treatments at 1000 mg L−1 in the vermicomposting was observed in the first 7 days, while removal of DCB for treatment at 1500 mg L−1 occurred at 14 days of vermicomposting (Fig. 1), keeping these values constant until 72 days. It was observed that in the two treatments there was a rapid adaptation to the toxicity of the earthworms accelerating the process of removal in the first days of vermicomposting. In relation to the removal of DCB in the vermicomposting system over 72 days, the mass balance allowed to determine that the highest concentration removal of DCB was 230.28 mg L−1 with 1000 mg L−1 and with 1500 mg L−1, the amount removal was 424.11 mg L−1 (Table 3). Therefore, these results allowed to determine the removal efficiency of the vermicomposting system which was the highest in the highest DCB concentration 1500 mg L−1 (P < 0.008). Also, the mass balance enabled to determine the remaining final concentration of DCB in the vermicompost for 1000 mg L−1 units was 450.87 mg L−1 and 366.38 mg L−1 for units with 1500 mg L−1 of DCB. Regarding the final concentration of bioaccumulated DCB by the earthworms, the Student’s test (P < 0.05) determined significant differences between the final concentrations contained in the earthworm. When the earthworms were treated with 1000 mg L−1, the highest bioaccumulation was recorded at 59.29 mg L−1.
3.4 Bacterial Diversity Associated with Earthworm E. fetida in Vermicomposting System
A total of 150 bacterial strains were isolated from the intestine of E. fetida at different cultivated times (0, 7, and 72 days) in a vermicomposting system with the highest concentration (1500 mg L−1 DCB). The isolates were grouped mainly within the class ɣ-proteobacteria and bacilli. Bacterial strains were identified by 16S rDNA gene sequences (Table 4). At the initial day, bacterial genera were identified, such as Bacillus, Paenibacillus, Pseudomonas, Solibacillus, and Staphylococcus and as the time of vermicomposting advanced, it was observed that the number of genera diminished. Thus on day 7, there were new bacteria identified as Acinetobacter, Bacillus, Klebsiella, and Staphylococcus, while at day 72, the genera found were Staphylococcus, Bacillus, Klebsiella, and Enterobacter, with Bacillus and Staphylococcus found at all three times of vermicomposting (0, 7, and 72 days). Corresponding to the diversity and abundance of bacterial species that are associated with E. fetida, the ARDRA allowed to determine the genomic fingerprinting of the different times of samples (Table 5). On day 0 (zero), the bacterial strains were grouped into seven groups with different genomic profiles. At 7 days, four groups were obtained and at 72 days, only five ARDRA groups with specific profiles were determined. The Shannon-Weaver index determined a high abundance or richness of bacterial species (d = 4.72) mainly on day 0. The abundance of the species was decreasing according to the sampling time. In the case of the bacterial diversity (H) associated with E. fetida, it was low in the three sampling times.
3.5 Removal Potential of Decachlorobiphenyl by Bacteria Associated with the Earthworm E. fetida
In this experiment, all the bacterial strains evaluated had the capacity to remove DCB in a vermicomposting system (Table 6). The strain Pseudomonas extremaustralis ADA-5 removed the highest concentration of DCB (326.49 mg L−1) from an initial concentration of 1500 mg L−1, which corresponded to 98.78% of DCB removal, determining that 66.47% was accumulated by the bacterial cellular biomass in comparison with the other strains evaluated in the experiment (P < 0.05). In contrast, the lowest amount of DCB removed (54.65 mg L−1) was recorded by the strain Acinetobacter schindleri ADA-15 (P < 0.05).
4 Discussion
The vermicomposting that use the earthworm Eisenia fetida (vermi-remediation) have shown a high efficiency in the removal of different toxic contaminants, taking into consideration the metabolic capacity of this organism (Rodriguez-Campos et al. 2014). In this work, the potential for removal of DCB by E. fetida and its symbiotic bacteria was evaluated in a vermicomposting system. Initially, the substrate formulated with rabbit manure and peat moss was composted for 72 days using E. fetida in the presence of high concentrations of 1500 mg L−1 DCB. At the end of the process, the vermicompost showed changes in its physicochemical properties (Table 1). The pH and electrical conductivity (EC) decreased significantly. The decrease in pH can be an important factor in the retention of nitrogen, since this element is lost in form of volatile ammonia at a higher pH (Hartenstein and Hartenstein 1981). The content of organic C and total N, and P content in the vermicompost showed a significant increase (P < 0.05). This phenomenon can be attributed to the release of humic acids, mucus, nitrogen compounds (urine), and other organic substances that contain high concentrations of C, P, and N from the worm’s metabolism (Curry and Schmidt 2007). Also, this may be due to the nitrogen mineralization process in the vermicompost, where commonly the organic N transformed to the nitrate (Atiyeh et al. 2000), while the increase of organic carbon content over time may be due to the accumulation or assimilation of an external carbon source such as DCB in the vermicomposting system. This result which had been confirmed when the content of organic C incremented in the vermicomposting with other types recalcitrant pollutants (Contreras-Ramos et al. 2006).
The survival of E. fetida in the presence of high concentrations of DCB during a period of 72 days is shown in Table 2. Although the worms showed a significant decrease in their weight (P < 0.05) during the vermicomposting due to natural senescence and high concentrations of DCB, they showed a 100% survival and succeeded in removing the DCB contaminant without showing any morphological alteration, such as surface lesions and mid-segmental swellings or general ulcerated areas on the surface, as stipulated by the standards methods for toxicity (OECD 2000). In the same way, Villalobos-Maldonado et al. (2015) reported that the weight of earthworms increased with concentrations of DCB at 100, 150, and 200 mg L−1 in the first 7 days. However, the weight of earthworms began to decrease during the process of vermicomposting due to exhaustion of the nutrients (mainly the carbon source) and the contaminant in the vermicompost. Also, this same phenomenon was reported by Xie et al. (2013), when they analyzed the effect of ether decabromodiphenyl (BDE-209) on the survival, growth, and reproduction of the earthworm (Eisenia fetida). Matscheko et al. (2002) reported at 5% decrease in E. fetida weight that was exposed to a soil that was contaminated with 16 different PAHs for 19 days. Earthworms have a complex interrelationship with microorganisms, which serve as their major source of nutrients, as well as earthworms that promote microbial activity by fragmentation and inoculation of decaying organic matter with microorganisms (Li et al. 2002).
The results obtained in this study have shown that in the vermicomposting system, there was high removal of concentration DCB during 72 days. Then, in this study, it was found that the highest DCB removal occurred with the highest initial concentration (1500 mg L−1) during the first 7 days, stirring 424.11 mg L−1 of which 43.88 mg L−1 were retained in the matrix of the earthworm, which could be due to the lipophilicity of the cellular components of their tissues and also by the chemical structure of the congener DCB and its chlorine atoms, which is characterized by having greater lipophilicity, resistance, and absorption strength (Arbeli 2009) (Table 3). Similar results were obtained by Villalobos-Maldonado et al. (2015) reporting high levels of bioaccumulation in the matrix of the earthworm in treatments evaluated with 200 mg L−1 DCB in a vermicomposting system. Also, in this study, high levels were obtained in the two treatments, the residual concentrations in the vermicompost obtained were 366.38 mg L−1 at the highest concentration (1500 mg L−1) (Table 3), caused by the minimal availability of substrate and the high levels of DCB that led to the contaminant being consumed as carbon source and also to the chemical characteristics of the DCB, since the congeners with substitutions of protons in the meta and para positions have been reported with greater susceptibility to biological removal processes (Llyas et al. 2013).
Diverse studies that are related with the potential removal of contaminants chemicals recalcitrants of the E. fetida were reported by (Tharakan et al. 2006; Contreras-Ramos et al. 2006; Lin et al. 2016). However, little is known about the removal capacity and biodegradation of the DCB of E. fetida and its symbiotic native bacteria. In this study, we managed to isolate and identify bacteria in the intestine of E. fetida that grew during 0, 7, and 12 days of vermicompost contaminant with high concentrations of DCB (Table 4). On day zero, there were seven different bacterial species that corresponded to ɣ-proteobacteria and bacilli. The sequences of 16S rDNA gen were similar to Pseudomonas alcaligenes and P. extremaustralis strains. Species of Pseudomonas, like P. aeruginosa TMU56 that were isolated in contaminant soil and with electrical transformer fluid (Askarel) had a capacity to degrade 73.3% of PBCs during a 4-day period of incubation (Hatamian-Zarmi et al. 2009). The strains ADA-2 and ADA-8 corresponded to Bacillus. Based on the sequence of the 16S rDNA gene, strain ADA-2 showed > 99.0% similarity with the sequence of Bacillus subtilis RD11 and the ADA-8 strain had 98.1% with the B. oceanisediminis H2 strain. Some strains, like Bacillus sp. JF8 showed degradation of PCB congeners including tetra- and pentachlorobiphenyl (Mukerjee-Dhar et al. 2005) and the Bacillus cereus JP12 that had the capacity to degrade decabromodiphenyl ether (Mang et al. 2013). ADA-3 showed > 98.2% similarities with the Paenibacillus tundrea A10b. ADA-6 strain had 99.15% similarity with the Solibacillus silvestris strain. ADA-9 strain was 98.65% to the Staphylococcus hominis DSM20328 (Table 4).
From day 7, the bacteria were identified and clustered within Klebsiella, Staphylococcus, Bacillus, and Acinetobacter. Based on the sequence of the 16S rDNA gene, strain ADA-11 showed > 98.1% similarity with the sequence of Klebsiella pneumoniae ATCC13884. ADA-12 strain had 99.7% genetic similarity to the Staphylococcus sciuri DSM20345. The ADA-13 and Bacillus cereus LP10-S6 strain were 100.0% similar to each other, and ADA-15 strain and Acinetobacter schindleri LUH5832 had 99.4% similarities to one another.
On day 72, Staphylococcus, Bacillus, Klebsiella, and Enterobacter were found. Noting that the isolates that were grouped in the genus Bacillus and Staphylococcus were found three times in vermicomposting (0, 7, and 72 days). Klebsiella and Staphylococcus are noticeable for their capacities to degrade different congeners of PCBs (Jianjun et al. 2016). As the case of Staphylococcus xylosus had the ability to degrade PCB to degrade PCBs during 168 h of incubation in liquid media (Leaes et al. 2006). The ADA-20 is the only bacterial species that had been found during this vermicomposting process and had 99.0% similarities to Enterobacter xiangfangensis BC3 (Ya-Ming et al. 2011).
Corresponding to the diversity and abundance of bacterial species are associated with E. fetida (Table 5). On day 0 (zero), the bacterial strains were grouped into seven groups with different genomic profiles. At 7 days, four groups were obtained and at 72 days, only five ARDRA profiles were determined. The Shannon-Weaver index determined a high abundance of bacterial species (H = 4.72) mainly on day 0. The abundance of the species was decreasing according to the sampling time. In the case of the bacterial diversity (d) associated with E. fetida, it was low in the three sampling times. The high concentration of DCB in the vermicomposting system acts as a chemical factor that limits the diversity and abundance of the species in the bacterial community that reside in the Eisenia fetida intestine. At the end of the vermicomposting process, only the bacterial species endowed with the enzymatic machinery capable of degrading this recalcitrant contaminant survive.
The maximum bioaccumulation of DCB was obtained with the bacterium Pseudomonas extremaustralis with 219.7 mg L−1 accumulated in its biomass while 98.78% de DCB was removed from the culture medium (Table 6), this bacterial genus has been reported to have a high potential for the degradation of organochlorine compounds. Komancová et al. (2003) reported that Pseudomonas sp. was able to eliminate congeners with lower chlorine content (tri and tetra-clorobiphenyl) with interference in the biphenyl structure with 48% of elimination. On the other hand, Hatamian-Zarmi et al. (2009) showed that Pseudomonas aeruginosa had the ability to eliminate up to 89% of hexa-chlorinated congeners, tolerating up to 200 mg L−1 of initial PCBs in the culture, which indicates that these results may be due to the obstruction and bioaccumulation observed in P. extremaustralis corresponds to an important initial process in the removal and possibly bacterial degradation of congener DCB (Alonso et al. 2008) where the lipids of its membrane and the lipophilicity of this congener are important characteristics to carry out the process of removal. The genera Acinetobacter, Klebsiella, and Bacillus have been reported as genera with potential for degradation of different persistent organic pollutants (Pieper 2005; Vasilyeva and Strijakova 2007). For instance, Borja et al. (2005) reported that these genera were isolated in the intestine of the earthworm and tolerated importantly the DCB, where the tolerance and aerobic degradation of these bacterial genera to different PCB congeners with lower chlorine content was evaluated. Also, in this study, the presence of 1,2-benzenedicarboxylic acid was detected qualitatively as an intermediate in the degradation pathway of PCBs in Pseudomonas such as was reported by Koubek et al. (2013).
5 Conclusions
In this study, we demonstrated for the first time that the earthworm E. fetida and its symbiotic bacteria in a vermicomposting system have a great potential to remove high concentrations of decachlorobiphenyl. In relation to the diversity and abundance of the bacterial species that inhabitant in the digestive tract, decrease significantly during the process of removal is due to the pressure given by contaminant DCB. Thus, the removal and bioaccumulation of DCB by Pseudomonas extremaustralis ADA-5 and Staphylococcus sciuri ADA-12 was greater at 1500 mg L−1 compared with other isolates. Therefore, it is important in the future to evaluate the presence of the biphenyl dioxygenase (bph) genes in the genome of the isolated bacteria to identify the potential of degradation of PCBs.
References
Alonso, M., Casado, S., Miranda, C., Tarazona, J., Navas, J. M., & Herradón, B. (2008). Decabromobiphenyl (PBB-209) activates the aryl hydrocarbon receptor while Decachlorobiphenyl (PCB-209) is inactive: experimental evidence and computational rationalization of the different behavior of some halogenated biphenyls. Chemical Research Toxicology, 21, 643–658.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
AOAC. (1996). Official methods of analysis of AOAC international (16th ed., Vol.1). Gaithersburg: AOAC International.
Arbeli, Z. (2009). Biodegradation of persistent organic pollutants (POPs): the case of polychlorinated biphenyls (PCB). Acta Biologica Colombiana, 14, 55–86.
Asgharnia, H., Jafari, A. J., Kalantary, R. R., Nasseri, S., Mahvi, A., Yaghmaeian, K., Esrafili, A., & Shahamat, Y. D. (2014). Influence of bioaugmentation on biodegradation of phenanthrene-contaminated soil by earthworm in lab scale. Journal of Environmental Health Science and Engineering, 12, 150. https://doi.org/10.1186/s40201-014-0150-2.
Atiyeh, R. M., Dominguez, J., Subler, S., & Edwards, C. A. (2000). Changes in biochemical properties of cow manure during processing by earthworms (Eisenia andrei Bouche) and the effects on seedling growth. Pedobiologia, 44, 709–724.
Borja, J., Taleon, D. M., Auresenia, J., & Gallardo, S. (2005). Polychlorinated biphenyls and their biodegradation. Process Biochemistry, 40, 1999–2013.
Bremner, J. M. (1996). Total nitrogen. In D. L. Sparks (Ed.), Methods of soil analysis chemical methods. Part 3 (pp. 1085–1122). Madison: Soil Science Society of America Inc, American Society of Agronomy Inc.
Contreras-Ramos, S. M., Escamilla-Silva, E. M., & Dendooven, L. (2006). Vermicomposting of biosolids with cow manure and oat straw. Biology and Fertility of Soils, 41, 190–198.
Costabeber, I., Sifuentes dos Santos, J., Odorissi, X. A. A., Weber, J., Leaes, F., Bogusz, S., & Emanuelli, T. (2006). Levels of polychlorinated biphenyls (PCBs) in meat and meat products from the state of Rio Grande do Sul, Brazil. Food and Chemical Toxicology, 44, 1–7.
Curry, J. P., & Schmidt, O. (2007). The feeding ecology of earthworms- a review. Pedobiología, 50, 463–477.
Field, J. A., & Sierra-Alvarez, R. (2008). Microbial transformation and degradation of polychlorinated biphenyls. Environmental Pollution, 155, 1–12.
Garrido-Sanz, D., Manzano, J., Martín, M., Redondo-Nieto, M., & Rivilla, R. (2018). Metagenomic analysis of a biphenyl-degrading soil bacterial consortium reveals the metabolic roles of specific populations. Frontiers in Microbiology, 9, 232.
Han, X., O’Connor, J. C., Donner, E. M., Nabb, D. L., Mingoia, R. T., Snajdr, S. I., Clarke, J. J., & Kaplan, A. M. (2009). Non-coplanar 2,2′, 3,3′, 4,4′, 5,5′, 6,6′-Decachlorobiphenyl (PCB 209) did not induce cytochrome P450 enzyme activities in primary cultured rat hepatocytes, was not genotoxic, and did not exhibit endocrine-modulating activities. Toxicology, 255, 177–186.
Hartenstein, R., & Hartenstein, F. (1981). Physicochemical changes in activated sludge by the earthworm Eisenia foetida. Journal of Environmental Quality, 10, 377–382.
Hatamian-Zarmi, A., Shojaosadati, S. A., Vasheghani-Farahani, E., Hosseinkhani, S., & Emamzadeh, A. (2009). Extensive biodegradation of highly chlorinated biphenyl and Aroclor 1242 by Pseudomonas aeruginosa TMU56 isolated from contaminated soils. International Biodeterioration & Biodegradation, 63, 788–794.
Hernández-Castellanos, B., Ortíz-Ceballos, A., Martínez-Hernández, S., Noa-Carrazana, J. C., Luna-Guido, M., Dendooven, L., & Contreras-Ramos, S. M. (2013). Removal of benzo (a) pyrene from soil using an endogeic earthworm Pontoscolex corethrurus (Müller, 1857). Applied Soil Ecology, 70, 62–69.
Horváthová, H., Lászlová, K., & Dercová, K. (2018). Bioremediation of PCB-contaminated shallow river sediments. The efficacy of biodegradation using individual bacterial strains and their consortia. Chemosphere, 193, 270–277.
Huang, L., Su, G., Liu, Y., Li, L., Liu, S., Lu, H., & Zheng, M. (2014). Effect of NiFe2O4 on PCDF by products formation during termal degradation of Decachlorobiphenyl. RSC Advances Home, 4, 25453–25460.
Hyun-Jung, K., Kwang-Hee, S., Chang-Jun, C., & Hor-Gil, H. (2004). Analysis of aerobic and culturable bacterial community structures in earthworm (Eisenia fetida) intestine. Agricultural Chemistry & Biotechnology, 47, 137–142.
Jianjun, S., Xurun, Y., Jing, Z., Ai-sheng, X., & Fei, X. (2016). Regional analysis of potential polychlorinated biphenyl degrading bacterial strains from China. Brazilian Journal of Microbiology, 47, 536–541.
Komancová, M., Jurćová, J., Kochánková, L., & Burkhard, J. (2003). Metabolic pathways of polychlorinated biphenyls degradation by Pseudomonas sp. 2. Chemosphere, 50, 537–543.
Koubek, J., Mackova, M., Macek, T., & Uhlik, O. (2013). Diversity of chlorobiphenyl-metabolizing bacteria and their biphenyl dioxygenases in contaminated sediment. Chemosphere, 931548–1555.
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., Mc Gettigan, P. A., Mc William, H., Valentin, F., Wallace, I. M., Wilm, A., Lopez, R., Thompson, J. D., Gibson, T. J., & Higgins, D. G. (2007). Clustal W and Clustal X version 2.0. Bioinformatics, 23, 2947–2948.
Leaes, F. L., Daniel, A. P., Mello, G. B., Battisti, V., Bogusz, S., Jr., Emanuelli, T., Fries, L. L. M., & Costabeber, I. (2006). Degradation of polychlorinated biphenyls (PCBs) by Staphylococcus xylosus in liquid media and meat mixture. Food and Chemical Toxicology, 44, 847–854.
Li, X., Fisk, M. C., Fahey, T. J., & Bohlen, P. J. (2002). Influence of earthworms invasion on soil microbial biomass and activity in a northern hardwood forest. Soil Biology and Biochemistry, 34, 1929–1937.
Lin, Z., Zhen, Z., Wu, Z., Yang, J., Zhong, L., Hu, H., Luo, C., Bai, J., Li, Y., & Zhang, D. (2016). The impact on the soil microbial community and enzyme activity of two earthworm species during the bioremediation of pentachlorophenol-contaminated soils. Journal of Hazardous Materials, 301, 35–45.
Llyas, M., Sudaryanto, A., Setiawan, I. E., Riyadi, A. S., Isobe, T., & Tanabe, S. (2013). Characterization of polychlorinated biphenyls and brominated flame retardants in sludge, sediment and fish from municipal dumpsite at Surabaya, Indonesia. Chemosphere, 93, 1500–1510.
Mang, L., Zhong-Zhi, Z., Xue-Jiao, W., Yu-Xin, X., Xiao-Li, S., Min, Z., & Jing-Xiu, W. (2013). Biodegradation of decabromodiphenyl ether (BDE-209) by a metal resistant strain, Bacillus cereus JP12. Bioresource Technology, 149, 8–15.
Matscheko, N., Lundstedt, S., Svensoon, L., Harju, M., & Tysklind, M. (2002). Accumulation and elimination of 16 polycyclic aromatic compounds in the earthworm (Eisenia fetida). Environmental Toxicology and Chemistry, 21, 1724–1729.
Mrozik, A., & Piotrowska-Seget, Z. (2010). Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiological Research, 165, 363–375.
Mukerjee-Dhar, G., Shimura, M., Miyazawa, D., Kimbara, K., & Hatta, T. (2005). bph genes of the thermophilic PCB degrader, Bacillus sp. JF8: characterization of the divergent ring-hydroxylating dioxygenase and hydrolase genes upstream of the Mn-dependent BphC. Microbiology, 151, 4139–4151.
Organization for Economic Co-operation and Development (OECD). (2000). Guideline for testing organic chemicals. Proposal for New Guideline: Earthworms Reproduction Test (Eisenia fetida/andrei). <http://www.oecd.org>.
Pereira, P., Nesci, A., & Etcheverry, M. G. (2009). Analysis of the bacterial seed treatments for the control of Fusarium verticillioides in maize. Biocontrol, 54, 109–111.
Perez-Gonzalez, E., Osuna-Martinez, U. G., Herrera-Moreno, M. N., Rodriguez-Meza, G. D., Gonzalez-Ocampo, H. A., & Bucio-Pacheco, M. (2017). Organochlorine pesticides in gonad, brain, and blood of mice in two agricultural areas of Sinaloa. Bulletin of Environmental Contamination and Toxicology, 98, 454–459.
Pieper, D. H. (2005). Aerobic degradation of polychlorinated biphenyls. Applied Microbiology and Biotechnology, 67, 170–191.
Polak, M. L., Zlatic, E., Demšar, L., Zlender, B., & Polak, T. (2016). Degradation of PCBs in dry fermented sausages during drying/ripening. Food Chemistry, 213, 246–250.
Qiu, L., Wang, H., & Wang, X. (2016). Isolation and characterization of a cold-resistant PCB209-degrading bacterial strain from river sediment and its application in bioremediation of contaminated soil. Journal of Environmental Science and Health, A, 0, 1–9. https://doi.org/10.1080/10934529.2015.1094324.
Rodriguez-Campos, J., Dendooven, L., Alvarez-Bernal, D., & Contreras-Ramos, S. M. (2014). Potential of earthworms to accelerate removal of organic contaminants from soil: a review. Applied Soil Ecology, 79, 10–25.
She, J., Holden, A., Sharp, M., Tañer, M., Williams-Derry, C., & Hooper, K. (2007). Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast Milk from the Pacific Northwest. Chemosphere, 67, 307–317.
Šmídová, K., & Hofman, J. (2014). Uptake kinetics of five hydrophobic organic pollutants in the earthworm Eisenia fetida in six different soils. Journal of Hazardous Materials., 267, 175–182.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA 5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739.
Tharakan, J., Tomlinson, D., Addagada, A., & Shafagati, A. (2006). Biotransformation of PCBs in contaminated sludge: potential for novel biological technologies. Engineering in Life Sciences, 6, 43–50.
USEPA. (2007). United States Environmental Protection Agency (USEPA). Method 8270D. Semivolatile organic compounds by gas chromatography/mass spectrometry (GC/MS).
Valenzuela-Encinas, C., Neria-González, I., Alcántara-Hernández, R. J., Enríquez-Aragón, J. A., Estrada-Alvarado, I., Hernández-Rodríguez, C., Dendooven, L., & Marsch, R. (2009). Phylogenetic analysis of the archaeal community in an alkaline-saline soil of the former Lake Texcoco (Mexico). Extremophiles, 12, 247–254.
Vasilyeva, G. K., & Strijakova, E. R. (2007). Bioremediation of soil and sediment contaminated by polychlorobiphenyls. Microbiology, 76, 639–653.
Villalobos-Maldonado, J. J., Meza-Gordillo, R., Mancilla-Margalli, N. A., Ayora-Talavera, T. R., Rodriguez-Mendiola, M. A., Arias-Castro, C., Vázquez-Villegas, P. T., Gutiérrez-Miceli, F. A., & Ruiz-Valdiviezo, V. M. (2015). Removal of decachlorobiphenyl in vermicomposting process amended with rabbit manure and peat moss. Water, Air, & Soil Pollution, 226, 159.
Weisburg, W. G., Barns, S. M., Pelletier, D. A., & Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology, 73, 697–703.
Xie, X., Qian, Y., Wu, Y., Yin, J., & Zhai, J. (2013). Effects of decabromodiphenyl ether (BDE-209) on the avoidance response, survival, growth and reproduction (Eisenia fetida). Ecotoxicology and Environmental Safety, 90, 21–27.
Ya-Ming, C., Li X., Ling-Yun, J. (2011). Analysis of PCBs degradation abilities of biphenyl dioxygenase derived from Enterobacter sp. LY402 by molecular simulation. New Biotechnology, 29(1), 90–98.
Yadav, A., & Garg, V. K. (2011). Recycling of organic wastes by employing Eisenia fetida. Bioresource Technology, 102, 2874–2880.
Zhao, L., Hou, H., Shimoda, K., Terada, A., & Hosomi, M. (2012). Formation pathways of polychlorinated dibenzofurans (PCDFs) in sediments contaminated with PCBs during the thermal desorption process. Chemosphere, 88, 1368–1374.
Acknowledgments
We thank Posgrado of Ingenieria Bioquimica-ITTG and CONAcyT for a fellowship to A. Zenteno-Rojas. We also thank Maria Celina Luján Hidalgo for technical assistance. We thank Phillip M. Barrios reviewing the English version of manuscript.
Funding
The work received financial support from the Tecnologico Nacional de México 6841.18-P.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zenteno-Rojas, A., Martinez-Romero, E., Rincón-Molina, C.I. et al. Removal of High Concentrations Decachlorobiphenyl of Earthworm Eisenia fetida and its Symbiotic Bacteria in a Vermicomposting System. Water Air Soil Pollut 230, 116 (2019). https://doi.org/10.1007/s11270-019-4170-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4170-5