Abstract
Developing urbanization, water shortage, watercourse pollution, and demands for more food due to population growth require a more efficient water irrigation and fertilizer application. Retaining nutrients and water in agricultural soils brings about higher crop yields and prevents pollution of water courses. Among different solutions, zeolites, which are environmental friendly, ubiquitous, and inexpensive, have been extensively employed in agricultural activities. These minerals are considered as soil conditioners to improve soil physical and chemical properties including infiltration rate, saturated hydraulic conductivity (K s), water holding capacity (WHC), and cation exchange capacity (CEC). Natural and surface-modified zeolites can efficiently hold water and nutrients including ammonium (NH4 +), nitrate (NO3 −) and phosphate (PO4 3−), potassium (K+), and sulfate (SO4 2−) in their unique porous structures. Their application as slow-release fertilizers (SRFs) are reported as well. Therefore, zeolite application can improve both water use efficiency (WUE) and nutrient use efficiency (NUE) in agricultural activities and consequently can reduce the potential of surface and groundwater pollution. This review paper summarizes findings in the literature about the impact of zeolite applications on water and nutrient retention in the agriculture. Furthermore, it explores benefits and drawbacks of zeolite applications in this regard.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water is one of the key and fundamental substances, essential for development especially in arid and semi-arid regions of the world, where renewable water resources are scarce (Akhtar et al. 2016; El Kenawy et al. 2016; Jlassi et al. 2016). Inadequate water resources coupled with increasing demand for freshwater, due to increasing population growth rate and improved living standards, has been a challenging issue for countries located in arid and semi-arid regions (Al-Busaidi et al. 2008; Omar et al. 2010; Roghani et al. 2016). Among the various sectors consuming freshwater, agricultural industries should be considered as the chief consumer of freshwater, because more than two-thirds of the renewable water resources are used in agricultural activities (Xiubin and Zhanbin 2001). This issue has resulted in unbalanced water allocation to various users particularly in arid and semi-arid regions that can endanger sustainable development in these areas (Sepaskhah and Barzegar 2010).
On the other hand, increasing population takes higher efficiency in agricultural activities to yield more food production. Besides population growth that asks for greater amounts of food, change of agricultural land use to urban areas brings about less arable farms. Thus, an efficient cultivation is crucial to sustain the urban developments. While high-efficient agricultural activities are needed to satisfy the current food demands, several issues have appeared to achieve this goal. These issues regard irrigation and fertilization (Nagaraja et al. 2016; Nyssen et al. 2015).
Unfortunately, not all soil types are appropriate for agricultural activities. Soil in farms can either be clayey that hampers water infiltration or sandy, which discharges irrigation water from root zones in high rates. Modifying soil physical properties, such as infiltration and hydraulic conductivity, is a method that can influence water and nutrient movement in soil, especially in light-textured soils (Mamedov et al. 2016; Wang et al. 2016a), because these soils have high infiltration rate and low potential to hold water. Therefore, there has been an interest among researches to improve soil physical properties such as infiltration and hydraulic conductivity to increase irrigation efficiency (Anderson et al. 2009; Thierfelder and Wall 2009).
Another issue in increasing the efficiency of cultivation is increasing the ability of soil for holding nutrients, most importantly including nitrogen (N), phosphorous (P), sulfur (S), and potassium (K). Presence of more nutrients in the root zone can lead to higher crop yields. Therefore, to elevate the growing rates, the problematic soils need some curing to boost efficiencies of both irrigation and fertilization (Wang et al. 2016b). In agriculture, although N is known as kingpin and widely used in all crops and cropping systems, rarely its conventional use efficiency exceeds 50% (Ramesh et al. 2015a). For instance, agricultural activities are recognized as an important source of NO3 − pollution. High NO3 − concentrations (> 3 mg L−1), which easily can be discharged from soils to the groundwater indicate anthropogenic reverse impacts on the groundwater quality (Hosono et al. 2013). Elevated NO3 − concentrations endanger public health through (1) spreading different diseases, such as methemoglobinemia disease, which causes defects in the vision or even death of infants (Greer and Shannon 2005), and cancer in digestive organs (Aschebrook-Kilfoy et al. 2013); (2) eutrophication of waterbodies; and (3) production of nitrous oxide (N2O), which is a greenhouse gas, through the denitrification process. Leaching of NO3 − from fertilizers has been known as the primary source of groundwater pollution (Peña-Haro et al. 2010). According to a field survey in China, more than 28% of groundwater samples had exceeded the maximum contaminant level (MCL) of NO3 − allowed by the World Health Organization (WHO)’s standard (Gu et al. 2013). Besides NO3 −, phosphate (PO4 3−) is another nutrient in the fertilizers, which can contaminate water resources (Delkash et al. 2014). Therefore, nutrients should be retained in the soil in order to provide the needed nutrients for plant growth, while leaching into the groundwater should be prevented (Bakhshayesh et al. 2014; Leggo 2015; Nakhli et al. 2014).
Applying additives to problematic soils is a popular approach, which has been pursued to improve physicochemical properties of soil (Al-Busaidi et al. 2008; Mahabadi et al. 2007; Sarkar and Naidu 2015). Natural zeolites are being considered as soil additives, in which because of their higher water holding capacity (WHC), they have the ability to improve water content of the treated soils. On the other hand, addition of excessive nutrients such as nitrogen to soils in order to enhance the level of nutrients might cause excessive foliage or soil contamination. Leaching of mineral fertilizers (nutrients) into water resources is a serious problem that causes water pollution (Abbasi et al. 2015). Therefore, one of the reasonable approaches to maintain clean water resources and meanwhile keep adequate level of nutrients in soil would be applying additives such as zeolite to soils.
Zeolites, which are hydrated aluminosilicates of alkaline and alkaline earth element, exist in more than 50 and 150 natural and synthetic forms, respectively (Jha and Singh 2016; Virta 2002). Natural and synthetic zeolites found a wide range of applications in a host of industries including soil construction and repair, soil amendment, animal nutrition and health, aquaculture, building materials, heat storage and solar refrigeration, absorption, adsorption, ion exchange, molecular sieves, ion exchange, and catalysts (Elliot and Zhang 2005; Ober 2017). Different zeolite markets are summarized and classified in Fig. 1 (Elliot and Zhang 2005). Among several zeolite markets, agricultural applications have the largest potential market volume for zeolites (Elliot and Zhang 2005). Zeolites are considered to be one of the widely used natural inorganic soil conditioners to improve physical and chemical properties of soil, such as water holding capacity, infiltration rate, saturated hydraulic conductivity, and cation exchange capacity (Chmielewska 2014b; Ebrazi and Banihabib 2015; Enamorado-Horrutiner et al. 2016; Inglezakis et al. 2012). Due to the unique characteristics of zeolites, their application can increase the water use efficiency (WUE) through increasing soil WHC (Xiubin and Zhanbin 2001). Zeolites improve different soil physical properties, in which infiltration and hydraulic conductivity are the most important ones (Gholizadeh-Sarabi and Sepaskhah 2013). It has been extensively acknowledged that soil amendment by means of zeolitic materials improves water holding and prevents it from deep percolation that can lower water usage in agricultural activities (Ming and Mumpton 1989; Mumpton 1999; Polat et al. 2004; Sharpley et al. 1994; Talebnezhad and Sepaskhah 2013). Their application has been tested in many studies in which the effects on soil hydraulic properties have been determined (Ibrahim-Saeedi and Sepaskhah 2013). It has been reported that adding zeolite to light-textured soils can improve their WHC (Bernardi et al. 2009; Bernardi et al. 2013).
Classification of different zeolite markets (Elliot and Zhang 2005)
Natural zeolites show a cation exchange capacity (CEC) of between 100 and 200 cmol(+) kg−1 (Inglezakis et al. 2015; Ming and Allen 2001). Zeolite is a silicate mineral whose characteristics differ from other silicate minerals such that it has spacious pores and channels within its structure (Chmielewska and Lesný 2012; Inglezakis and Grigoropoulou 2003). Natural zeolites are loaded with cations such as sodium (Na+), potassium (K+), and calcium (Ca2+). They have different significant and well-known properties. The first one is high CEC, which is greater than that of soils (Inglezakis et al. 2015; Stylianou et al. 2015), the second one is a large amount of free water within their structural channels, and the third property is great ability of adsorption with a high surface area. These properties have been used in environmental science, such as improving water quality and ameliorating soil (Cobzaru and Inglezakis 2012). Potential applications of zeolites and their modified forms for capturing cations, anions, and molecular species have been reported. The plant growth media where zeolite is mixed with soil is called zeoponic. This media is nutrient rich and has high capacity of cation exchange. Therefore, there is no drastic demand for providing nutrients with irrigation water (Allen and Ming 1995; Gruener et al. 2003; Rivero and Rodríguez-Fuentes 1989). This type of soil additive is called slow-release fertilizer. This concept will be extensively discussed later.
Zeolites can exchange or adsorb different cations such as cesium (Cs) and strontium (Sr) as well as heavy metals such as cadmium (Cd), lead (Pb), nickel (Ni), manganese (Mn), zinc (Zn), chrome (Cr), iron (Fe), and copper (Cu) (Faghihian et al. 1999a; Faghihian et al. 1999b; Ghasemi Mobtaker H, Kazemian H, 2006; Kazemian et al. 2001; Kazemian and Mallah 2006); anions such as chromate (CrO4 2−) and arsenate (AsO4 −3) (Kazemian and Mallah 2008; Menhaje-Bena et al. 2004); and organic pollutants such as volatile organic compounds (VOCs) including benzene, toluene, ethyl benzene, and xylene (BTEX) (Ghadiri et al. 2010; Seifi et al. 2011a; Seifi et al. 2011b; Youssefi and Waring 2012; Youssefi and Waring 2014; Youssefi and Waring 2015).
Worldwide zeolite mine production of large deposits of different natural zeolites in 2012–2016 is shown in Fig. 2 (Dolley 2014; Jewell and Kimball 2016; Ober 2017; Summaries 2015). The annual worldwide mine production of natural zeolites remains almost constant during the last 5 years and is about 2800 kilo metric ton. This high production rate reveals both their variety and interest in their potential application in agricultural and other industrial sectors.
Zeolites have been historically utilized to enhance agricultural efficiency. Mumpton (1980) mentioned that zeolites were used in Japan three centuries ago for several purposes including soil conditioning. Mumpton (1972) noted that zeolites were widely mined from the USA and Japan in 1950s and employed as soil conditioners. Several seminars held between the researchers of these two countries caused a noticeable progress in this field. Mumpton (1985) elaborately enumerated the application of natural zeolites in agricultural activities in that era. Acknowledging all the efforts that have been made for this research field, the applications of zeolites for improving agricultural activities have been widely paid attention to over the last two decades. The chronological trend of the publication rate in “Scopus” online database with keywords “zeolite” and “water retention” or “nutrient retention” is depicted in Fig. 3a. This trend reveals an annual ascending trend in number of publications of this topic that justifies a comprehensive review about it. The subject areas that researches about zeolites have been published are shown in Fig. 3b. According to this figure, about 23 and 11% of the total zeolite research papers deal with environmental and agricultural sciences, respectively. Different review papers have summarized the applications of zeolites in environmental sciences. The application of different zeolites in treating various polluted wastewaters such as urban runoff, landfill leachate, nuclear fallout, and acid mine drainage has been reviewed (Delkash et al. 2015). A book chapter covered some eco-friendly applications of zeolites for treating contaminated water and wastewaters (Kazemian et al. 2012). Latest scientific reports on the applications of zeolitic materials in aquaculture industry for selective capturing of ammonia and toxic heavy metals, for live transportation of fish, and for feed additives are discussed in a review article (Ghasemi et al. 2016). Some other review papers summarized roles of zeolites in cation exchange and treatments of some heavy metals from the aqueous phase. However, to the best of authors’ knowledge, there is not any comprehensive review article that covers recent achievements in the last two decades on zeolite applications in improving soil nutrients and water holding capacities in agricultural Industries. It is noteworthy that impacts of various zeolites on the behaviors of soil are very subjective. It means that each zeolite has its own effect on a specific soil and this conclusion might not be true for another soil type. This fact renders this review article necessary to individually summarize the recent findings in the literature to pave the ways for future investigations.
a Trend of annual publications on zeolite applications in soil modifications. b Subject areas published in zeolite field (Scopus.com, last accessed August 25, 2016)
This paper reviews applications of zeolites in holding water and modifying infiltration rate and hydraulic conductivity. Besides, zeolites have been applied to hold nutrients in the soil and reduce their leaching into water bodies. Regarding these issues, scientific progress and development will be addressed and compared to illuminate the current state of the art in this field. Effects of different zeolite, and with or without surface modification on the different soils, will be discussed. The mechanisms that cause alteration in water and nutrient dynamics in soils will be argued and summarized too.
2 Impact on Water Retentions
2.1 Effects of Zeolite on Infiltration Rate
Infiltration rate is a measure of the rate of water entering the soil surface under a vertical hydraulic gradient, which varies in space and over the time. It is notable that neither high nor low infiltration rate is desirable for agricultural activities. In light-textured soils such as sandy soils, infiltration rate is typically high, which might lead to a significant water lost. On the contrary, in soils with lower infiltration rates such as loam and clay, high intensity of rains can generate a great deal of surface runoff, leading to soil erosion and nutrient lost. Depending on the purpose, various methods can be used to increase or decrease soil infiltration rate, including increasing soils organic matter content and vegetation cover and managing the crop residue. The application of these methods leads to a change in soil structure because of which infiltration rate alters. However, the application of these methods only modifies the soil structure and is considered as a short-term practice, whose efficiency can decrease over time (Franzluebbers 2002). Hence, in order to improve soil infiltration rate, soil texture as an inherent characteristic should be modified. One of the long-term method by which soil infiltration can be improved is applying a suitable natural zeolite to soils. Given the unique characteristics of natural zeolites (e.g., clinoptilolite) such as high pore volume and low bulk densities, they can be used to modify soil texture and consequently their infiltration rate. Therefore, their application in improving soil infiltration rate has been the subject of numerous studies worldwide.
According to the results of the related studies, soils treated with natural zeolites alter the infiltration rate compared to unamended soils, depending on soil, zeolite, and water characteristics and also experimental conditions (Gholizadeh-Sarabi and Sepaskhah 2013; Ippolito et al. 2011; Razmi and Sepaskhah 2012; Xiubin and Zhanbin 2001). In a study, the effects of natural mordenite with a particle size of less than 0.25 mm on infiltration rates of fine-grained calcareous loess soil was determined and compared to untreated soil under laboratory and field conditions (Table 1) (Xiubin and Zhanbin 2001). The zeolite had a CEC of 136.35 cmol(+) kg−1 and a WHC of 121.32%. The organic content of the soil was 0.76% by weight. Particles with a size range of 0.05–0.01 mm fraction were 46% and the clay fraction was about 24%. To determine the effects of the zeolite on soil infiltration, water was applied with sprinkler irrigation method with an intensity of 2 mm min−1 for amended soils at three different slopes (5°, 10°, and 20°). According to the results, the application of zeolite resulted in an increase in infiltration rate such that the beginning time of runoff for slopes between 5° and 20° went up. The extent of increase was higher for steep slope compared to gentle slope. The infiltration rate on gentle slopes increased by 7–30%, whereas it went up by more than 50% on steep slope. The observed increase in infiltration rate can be attributed to zeolites porous properties that can generate more routes for water movement (Szerement et al. 2014; Xiubin and Zhanbin 2001). It is suggested that the open network of the zeolites structure can lead to formation of new routes for water movement, which can consequently improve infiltration rate.
In another study (Table 1), the effects of natural zeolite application rate and saline water on sorptivity of three different soils (sandy loam, loam, and clay loam) in a column study under laboratory conditions was determined (Gholizadeh-Sarabi and Sepaskhah 2013). It should be noted that the Philip equation is a two-term infiltration relationship and can be expressed as follows:
where z is cumulative infiltration, s is the sorptivity, K fs is the field saturated hydraulic conductivity, and t is the time. Sorptivity, which is an important factor in Philip’s infiltration equation, controls the infiltration rate of saline water and depends on both initial and final moisture content of soil (Bagarello et al. 2006; Moosavi and Sepaskhah 2012; Suarez et al. 2006). In this study, the zeolite application rate and salinity levels were 0, 4, 8, and 16 g kg−1 and 0.5, 1.5, 3.0, and 5.0 dS m−1, respectively. Then, soil samples modified with the zeolite were put in a column. The results illustrated that sorptivity was not affected in non-saline water for all three soil textures studied at different zeolite application rates. However, other results of this investigation demonstrated that in addition to zeolite, other factors play a significant role in the effects of zeolite on infiltration rate. For instance, salinity can cause interference with the zeolite application. It has been shown that saline water application can have a negative effect on soil infiltration rate. According to the findings, in clay loam salinity levels of 0.5 and 3.0 dS m−1 with application of 16 g kg−1 zeolite and a salinity level of 5.0 dS m−1 with application of 8 g kg−1 zeolite resulted in the lowest values of sorptivity (s = 0.5711 and 0.5423 cm min−0.5 for 16 g kg−1 and s = 0.5191 cm min−0.5 for 8 g kg−1 application rates, respectively). In loam, all salinity levels with application of 16 g kg−1 zeolite increased sorptivity compared to other zeolite application rates (s = 2.003 cm min−0.5 was the highest sorptivity). For sandy loam, only a salinity level of 0.5 dS m−1 with application of 4 g kg−1 zeolite increased sorptivity (s = 1.530 cm min−0.5). Other zeolite applications decreased sorptivity in sandy loam. It was concluded that zeolite application rate along with soil texture and water salinity level affect infiltration rate in soils. The authors determined a relationship between sorptivity, Z (zeolite application rate), ECiw (electrical conductivity of irrigation water), SARiw (sodium adsorption ratio of irrigation water), and mean soil particle diameter standard deviation (σ g) as follows (Gholizadeh-Sarabi and Sepaskhah 2013):
In addition to natural zeolites, synthetic zeolites have been used as amendments for improving the infiltration rate in soils. In a study (Table 1), Ca2+-type zeolite was added to a sand dune soil with a bulk density of 1.47 g cm−3, at the rate of 0, 1, and 5% (equivalent to 0, 1, and 5 kg m−2). The experiments were conducted in plots and pots with a height and diameter of 30 and 16 cm, respectively. According to the finding of this study, the infiltration rate of the soil tested was affected negatively with zeolite application, such that the higher application led to the lower infiltration rate (Al-Busaidi et al. 2008). Over about 4.5 min, the infiltration rate of the control soil, zeolite (1%), and zeolite (5%) decreased 41, 57, and 59%, respectively. The authors attributed this finding to the micro-pores of the zeolite particles, which slowed the percolation of water within the soil structure.
In another column study (Table 1), the effect of a polymer of lignin and natural zeolite on rainwater infiltration rate was determined. The natural zeolite used was obtained from a mine in Jinyum in Zhejiang province, China, and had a water content of 12 g 100 g−1, specific gravity of 2.16 and specific surface area of 230–320 m2 g−1. The lignin was powdered and mixed with the natural zeolite. According to the results, both tested materials delayed and decreased the amount of surface runoff, which is an indication of an increase in infiltration. The combination of lignin and zeolite reduced the quantity of runoff by 44.4 to 50.55% (Zheng et al. 2006).
In general, infiltration is directly related to soil pore structure and structural stability. Zeolite application might alter the soil bulk density, total porosity, and aggregate stability, thereby changing soil structure and the numerous factors affecting the infiltration rate (Azooz and Arshad 1996). In structured soils, macropores and larger mesopores play the key role in conducting water, although they form only a small portion of total porosity (Schwen et al. 2011). It is reported that increasing zeolite application rate can decrease crack area density and therefore reduce the macropores in the soil surface (Razmi and Sepaskhah 2012). The authors did not mention the reason for this alteration. For future studies, more laboratory and field experiments are needed to mechanistically understand the effect of zeolite on infiltration rate, with specific attention on important factors affecting soil structure. It is also recommended to investigate spatial and temporal variation of infiltration in field works, which are common characteristics of the soil infiltration (Khatri and Smith 2006).
2.2 Effect of Zeolite on Hydraulic Conductivity
Hydraulic conductivity is another physical property of soil showing the easiness of water movements within the soil and is used for designing irrigation and drainage systems (Gholizadeh-Sarabi and Sepaskhah 2013). Saturated hydraulic conductivity, K s, is the ability of soil to conduct water when water fills all pores (Lal and Shukla 2004). It is a parameter that is used for modeling water and solute movements in soil. This parameter has a significant effect on water deep percolations, as a factor playing an important role in efficient water use in the agriculture. In addition, movement of solutes within soil is controlled by hydraulic conductivity (Sepaskhah and Yousefi 2007). Improvement of soils hydraulic properties can lead to efficient water use (increased WUE) in the agriculture and an increase in crop production (Gholizadeh-Sarabi and Sepaskhah 2013). This can be done via improving soil physical properties, which can be achieved by applying soil amendments like zeolite (Gholizadeh-Sarabi and Sepaskhah 2013).
Zeolites can modify the hydraulic conductivity due to the existence of channels within their structure. However, it should be noted that zeolites have different effects on different soils textures. In heavy-textured soils, they are able to increase the hydraulic conductivity, while in light-textured soils, they lower the hydraulic conductivity. In a study (Table 1) clinoptilolite was added to four different soil textures including clay, loam, loamy sand, and sand. Zeolite with particle size of 0.05–0.1 mm was added to soils at different application rates of 0–15 g 100 g−1 soil. The results indicated that application of zeolite decreased the hydraulic conductivity of sandy and loamy soils. On the contrary, it increased the hydraulic conductivity of clay soil. Change in the hydraulic conductivity was highly attributed to change the average particle size of the soil (Mahabadi et al. 2007). Larger particles create larger pores, in which water transport can be conducted faster. For sandy and loamy soils, zeolite application can effectively reduce the average particle size, resulting in lower hydraulic conductivity compared to control soils. In contrast, the addition of zeolite into clay soils might increase the average particle size, therefore resulting in higher hydraulic conductivity. Similar results were also reported in another study, supporting these observations (Lin et al. 1998).
In another column study (Table 1), the effects of natural zeolite (with application rates of 0, 4, 8, and 16 g kg−1) and saline water (salinity levels of 0.5, 1.5, 3.0, and 5.0 dS m−1) on K s of sandy loam, loam, and clay loam soils were examined (Gholizadeh-Sarabi and Sepaskhah 2013). According to the findings, soils responded differently to different zeolite application rates and salinity levels. For clay loam and loam at salinity levels of 0.5–1.5 dS m−1, saturated hydraulic conductivity increased with 8 and 4 g kg−1 zeolite, respectively. For clay loam, this increase was 180 and 166% for the mentioned application rates, while for loam the extent of the increase was 20 and 26% for those application rates, respectively. Moreover, in sandy loam soil for 8 g kg−1 zeolite at low salinity levels and for 16 g kg−1 zeolite at high salinity levels saturated hydraulic conductivity dropped compared to the control soil. The amount of decrease for 8 g kg−1 zeolite was 67 and 54% at salinity levels of 0.5–1.5 dS m−1. The extent of decrease was 58 and 50% for 16 g kg−1 zeolite at 3.0–5.0 dS m−1, respectively (Gholizadeh-Sarabi and Sepaskhah 2013). In terms of the effect of zeolite application, an increase in conductivity of clay loam and loam soil and a decrease in conductivity of sandy loam might be attributed to increase and decrease of the average particle size, respectively. In terms of the effect of salinity, the adverse effect of salinity on saturated conductivity of soil can be due to plugging of pores by dispersed clay particles in the high saline water (Frenkel et al. 1978). However, the property of solution alone is not enough to characterize the effect of salinity on soil hydraulic conductivity and soil chemical composition is also a key factor that should be considered (Levy et al. 2005; McNeal and Coleman 1966).
In a column study (Table 1), the effect of a natural zeolite application on saturated hydraulic conductivity of a silty clay soil was determined. The zeolite application rate was 0, 4, 8, and 12 g kg−1. Results showed that with increasing zeolite application rate to 8 g kg−1, saturated hydraulic conductivity of the soil tested increased 130% compared to the control soil. However, results showed that further increase in zeolite application could decrease saturated hydraulic conductivity (67% reduction in 12 g kg−1 in comparison to 8 g kg−1 application rate). The authors concluded that the application rate of 8 g kg−1 zeolite improved soil porosity and pore size, while higher application rate (12 g kg−1 zeolite) had a negative impact on pore size and porosity of the soil sample (Razmi and Sepaskhah 2012). However, soil porosity and soil and zeolite particle size were not reported in that paper and without these information, making strong conclusion is impossible. A similar result was reported in another study illustrating that with an increase in zeolite application rate pore water velocity increased (Sepaskhah and Yousefi 2007). They showed that 4 and 8 g kg−1 zeolite application rate increased the pore water velocity by 35 and 74%.
In another investigation (Table 1), the effects of adding two different natural clinoptilolite minerals (i.e., Clinolite and Ecolite) on hydraulic properties of a sand at 15:85% volume fraction (v v −1) was determined (Githinji et al. 2011). Bulk density of Ecolite and Clinolite were 0.95 and 0.97 g cm−3, respectively. The bulk density of the Ecolite-sand and Clinolite-sand were 1.56 and 1.57 g cm−3, while the bulk density of the sand was 1.67 g cm−3. The experiments were conducted in a column. Results showed that zeolite application increased hydraulic conductivity (Githinji et al. 2011). The saturated hydraulic conductivity of Clinolite and Ecolite was 1.42 and 1.29 m h−1, respectively, which was higher than the saturated hydraulic conductivity of the sand (0.41 m h−1). These results were in agreement with those reported by Waltz et al. (2003). They had shown that an increase in hydraulic conductivity value for sand-amendment compared to pure sand. This can be attributed to the improvement of soil texture due to the zeolite application. According to the results, geometric mean diameter, particle density and the bulk density of Ecolite-sand were 0.35 mm, 2.65 g cm−3, and 1.56 g cm−3. These parameters were 0.55 mm, 2.40 g cm−3, and 1.49 g cm−3 for Clinolite-sand. While these parameters were 0.31 mm, 2.67 g cm−3, and 1.67 g cm−3 for 100% sand. As can be seen, the amended sand had larger particles than that of sand and lower bulk density, which are the reasons for higher saturated hydraulic conductivity.
In addition to natural zeolites, modified zeolites can be used in soils to improve their hydraulic conductivity. In a research, the effect of modified zeolite on soil hydraulic conductivity was determined. Results showed that adding Ca2+-zeolite to sodic soils increased the hydraulic conductivity compared to unamended soil (Pal et al. 2006). The hydraulic conductivity of the treated soil was more than 10 mm h−1. Moreover, the infiltration rate of the amended soil was higher. The authors linked this finding to Ca2+ release that facilitates the infiltration rate.
In general, saturated hydraulic conductivity is a function of several factors including total and effective porosity, pore size distribution, pore connectivity, and tortuosity (Jabro 1992; Vervoort and Cattle 2003). Zeolite application can alter the soil bulk density, particle density and particle size distribution, resulting in change in the factors affecting saturated hydraulic conductivity. However, the number of publications investigating the effect of zeolite application on hydraulic conductivity is not sufficient to make a general conclusion. It is recommended to assess change in pore size and connectivity, tortuosity, and potential segregation of zeolite particles using advance methods such as image processing in the future works.
2.3 Effect of Zeolite on Soil Water Content
Soil moisture is defined as the water held between soil particles and is considered as the major component of the soil characteristic playing a critical role in plant growth. Soil water content is critical for plant growth, microbial activity, regulating soil temperature, and runoff (Bittelli et al. 2015).
Zeolites can modify soils water content by altering the bulk density and total and aeration porosity. Bulk density is a basic soil physical property that can have an effect on the total porosity and topsoil stability, such that the bulk density of light-textured soils can be lowered with the application of zeolites (Ramesh et al. 2011). The lower the particle size of the zeolite and higher the application rates, the lower the bulk density of a sandy soil. Moreover, soils’ total and aeration porosity can be modified by using zeolites. However, the extent of this modification is higher for aeration porosity than that of total porosity (Ramesh et al. 2011). Furthermore, in sandy soils, decreasing of particle sizes of zeolites can lead to higher water holding capacities, which can be attributed to zeolites high pore volumes that enable them to hold more water in their structures (Ramesh et al. 2011).
Soil amendments are able to hold water and prevent it from deep percolation that can improve WUE in agricultural activities. Their application has been tested in a number of studies in which their effects on soil hydraulic properties have been determined (Ibrahim-Saeedi and Sepaskhah 2013). It has been illustrated that their application to light-textured solids can improve the WHC (Bernardi et al. 2009; Bernardi et al. 2013). It is noteworthy that one of the drawbacks of light-textured soils in agriculture and horticulture is attributed to its lower irrigation efficiency. This is because sandy soils do not have a proper water retention capacity. Treating light-textured soils with soil amendments such as zeolites can improve their WHC.
The capability of zeolites in improving soil WHC has been the subject of a number of studies. In a study, the effects of treating soils with a natural zeolite on WHC was determined (Table 1) (Xiubin and Zhanbin 2001). The natural zeolite used in this study was mainly mordenite with a size of less than 0.25 mm. The zeolite had a CEC of 136.35 cmol(+) kg−1 and saturation water content of a 121.32%. Organic content of the soil was 0.76% by weight. The extent of particles with a size range of 0.05–0.01 mm fraction was 46% and the clay fraction was about 24%. Zeolite was added to fine-grained calcareous loess that had low WHC. Typical effects of zeolite on soil WHC is illustrated in Fig. 4. According to this figure, zeolite application increased the soil water content: after 25 h of adding water to treated and normal soils, the WHC of soil and zeolite was higher than that of the normal soil. This study showed that the capacity of the treated soil with natural zeolite in holding water in drought and general conditions, increased 0.4–1.8 and 5–15%, respectively, compared to control soil (Chmielewska 2014a; Xiubin and Zhanbin 2001).
Soil water releasing processes of the control soil and zeolite-treated soil (Xiubin and Zhanbin 2001)
In another study (Table 1), a Brazilian natural zeolite, that was composed of stilbite, mixed with a smectite clay mineral and quartz, was added to a sandy soil (890, 30, and 80 g kg−1 of sand, silt, and clay) at the levels of 33.3, 66.7, and 100.0 g kg−1. According to the results, the amount of available water content of the treated soil was 10, 38, and 67% higher than that of the control for the mentioned zeolite application rates. Moreover, the amount of the readily available water is another soil characteristic that can be improved with zeolite application. It was illustrated that an increase in available water for treated soil samples compared to the control, such that easily available water went up by 15, 51, and 111% for noted zeolite application rates compared to the control, respectively (Bernardi et al. 2013).
In another study (Table 1), it is reported that with zeolite application WHC of sandy soil increased (Nus and Brauen 1991). The bulk density and CEC of the soil tested were 1.68 g cm−3 and 2.6 cmol(+) kg−1, respectively. The zeolite used in this study was a natural clinoptilolite with a bulk density and CEC of 1.11 g cm−3 and 61.3 cmol(+) kg−1, respectively. Distribution of zeolite particle size showed that 54.7% of the particles had a diameter of higher than 54.7 mm. In this study, the effects of increasing natural zeolite application rate (5, 10, and 20 v v −1) on water content of a sandy soil was determined. It was revealed that with an increase in zeolite application rate, the volumetric water content of the sand soil improved at − 10 KPa matric potential at each application rate. For 10% v v −1 zeolite application rate, moisture content was 5.85%, while it was about 3.51% for sandy soil (Nus and Brauen 1991).
Influence of natural zeolite application on a sandy soil water content was determined in another study (Table 1). Sandy soil was amended with (10% v v −1) natural clinoptilolite zeolite in which turfgrass was grown. The bulk density, porosity, hydraulic conductivity, and CEC of the sand soil were 1.66 Mg m−3, 0.413, 317 cm h−1, and 0.8 cmol(+) kg−1, respectively. The same parameters for zeolite-treated soil were 1.60 Mg m−3, 0.437, 212 cm h−1, and 1.6 cmol(+) kg−1, respectively. Based on the findings, natural zeolite application resulted in an increase in the soil volumetric water content such that the volumetric water content of the amended sandy soil was 20% higher compared to untreated sand (Bigelow et al. 2001).
In a study (Table 1), the effects of four natural clinoptilolite zeolite products on transpiration of “Tifdwarf” Bermuda grass, which was planted in sandy soil was examined (Miller 2000). All zeolites were added to soil at a rate of 8.5% weight fraction (w w −1). The author noted that zeolites increased the amount of transpirational water by 1% to 16% in sand (Dwairi 1998; Miller 2000). This is an indication of an increase in soil water content as a result of zeolite application.
One of the factors that might affect the efficiency of zeolite is the method whereby it is applied to soil. Effect of zeolite application on the water content of mixed zeolite soil versus banded zeolite soil was determined in another study (Table 1). The zeolite studied was natural clinoptilolite with CEC, bulk density, pore size, pore volume and permeability of 155 cmol(+) kg−1, 0.76 g cm−3, 0.5 nm, 51%, and 10−3 m s−1, respectively. The zeolite application rate for both methods (mixed and banded) was 0, 6.7, 13.4, 20.2, and 44.8 Mg ha−1. Results showed that soils mixed zeolite contained 1.3% more soil moisture as compared to band zeolite applications. Furthermore, authors reported that 44.8 Mg ha−1 application rate showed the highest water content, such that it had 2.1% more water than the 0 Mg ha−1. According to the results, the differences among the application rates were minimal at potentials of 0 and − 10 KPa. Water retention at matric potentials of − 100 and − 300 KPa were the greatest for a mixed zeolite rate of 44.8 Mg ha−1, compared to a lower zeolite rate and the control. This is an indication of having more water retained in the pore spaces of the sandy soil in 44.8 Mg ha−1 (Ippolito et al. 2011).
Modified zeolites can also be used to improve soil water content. In a study (Table 1), the effect of a modified Ca+2-type zeolite on sand dune soil irrigated with saline water was determined. Sand dune soil samples were treated with the zeolite at the rate of 0, 1, and 5% (equivalent to 0, 1, and 5 kg m−2) and irrigated with seawater diluted to electrical conductivity (ECw) levels of 3 and 16 dS m−1. According to the results, a 5% zeolite application rate increased both the content of soil salt and water (Al-Busaidi et al. 2008). Authors attributed this finding to the fact that zeolite can enhance CEC of soils, hold cations on the surface, and release them at the expense of salts in the saline water, and the amount of the increase was more significant at the soil surface (Inglezakis 2005). The extent of increase for water content and salinity were 20 and 1.4%, respectively.
Generally, zeolite can decrease the bulk density and increase total porosity, which consequently increase soil water content. Its application changes the inter-particle porosity of soil. Zeolite is a porous medium with open pore network channels into its structure, which can also play an important role in water retention. For future researches, it is recommended to investigate the effect of zeolite on full range of water retention curve from saturated zone to dry zone, by distinguishing the effect of inter-particle and intra-particle of zeolite.
Effects of zeolite application on discussed physical properties of soils (i.e., infiltration rate, hydraulic conductivity, and water content) reported in the selected scientific papers are summarized in Table 1.
3 Impact on Nutrient Retention
3.1 NH4 +/NH3 Retention and Release in Soil
Adding zeolites to soils can improve nutrient holding capacities by directly or indirectly affecting physical, chemical, and biological properties of soils, which in turn control the nutrient dynamics in soils. Because of high CEC of zeolitic minerals, these compounds have demonstrated high NH4 + sorption selectivity and capacity as a result of electrostatic attractions between positively charged NH4 + and negatively charged sites of zeolites structure (Aiyuk et al. 2004; Englert and Rubio 2005). The strong affinity of zeolites for NH4 + cations can be extensively used to improve its retention and release in the soil media (McGilloway et al. 2003; Rabai et al. 2013; Sfechis et al. 2015). On the other hand, addition of zeolites to soils can increase the amount of NH4 + adsorption into soils; that may be due to a high affinity of zeolites to entrap NH4 + molecule by ion exchange processes (Demir et al. 2002). Nevertheless, the degree of retention of NH4 + by zeolitic minerals can vary depending on silicon/aluminum (Si/Al) ratio, mobile and exchangeable cations of zeolites, pore size and structure of these materials, pH, contact time, temperature, and the concentration of other ions in the soil and water (Barros et al. 2003; Sarkar and Naidu 2015; Torma et al. 2014; Watanabe et al. 2005). Although the CEC of some natural zeolites are 2 to 3 times greater than other types of minerals existing in soils, there is a wide diversity among zeolites because of the difference in the nature of zeolite-cage structures, natural structural defects, adsorbed ions and their associated minerals (Kazemian 2002; Kazemian et al. 2012; Malekian et al. 2011b; Ramesh et al. 2015b). Therefore, understanding the adsorption and release kinetics and mechanisms in different sources of zeolites is important in order to increase NH4 + retentions and decrease its leaching losses in soils.
3.1.1 Effect of Natural Zeolites on NH4 + Retentions and Release in Soils
The peerless chemical and physical properties of natural zeolites among other aluminosilicate minerals, especially high CEC and strong affinity for NH4 +, have been exploited to minimize the N loss through leaching and to maximize the nitrogen use efficiency (NUE) in agricultural applications (Sarkar and Naidu 2015). NUE and N leaching are a function of each other; i.e., reducing NH4 + leaching to groundwater and surface runoff by zeolite amendment will likely increase the NUE and vice versa (Ming and Allen 2001). Clinoptilolite is one of the naturally existing zeolites, with a theoretical CEC of 2.16 cmol(+) kg−1 (Jha and Hayashi 2009). Although the ion exchange capacity of clinoptilolite is lower than some other zeolites, it generally exhibits a high selectivity for NH4 + ion. This zeolite is an inexpensive mineral with huge sedimentary deposits, which is available in most regions of the world. Clinoptilolite has been utilized in several studies to decrease leaching of N, which leads to reducing the risk of groundwater contamination. For example, a column leaching experiment was conducted under a pulse application of urea and ammonium nitrate solution (UAN32) with a total N concentration of 443 mg-N L−1 (NH4 + concentration of 37 mg-N L−1) (Piñón-Villarreal et al. 2013). From the total applied NH4 +-N pulse, only 3% was leached from the pure clinoptilolite zeolite column in comparison to 17% in the column of the loamy sand. Therefore, an average leaching reduction of 82% was observed (Table 2). It is also noted that the leaching and retention of NH4 + in treatments of loamy sands with clinoptilolite using mass ratios of 60:40 and 80:20 insignificantly deviate from pure zeolites. Similar reductions in NH4 +-N leaching after application of the clinoptilolite zeolite have been reported. In another leaching experiment, after application of an NH4NO3 pulse at a rate of 350 kg-N ha−1 under saturated conditions, the maximum relative concentrations of NH4 +-N breakthrough curves (BTCs) decreased by 43 and 50%, respectively, in the columns amended with 2 and 8 g kg−1 of the clinoptilolite zeolite as compared to the control loam soil (Sepaskhah and Yousefi 2007). In another experiment, NH4 + movement in a Rositas loamy sand amended with clinoptilolite was evaluated (MacKown and Tucker 1985). It was observed that in the column filled with a 50 g kg−1 zeolite-soil-mix, the NH4 + leaching was 83% less than the control column (Table 2). The leached NH4 + ranged from 0.01 to 0.6% of the total applied N fertilizer. This result can be attributed to an increase in the CEC of soils. The sorption of NH4 + in powder natural clinoptilolite zeolite was monitored in another study. The zeolite with an average particle size of 45 μm was applied to a soil at a rate of 600–1200 kg ha−1 (Torma et al. 2014; Vilcek et al. 2013). In a sorption experiment, it was revealed that more than 90% of NH4 + was adsorbed by zeolite in the first several minutes. Stabilization of the exchange equilibrium was reached in only a few hours, which can be attributed to a very small particle size and high surface area of zeolite materials. Furthermore, the results of mixing with a soil revealed a reduction of NH4 + content in the soil (92.5 mg kg−1 in the control soil and 77.2–81.0 mg kg−1 of soil in the zeolite-soil-mix) 1 month after the zeolite application. This reduction might be attributed to entrapping of NH4 + ions at some ion exchange sites within zeolite crystal lattice that are not easily accessible. Three months later, the opposite was true and the amount of NH4 + contents in the soil with zeolites were found to be 24–59% more than in the soil without any zeolite.
Zeolite minerals also can protect NH4 + in soils from biological conversions to NO3 − through the nitrification process. The latter is more prone to leach out of the soil. This transformation can contribute to surface and groundwater NO3 − contaminations (Sepaskhah and Yousefi 2007). In zeolites with small pore sizes of the crystal lattice structure (4–5 Å) such as clinoptilolite, cations, like NH4 +, can fit in. However, microorganisms, especially nitrifiers, which are a precursor in microbial conversions of NH4 + to NO3 −, are not able to access the pores (Baerlocher et al. 2007). Therefore, when zeolites selectively adsorb NH4 + in soils, the retained NH4 + ions in the internal voids of zeolites are physically inaccessible to nitrifying bacteria and thus NH4 + is protected against the nitrification process. Zeolites can effectively inhibit the nitrification process and as a result, reduce N leaching especially in well-aerated sandy soils, where nitrifying microorganisms are more active (Gholamhoseini et al. 2013). The effect of adding 10% (w w −1) clinoptilolite-rich tuff to sand-based putting greens on N leaching was evaluated. While (NH4)2SO4 was applied as a source of N, regardless of the N application rates, zeolites reduced the both NH4 +-N and NO3 −-N leaching. At the highest N application rate (293 kg-N ha−1), NO3 − and NH4 + leaching from the clinoptilolite zeolite-amended sand was 86 and 99% lower than those from the unamended sand, respectively (Table 2). The NO3 − concentration in leachate from the amended sand did not exceed 10 mg NO3 −-N L−1 (Huang and Petrovic 1994). In an incubation experiment, it is found that application of pre-adsorbed clinoptilolite with large particle sizes (2.0 to 1.0 mm) decreased nitrification in Rositas loamy sand and Gila silty clay loam by 24 and 31%, respectively (MacKown 1978). Another experimental result also revealed a less intense nitrification rate after application of clinoptilolite (600–1200 kg ha−1) to a soil. NO3 − content decreased by 66–78% in comparison with the unamended soil in the consecutive months (in an autumn period) (Torma et al. 2014; Vilcek et al. 2013).
Zeolites also have been widely applied along with other materials to increase the soil nutrient availability, plant growth as well as yield (Colombani et al. 2015; Lim et al. 2015). For example, it has been observed that an increment of the exchangeable soil N concentration (mostly NH4 +, and NO3 − was not detected) after a co-application of the clinoptilolite zeolite and fly ash to a coarse loamy soil was more than the application of those alone. This substantial increase in the nutrient retention in soil is beneficial in terms of prevention of water contaminations by reducing the nutrient loss. However, it might not always improve the plant growth and yield productivity due to the nutrient limitation, especially in a low fertile soil (Lim et al. 2015). In another study, application of the Italian chabazite-rich tuff of Sorano (Grosseto) as a soil conditioner and slow nutrient fertilizer was evaluated using batch and column experiments. Zeolite was added to a silty clay soil and a sandy soil at the rate of 95:5 v v −1. A natural zeolite was mixed with a swine manure, with a solid fraction of 1% w w −1, in order to gain an NH4 +-pre-charged zeolite. Using an artificial rainwater (deionized water Milli-Q plus CaCl2 0.01 mM and NaCO3 0.01 mM, pH = 7.6) as eluent, NH4 + was never detected in the leachate of a batch and in the effluent of column experiments (Colombani et al. 2015). It has been suggested that sewage sludge compost, combined with zeolites can be used as a potting soil for greenhouses and nurseries (Bugbee and Elliott 1998). Composting is one of the most popular biological technologies in the sludge treatment; in which sewage sludge nutrients can be recycled for plant growth and soil fertility improvements (Doublet et al. 2010; Jiang et al. 2014; Wang et al. 2014; Wei et al. 2000). However, a direct application of sewage sludge compost onto fields may lead to an increase in water pollutions by leaching of nutrients such as NH4 +. In a greenhouse study, the combination of the sewage sludge compost, sphagnum peat, and clinoptilolite-rich tuff, by volume ratio of 50, 30, and 20%, respectively, reduced the total N leached through the pot to 0.4 mg, compared with 1.4 g leached through the pot of the control mix consisting the sewage sludge compost, sphagnum peat, and sand.
Zeolite amendments may influence coarse-textured soils to a greater extent than fine-textured soils. Many of the beneficial effects are due to a significant change in CEC and an improvement in the physical characteristics of soils (Ippolito et al. 2011; Sarkar and Naidu 2015). Sandy soils have low water and nutrient retention capacities. Thus, zeolite amendments can demonstrate the remarkable influence on the reduction of NH4 + leaching (Zwingmann et al. 2009). It is reported that the addition of 0.2% by weight of a clinoptilolite zeolite to a loamy soil is enough to absorb the applied NH4 + and prevent its leaching by the inflow water (Sepaskhah and Yousefi 2007). Similar studied obtained a significant reduction of NH4 + leaching rates after application of small amounts of the clinoptilolite zeolite in sandy and loamy soils (Huang and Petrovic 1994; MacKown and Tucker 1985; Pepper et al. 1982; Perrin et al. 1998b). In comparison, it is noted that a clinoptilolite zeolite reduced NH4 +-N leaching in a Nunn clay loam (Aridic Argiustoll) only at a high application rate (135 Mg ha−1) compared to an unamended control, indicating that large quantities of the clinoptilolite is needed to diminish NH4 + leaching in fine-textured soils (Weber et al. 1983).
In addition to the soil texture, the zeolite particle size may also have a significant impact on the rate and kinetics of NH4 + adsorption and desorption in soils. In a greenhouse study, N leaching from three different particle sizes of NH4 +-loaded clinoptilolite, including small (< 0.25 mm), medium (0.25–2 mm), and large (2–4 mm) was investigated. Zeolites were banned with rounded quartz sands at the rates of 112, 224, or 336 kg-N ha−1 (Perrin et al. 1998a). It was found that the larger zeolite particles, the less N leached from amended soil. After 40 days of the simulated leaching experiment, the sand modified with large clinoptilolite particles leached 24.9–32.5% of the total N. In comparison the medium and small-sized clinoptilolite-amended sands had leaching rates of 53.4–68.4 and 44.4–72.1% of the total N, respectively. This inverse relationship between zeolite particle sizes and NH4 + exchange rates can be attributed to the required time for intra-particle diffusion, which increases as the particle size increases. However, this result is not consistent with the result of another study, in which the NH4 + concentration in leachate from a sandy soil amended with millimeter particle sizes of clinoptilolite zeolites was slightly greater than that amended with nanometer particle sizes (Malekian et al. 2011a).
The other factor that might affect nutrient retentions is the method of zeolite incorporations to soils. The effect of banding or fully mixing of clinoptilolite zeolites (up to 90 Mg ha−1) and urea fertilizers (224 kg-N ha−1) with a silty loam soil on N dynamics was investigated during 35 days of an incubation study. The results revealed a greater extent of urea mineralizations and more effective NH4 + sorptions at a mixing application of zeolites in comparison to band applications (Ippolito et al. 2011). This result is consistent with another column leaching study conducted by the same authors; in which mixed versus band applications of zeolites and fertilizers to a silty loamy soil was evaluated. It is reported that when the N fertilizer (224 kg-N ha−1) and clinoptilolite zeolite (up to 20.2 Mg ha−1) were fully mixed into the silt loam soil, less NH4 +-N was leached as compared with a control, regardless of zeolite application rate (Tarkalson and Ippolito 2010). In contrast to the abovementioned observation, another researcher stated that the application of the natural zeolite to soils in a layered treatment is more efficient in pollution retentions in soils and consequently reduces the risk of groundwater contaminations (Taheri-Sodejani et al. 2015).
3.1.2 Effects of Manipulated Zeolite on Improving NH4 + Retention in Soil
In addition to natural zeolites, modified and synthetic zeolites have been used to reduce the NH4 + leaching and improve the NUE. MesoLite is an artificial zeolite, which is made by a caustic treatment of kaolin at 80–95 °C. It has a very high CEC (~ 500 cmol(+) kg−1) and a moderately low surface area (9–12 m2 g−1) (Mackinnon et al. 2003; Thornton et al. 2007a; Thornton et al. 2007b). The effectiveness of the K-MesoLite and NH4 +-MesoLite compared to a natural clinoptilolite in retaining and releasing NH4 + in columns of amended sandy soils was investigated (Zwingmann et al. 2009). The additions of 8 g kg−1 natural zeolites and K-MesoLite both reduced leaching of the applied dissolved NH4 + by bore waters. However, as it is shown in Fig. 5, a K-MesoLite-amended soil exhibited a much higher efficiency in NH4 + retentions. The column of the K-MesoLite-amended soil retained more than 90% of the added NH4 + compared to 30 and 4% retained by the clinoptilolite-amended soil and unamended soil, respectively (Table 2). Based on the amount of amendment needed to retain 25% of added NH4 + after six pore volumes of leaching, the effectiveness of K-MesoLite was about 11.5 times higher than that of clinoptilolite zeolite. The higher efficiency of K-MesoLite might be explained as the CEC of K-MesoLite is about five times higher than that of clinoptilolite zeolite. Furthermore, the release of NH4 + adsorbed on zeolites showed dependency on the existing exchangeable cations in the irrigation water. When Ca2+-rich bore water was used, 15% of the NH4 + was released by six pore volumes. But only one-quarter of this amount was released when deionized water was used. This higher concentration of NH4 + in leaching by bore water occurred in all treatments in this study. Thus, under field conditions, where irrigation or surface water contains abundant dissolved cations, both the K-MesoLite and NH4 +-MesoLite material will give the most benefits in N loss reduction from soils.
The effect of natural zeolite and K-MesoLite on NH4 + ion retention (Zwingmann et al. 2009)
In another research experiment, a natural clinoptilolite from Japan was modified chemically with NaOH treatment to increase its content of alkaline metal cations (Na+ ion which is more easily exchanged with NH4 + ion); and also was modified mechanically with a wet ball milling to decrease particle sizes (increase the specific surface area) (Jha and Hayashi 2009). Either with a physical or chemical modification of the clinoptilolite, the NH4 + retention capacity was sharply increased. The maximum NH4 + retention capacities of natural clinoptilolite (NZeo), clinoptilolite treated with NaOH solution for 72 h (Zeo-72), and wet-milled clinoptilolite (WM-50) were 0.89, 1.15, and 1.39 mmol g−1, respectively (Fig. 6). Figure 6 also illustrates the Langmuir and Freundlich sorption isotherms, indicated as solid and broken lines. In the case of natural and chemically modified clinoptilolite, the sorption isotherms were good fit to the Langmuir model, while in case of mechanically modified clinoptilolite the Freundlich model was favorable. This behavior is expected because during chemical modification the crystalline structure of zeolite was not altered, while the crystalline structure was crushed during mechanical modification. Because of a higher adsorption capacity of the physically treated zeolite, it was concluded that the enhancement of NH4 + retention capacity of the natural clinoptilolite just by a decrease in the particle size is possible, without incorporating any further exchangeable cations within the framework of zeolites. Selected studies that have used natural and manipulated zeolites to reduce NH4 + leaching through soil are summarized in Table 2.
NH4 + sorption of natural and modified clinoptilolite zeolites. Solid and broken lines illustrate predicted data obtained from the Langmuir and Freundlich sorption isotherm parameters (Jha and Hayashi 2009)
3.1.3 Using Zeolite as an NH4 +-Slow-Release Fertilizer (SRF)
The imbalance between the rate of nutrient releases from fertilizers and the rate of nutrient uptake by plants causes a lower NUE of the fertilizers. An effective way to mitigate the aforementioned problem is to develop SRFs, which can release nutrients slower than commonly used fertilizers (Behin and Sadeghi 2016; Bhardwaj et al. 2012). SRFs have better performance over commonly used fertilizers, they have lower fertilizer loss rate and sustainable nutrient supply, reduce the application frequency of fertilizers, and minimize the potential of negative effects associated with over-dosages (Guo et al. 2006; Ni et al. 2010a; Ni et al. 2010b; Zhang and Saito 2009; Zhang et al. 2010). The prominent large CEC and conspicuous selectivity of natural, synthetic, and modified zeolites including natural clinoptilolite and synthetic MesoLite for plant nutrients, especially NH4 +, can be exploited in the preparation of fertilizers to improve the capacity of the soil nutrient retention. This can be done by promoting a slower release of these elements synchronized with plant uptake (Sepaskhah and Yousefi 2007; Sfechis et al. 2015). Although the aluminosilicate framework of natural zeolites is relatively not affected when exposed to water, NH4 + may be slowly exchanged and released from exchange sites of zeolites (Rehakova et al. 2004). A slow-release fertilizing not only increases the NUE and promotes crop yielding, but it also diminishes the potential migration of these elements into groundwater and runoff from plant root zone, and subsequently reduces environmental pollution. Zeolites have been extensively used as SRFs, either in the combination of another N fertilizer or in the form of a pre-loaded NH4 + zeolite (Sepaskhah and Barzegar 2010; Zwingmann et al. 2009). In both ways, exchange sites within zeolite structures act as sinks for NH4 +. Over time, this retained exchangeable cation can be slowly replaced by another cation and thereby become available for plant uptake (Ming and Allen 2001). As an example in a greenhouse experiment, more N was assimilated when an NH4 +-loaded clinoptilolite was exploited as an amendment for a sandy soil at rates of 112, 224, or 336 kg-N ha−1, compared to the (NH4)2SO4. Less than 5% of the added N leached out from the amended sandy soil, regardless of the N rate and zeolite particle size, while 10% to 73% of the N leached when (NH4)2SO4 was applied (Perrin et al. 1998a).
3.1.4 Effect of Zeolites on NH3 Volatilization in Soils
In addition to N loss from soils through leaching, a significant portion of the loss could be due to NH3 volatilization (Piñón-Villarreal et al. 2013). Natural zeolites such as clinoptilolite are renowned adsorbents for ammonia, in which they are selectively trap ammonia even at very high relative humidity (Asilian et al. 2004). In agricultural applications, this gaseous NH3 loss will lead to a decrease in the N uptake efficiency and an increase in production costs (He et al. 2002; Omar et al. 2010). As part of a study, the effects of clinoptilolite zeolites, cellulose and combination of both on NH3 volatilizations and N transformations in agricultural calcareous sandy soils were investigated. With the application of 15 g kg−1 clinoptilolite zeolites, NH3 volatilization from NH4NO3, (NH4)2SO4, and urea (applied at the rate of 200 mg-N kg−1 soil) decreased by 4.4-, 2.9-, and 3.0-fold, respectively, compared to that from the respective sources without treatments. It is also reported that a co-application of clinoptilolite and cellulose (at a rate of 15 g kg−1 each) was the most effective way in decreasing NH3 volatilizations. The effect of a clinoptilolite and cellulose amendment on the N holding capacity of soils may be attributed to increase in NH4 + retentions in the ion exchange sites of zeolites and increase in the microbial biomass, which is responsible for the immobilization of N. However, it should be noted that NH3 volatilization is a function of pH and temperature. It enhances in a higher pH and temperature (Meisinger and Jokela 2000). Other studies also reported similar reductions in NH3 volatilization after amending sandy soils with zeolites. It is reported that by applying 1 g kg−1 of clinoptilolite to a sandy clay loam and sandy loam, NH3-N losses reduced by 49.23 and 51.09%, respectively. In this research, urea and an acidic agent (triple super PO4 3−) were utilized as fertilizers (Ahmed et al. 2010; Haruna Ahmed et al. 2008).
Besides, composting of animal manures is considered an effective treatment method for naturally recycling organic matters. It generates high NH3 emissions because of the microbial decomposition of nitrogenous organic compounds in the manures. NH3-N loss decreases N content of the product and also causes environmental pollutions (Baek et al. 2002; Kuroda et al. 2004; Tiquia and Tam 2000). Without an appropriate control, 33–62% of the total N content of the manure may be lost during composting. Therefore, it is required to utilize proper methods to diminish NH3 emissions and N loss from the compost (Hong and Park 2005). Zeolite amendments, such as clinoptilolite, have demonstrated a great ability in reducing NH3 emissions from animal manures, both in lab-scale and large-scale composting processes (Bautista et al. 2011; Li et al. 2008). Reduction of volatilization of gaseous N, such as NH3 or N2, can be attributed to the exchange of NH4 + into zeolite exchange sites; so that it is unavailable for conversions to these gaseous phases via microbial processes (Ming and Allen 2001). The addition of alum and zeolite to swine manures has shown a great ability in reducing NH3 emissions by 85–92%. The final compost retained three-folds more NH4 +-N than the unamended control (Bautista et al. 2011). Furthermore, the zeolite substantially improved the quality of the compost by sequestering 44% of retained NH4 + at exchange sites, which can be exploited as a slow-release fertilizer for applications to agricultural soils and improve the crop productivity (Leggo 2015).
3.2 NO3 − Retention and Release in Soil
NO3 −, because of its negative charge, typically does not have much affinity for soil particle surfaces and does not readily adsorb in soil (Feigin et al. 2012), although sorption of NO3 − on a few acidic types of soils has been reported (Cahn et al. 1992; Eick et al. 1999). Because of anion repulsion between NO3 − and soil surfaces, the application of a fertilizer containing anions, such as UAN 32 or KNO3 to soil, can result in higher N loss and lower NUE (Li et al. 2006; Li 2003). Furthermore, it can be lost via denitrification, especially in moist soils. Denitrification losses also reduce the NUE and are an environmental concern for the potential production of N2O; that may play in stratospheric ozone depletion (Qian et al. 1997; Ravishankara et al. 2009). Due to the low retention and high potential to loss in soil, an increasing amount of applied fertilizer is therefore required for appropriate plant growth. However, this increased dose will contribute more to contaminate water, due to elevated highly soluble NO3 − concentrations in surface and groundwater. Therefore, it has been recommended to apply anionic fertilizers at lower rates but at a higher frequency, use other types of fertilizers that do not contain NO3 −, or utilize lower soluble fertilizers and SRFs. Another possible approach is incorporation inexpensive soil modifiers such as natural zeolites that can increase NUE and decrease NO3 − leaching (Malekian et al. 2011a).
3.2.1 Effect of Natural Zeolite on NO3 − Retention and Release in Soil
Zeolite minerals do not have an evident affinity for NO3 − ions, due to negative charges on the surface of zeolitic framework. Nevertheless, contradictory results can be found among published literature. While various studies from the literature suggest that addition of natural zeolites promotes NO3 − leaching from soils (Piñón-Villarreal et al. 2013; Sutherland et al. 2004), several others have shown opposite effects (Aghaalikhani et al. 2012; Rabai et al. 2013; Sepaskhah and Yousefi 2007). In most of the reported studies, leached NO3 − cannot be differentiated among one of these two forms: direct application of inorganic fertilizer containing NO3 − or zeolites pre-loaded with NO3 −, and NO3 − produced from biological nitrification after incorporation of NH4 +/urea fertilizers or zeolites pre-loaded with NH4 +. To determine whether or not the addition of natural zeolite, especially clinoptilolite to soil has a positive effect on NO3 − retention and leaching, N source in soil should be considered as the main factor.
In the case of direct application of NO3 − to soil, due to negatively charged the surface of natural zeolite molecules, anion repulsion might occur. In this case, zeolites not only have no positive effect on NO3 − retention in soil but rather increase its leaching rate. In a column leaching experiment with a pulse application of 443 mg-N L−1 of UAN 32 fertilizer, 92% of the total applied NO3 −-N was leached from clinoptilolite zeolite, in comparison to 83% leached from loamy sand (Table 2) (Piñón-Villarreal et al. 2013). In another leaching study, the maximum value of relative concentration occurred in the pore volume of about 0.5 (Sepaskhah and Yousefi 2007). While theoretically it should occur at pore volume of 1.0 for non-absorbent ions, such as NO3 −. This was attributed to anion exclusion by negatively charged zeolite and soil surface. When solute molecules are repulsed from the surfaces of the porous media, they tend to migrate toward the center of the pore line, where the pore velocity reaches its maximum. It causes the solute to move faster than the average pore velocity (Leij and van Genuchten 2001). Nevertheless, it is reported that after pulse application of NH4NO3 (350 kg-N ha−1) under saturated conditions, the maximum relative concentration for NO3 − breakthrough curve was reduced by 15%, when clinoptilolite zeolite was added at a rate of 8 g kg−1 soil. This indicated that some portion of the applied N was held in the soil by amending zeolite and was not leached by water (Sepaskhah and Yousefi 2007). Some studies have reported enhancement in NO3 − retention by zeolite incorporation in soil. In a particular report, the reduction has attributed to the trapping of NO3 − into the pores of zeolite framework (Sadeghi et al. 2010). Adsorption properties of the clinoptilolite zeolite were utilized to improve filtering properties of a silt loam soil, irrigated with a wastewater containing NO3 − at the concentration of 14.2 mg-N L−1 (Taheri-Sodejani et al. 2015). After passing through a 40-cm column, NO3 − concentration of the leached wastewater in the control sand, mixed, and layered soil amended with clinoptilolite, decreased by 12.18, 32.19, and 54.90%, respectively (Table 2). The result from this experiment revealed that zeolite application in band method, smaller zeolite particle size (less than 63 μm) and higher dosage (4%), is more effective in reducing NO3 − in wastewater leachate. Similarly, it has been reported that the mixed method of clinoptilolite zeolite application remarkably reduces NO3 − leaching; when NH4NO3 was used as a fertilizer to a silt loam soil at a rate of 350 kg-N ha−1 under saturated conditions. By adding 2, 4, and 8 g of zeolite to 1 kg of soil, NO3 − leaching is reduced to 6, 28.7, and 47.6%, respectively (Sadeghi et al. 2010). Another study reported that odorless and cohesive zeo-sewage sludge can be produced, either by commixture of sewage sludge with the Hellenic Natural Zeolite (HENAZE) or as a precipitate from HENAZE-treatment of urban wastewaters, which is suitable for the reclamation of agricultural soils. Addition of HENAZE with an NH4 + ion exchange capacity of 226 cmol(+) kg−1 in agricultural soils, reduced seepage of NO3 − to water environment by 55–92% and thus protected the quality of surface and groundwater (Filippidis 2010).
When other types of nitrogenous compounds, like urea and NH4 +, are incorporated to soils the presence of NO3 − in the leachate can be attributed to nitrification process (Piñón-Villarreal et al. 2013). Zeolite addition to soil might reduce NO3 − leaching because of its unique physiochemical properties. It renders NH4 + unavailable to nitrobacteria. During an experimental study, NO3 − leaching after application of urea fertilizer at three N levels (90, 180, and 270 kg-N ha−1), and clinoptilolite zeolite at four rates (0, 3, 6, and 9 t zeolite ha−1) to a sandy loam was monitored (Gholamhoseini et al. 2012). Minimum NO3 − leaching was obtained at the higher zeolite application rate (9 t zeolite ha−1). Compared with the control sand, this treatment caused a 36 and 37% decrease in NO3 − leaching loss in the first and second year, respectively (Table 2). A linear relationship between N application rate and its leaching loss was also observed, similar to the result of the other study (Li et al. 2007). NO3 − leaching reduction after application of a mixture of zeolite and urea fertilizer to a soil was explained by different mechanisms including placement of urea in pores of zeolite crystals, reduction in the transformation of urea to NO3 − by nitrification, and a decrease in the nitrification process by NH4 + adsorption on zeolite (Sepaskhah and Yousefi 2007). In another study, the effects of applying clinoptilolite zeolite on nutrient leaching under two irrigation regimes (full and limited) were determined (Gholamhoseini et al. 2013). Several combinations of clinoptilolite, cattle manure and urea were added to a sandy soil as chemical fertilizer. The integrated application of zeolite and cattle manure significantly decreased NO3 − leaching as compared to the unamended soil, particularly under full irrigation regime. Maximum NO3 − leaching (36 kg ha−1) was obtained in the soil without zeolite and cattle manure at full irrigation regime. While minimum NO3 − leaching (11 kg ha−1) was observed in a soil amended with urea, cattle manure and highest zeolite application rate (21%) at limited irrigation regime. In some studies, NO3 − leaching rate decreased by modifying soils with clinoptilolite zeolite, when N sources containing NH4 + (such as (NH4)2SO4) were applied as fertilizer (Huang and Petrovic 1994; Perrin et al. 1998a). Similarly, it is found that the NO3 − leaching decreased by 30% when a loamy sand soil (6% clay) was amended with NH4 +-loaded clinoptilolite zeolite (Wang and Alva 1996). These results are not consistent with the result of other study, where clinoptilolite zeolite was added to golf course sand at two rates of 100 and 200 mL L−1 and two particle sizes of fine and coarse. In this case, (NH4)2SO4 in addition to two forms of urea was added to the soil at a rate of 97.6 kg ha−1 per month. It is reported that significantly more NO3 −-N leached from the sand amended with coarse clinoptilolite zeolite particles than control sand, while no difference was observed between sand amended with fine clinoptilolite zeolite and control (Sutherland et al. 2004). However, in this case, the leached NO3 −-N might have formed faster in the sand by speciation paths other than nitrification causing NO3 − to experience anion exclusion and to be leached out from the amended soil in larger amounts than with control soil (Piñón-Villarreal et al. 2013).
3.2.2 Effects of Manipulated Zeolite on Improving NO3 − Retention in Soil
While almost all of naturally occurring zeolites and most of the artificial zeolites do not possess high affinity for trapping anionic species, by modifying their surface chemistries using cationic surfactant multifunctional adsorbents with capability to trap anions and non-polar organics can be manufactured (Seifi et al. 2011c). Schematic diagram of a typical surfactant-modified zeolite (SMZ) is illustrated in Fig. 7.
Scheme diagram of surfactant-modified zeolite (SMZ), a multifunctional adsorbent for trapping organic molecules, anionic species, and cations (Kazemian et al. 2012), Copyright 2012, Bentham Science Publishers
It has been reported that SMZ, an inexpensive anion exchanger, can be exploited as a fertilizer carrier to comply this requirement and control NO3 − release from soil (Malekian et al. 2011a). For instance, the quaternary amine hexadecyltrimethylammonium (HDTMA) is a long-chain surfactant with a permanent positive charge (Bowman 2003). The maximum loading of HDTMA is about 200% of the zeolites external CEC. At the HDTMA sorption maximum, the surfactant molecules form bilayers on zeolite surfaces with the lower layer held by electrostatic interactions between the negatively charged zeolite surface and the positively charged surfactant head groups, while the upper layer is bound to the lower layer by hydrophobic forces between the surfactant tail groups in both layers. The surfactant loading on the zeolite is a function of the external CEC of zeolite and the chain length of the cationic surfactant (Thirunavukkarasu and Subramanian 2014). Under the surfactant bilayer configuration, the positive surface charge of zeolites is reversed, making it as a mineral with greater potential of sorption and retention of negatively charged NO3 − that is attributed to surface anion exchange (Cordoves et al. 2008; Zhang et al. 2007). By application of surface modified zeolite, therefore, the problem of low NO3 − retention due to lack of significant positive charges in many soils can be effectively addressed.
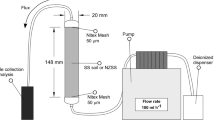
In a study, the performance of SMZ in comparison to clinoptilolite zeolite in NO3 − leaching reduction and crop growth enhancement was investigated (Malekian et al. 2011a). Both zeolite types were added to a sandy soil at different application rate (20 and 60 g kg−1) and with a different particle size (millimeter and nanometer). The clinoptilolite zeolite was modified by HDTMA to 200% of the external cation exchange capacity (ECEC). NH4NO3 was applied as N fertilizer via irrigation water at a rate of 150 kg-N ha−1. They found that both maximum and mean concentration of NO3 − in the leachate of SMZ-amended soil were significantly lower than those of clinoptilolite-amended soil. For example, at the application rate of 60 g kg−1 the leaching of NO3 −-N from SMZ- and clinoptilolite-amended soil was approximately 26 and 22% lower than that from the control system, respectively (Table 2). The decreased NO3 − concentration in clinoptilolite-amended soil may be attributed to the increasing CEC of the soil by mixing with zeolite and the high affinity and selectivity of clinoptilolite zeolite for NH4 +. Therefore, it leads to decreasing NH4 + availability to nitrifying bacteria. Although theoretically adsorbents with smaller particle size should exhibit greater capacity of adsorption due to the greater specific surface area (Huang et al. 2010), no significant difference in the amount of NO3 − leaching from amendments with different particle size was observed. It might be attributed to the fact that particle size affects both adsorption and desorption of NH4 + and NO3 − from unmodified and modified clinoptilolite, respectively. While more N was adsorbed in smaller particle size zeolites, more N was also released from them. However, as it is depicted in Fig. 8, the results revealed that the application of both surfactants modified and unmodified zeolites to agricultural soil can reduce N leaching and increase the ratio of N uptake to the applied N fertilizer.
The amount of NO3 − leached from different soil amendment types (S, SMZ; Z, zeolite clinoptilolite; C, unamended soil control), size (M, millimeter; N, nanometer), and rate (a, 20 g kg−1; b, 60 g kg−1). Different letters above each column represent significant difference at p < 0.05 (Malekian et al. 2011a)
The synthetic clinoptilolite and its surfactant-modified type by using solutions of hexadecyltrimethylammonium bromide (HDTMAB) and dioctadecyldimethylammonium bromide (DODMAB) were introduced. In addition, its potential in adsorption of NO3 − and using as SRF was evaluated (Bhardwaj et al. 2012). The anion exchange equilibrium between NO3 −ions and counter bromide ions can be schematically expressed by:
The powder X-ray diffraction (XRD) patterns of clinoptilolite and its modified forms are shown in Fig. 9. According to XRD patterns, a close resemblance observed on comparing the diffraction patterns of clinoptilolite with its modified surfactant forms indicated the structural integrity of material before and after surfactant treatment. Therefore, surface modification of zeolite does not alter zeolite structure, which is desirable. In aqueous solution with NO3 − concentration of 160–280 mg L−1, the surfactant-modified material exhibited much higher adsorption capacity than the unmodified clinoptilolite. The slow NO3 − release studies have been performed using thin layer-funnel analytical test and soil column percolating system. In the funnel analytical test, unmodified zeolite released 25–35% of NO3 − after first irrigation, while SMZs demonstrated slow release tendency with only 15–20%. Furthermore, at the first day of the column study, NO3 − desorption was 61%, 30–35%, and less than 18% in the soil, clinoptilolite, and surfactant-modified clinoptilolite, respectively. Even after 15–20 days of leaching, the SMZs still released NO3, clearly indicating that a slow release of N is achievable by application of these type of modified zeolite to soil. It is also noted that the presence of humic acid reduced NO3 − adsorption. It can be attributed to the high affinity of surface modified zeolite for humic acid adsorption, which cause low availability of the adsorption site on zeolites for NO3 − ion to be adsorbed (Klučáková 2010; Zhan et al. 2010). In another greenhouse study, NO3 − and NH4 + ions retention in a soil amended with 2 g kg−1 of clinoptilolite zeolite (Z), iron oxide in the form of goethite (G), and SMZ with goethite at two forms (Z-G I and Z-G II) was examined (Molla et al. 2014). NH4NO3 was used as N source at two rates of 75 mg-N kg−1 (300 kg-N ha−1) or 150 mg-N kg−1 (600 kg-N ha−1). The results revealed that the modification of zeolite with goethite led to a positively charged modified zeolite surface, which can be used to promote NO3 − retention in the soil. Consequently, the use of binary systems (Z-G) as an amendment to soil with high concentration of NO3 − ion will reduce the amounts of nitric compounds to significant levels. A summary of selected studies are listed in Table 2.
XRD patterns of clinoptilolite and its modified forms by HDTMAB and DODMAB (Bhardwaj et al. 2012)
3.2.3 Using Zeolite as NO3 −-Slow-Release Fertilizer
Presumably if cations, such as NH4 +, can be released slowly from zeolite due to cation exchange, a similar result can be obtained for NO3 − release from SMZ due to an anion exchange reaction, even though the location of the ion exchange sites may differ. The feasibility of using SMZ as a slow-release NO3 − fertilizer was evaluated (Li 2003). Clinoptilolite was modified using HDMTA at different loading rate (25–200% ECEC). Batch sorption experiment revealed that NO3 − sorption onto SMZ increased by increasing the surfactant loading. In batch desorption study, 30–40% of sorbed NO3 − still retained by modified zeolite after 100 pore volumes of water; indicating that this type of zeolite can be utilized as SRF. Furthermore, column leaching tests showed that a 95% decrease in the effluent NO3 − concentration could be achieved, compared to that when soluble NO3 − was mixed with zeolite at the same NO3 − loading (Table 2). In the treatment with a mixer of soluble NO3 − and clinoptilolite after two pore volume of water the effluent NO3 − concentration was rapidly decreased to < 0.2 mM. While the effluent NO3 − concentration remained > 0.3 mM after more than 50 pore volumes in the treatment with NO3 −-loaded SMZ. The result from both batch desorption and column study demonstrate the high ability of a NO3 − loaded SMZ to control NO3 − retention and release in agricultural soil (Li 2003).
It is noteworthy that most of the surfactants that are being used to modify zeolites are toxic chemicals. Furthermore, there are not enough scientific studies on potential leaching of the loosely attached surfactant from the zeolite surface. Therefore, further studies are needed prior to any suggestion for commercializing this type of modified products. Nevertheless, using more eco-friendly and less toxic compounds as potential surface modifiers can be considered as an alternative path.
3.3 Phosphorous (P) Retention and Release in Soil
P is one of the most critical major plant nutrients. Among its different compounds, PO4 3− is the main constituent in the soil matrix. Although PO4 3− exists as negatively charged ion in the soil solution, it has a large affinity to the soil matrix. It reacts quickly in soils and becomes immobile and insoluble (Gholamhoseini et al. 2013). PO4 3− fixation in soils occurs via adsorption and precipitation reactions. Adsorption is the dominant reaction in a short period of time, and generally it is attributed to hydrous oxides of iron (Fe) and aluminum (Al) (Moharami and Jalali 2014). Nevertheless, several recent studies clearly have demonstrated that PO4 3− can be leached out from soils under different conditions (Heckrath et al. 1995; Jalali and Kolahchi 2009; Jalali 2009). PO4 3− accumulates when is added to soils in the amounts of more than those removed by the plant. Consecutive applications of the excess PO4 3− in fertilizers will saturate adsorption sites of soils. In this condition, PO4 3− can be washed off and leads to high concentration of this element in the surface runoff. Furthermore, it can be leached to deeper soil layers and eventually leads to increasing PO4 3− concentrations in the groundwater (Bansiwal et al. 2006; Coblentz et al. 2004; Sharpley et al. 2007).
3.3.1 Effect of Zeolites on P Retention and Release in Soils
Zeolites are aluminosilicate minerals with poor amounts of Fe and Al hydrous oxides. They have a large negatively charged surface and no anion exchange capacity (Elliot and Zhang 2005). Therefore, negligible or no effect on PO4 3− leaching is expected when it is added to the soil. For example, in a leaching experiment with (NH4)2HPO4 solution, the addition of clinoptilolite zeolite to A (0 to 10 cm) and B (30 to 40 cm) horizons of a coarse sandy soil at a rate of 10% (w w −1) showed P sorption similar to unamended soils. This was attributed to the high pH and lack of sorption sites in the natural clinoptilolite zeolites (Phillips 1998). Nevertheless, the modification of zeolite could lead to an improvement in its ability to sorb more P. In a leaching experiment, P leaching from a sandy soil was evaluated in the presence of unmodified and modified zeolites with Fe3+ ions, amending at rates 10 and 3%, respectively. P retentions in the zeolite-Fe and zeolite were 14.2 and 10.5%, respectively, in comparison to 8.1% in the control soil. Compared with the untreated soil, the soil amended with zeolite-Fe leached slightly less amount of P. This reduction in P leaching is possibly attributed to the P adsorption by Fe and Al oxides and precipitation of stable form of Fe, Al, and Ca compounds of P (Moharami and Jalali 2014). In another experiment, the feasibility of using SMZ as a carrier for P was investigated. The HDTMAB was used to modify the surface of a commercial zeolite-A type, to increase its capacity of PO4 3− retention. The P loading on the SMZ increased by a factor of 4.9, compared with the unmodified zeolite-A. The results also revealed that P released from PO4 3−-loaded surface SMZ was available even after 1080 h of continuous percolation, while P from KH2PO4 was depleted within 264 h (Bansiwal et al. 2006).
3.3.2 Using Zeolite as PO4 3−-Slow-Release Fertilizer
An effective way to alleviate the imbalance between the rates of nutrient uptakes by plant roots and the rates of nutrient releases from fertilizers is to use SRFs (Ni et al. 2010a; Zhang and Saito 2009; Zhang et al. 2010). Using zeolites as SRFs is limited to cationic nutrients which can be loaded on the zeolite sites, such as NH4 + and K+. Therefore, loading of anionic nutrients like PO4 3− is negligible on unmodified zeolites. Nevertheless, several studies showed that a controlled and gradual release of PO4 3− is achievable by a combination of zeolite ion exchange and mineral dissolution (like apatite-rich phosphate rock (PR)) (Chesworth et al. 1987; Lai and Eberl 1986). Ion exchange and dissolution reactions drive each other. Zeolites capture dissolved cations by an ion exchange mechanism, thereby increase dissolution and mineral dissolution provides cations for ion exchange. The reactions occurring in these systems can be described by the following reactions in which Ca5(PO4)3F is fluoroapatite, a frequent source of P, and K+- and NH4 +-zeolite are zeolites enriched with potassium and ammonium ions, respectively (Lancellotti et al. 2014):
The first reaction shows a weak dissolution of the PR. In the second and third reactions, because of high CEC of zeolites, released Ca is adsorbed on the zeolite surface. Therefore, more PR will be dissolved with lowering Ca2+ activity in the solution. This system releases PO4 3−, NH4 +, and K+ ions. As a result of uptaking the released nutrients by plant roots, dissolution and ion exchange reactions will continue (Lancellotti et al. 2014). Although the P release rate from induced dissolution-ion exchange system may not be sufficient for intensive cropping systems, it is suitable to use where a low concentration of P must be maintained in soils (Pickering et al. 2002).
Clinoptilolite is one of the most abundant natural zeolites that has been used with the PR in several studies. A glasshouse study was undertaken to assess the enhancement of P uptake by sunflower after using clinoptilolite in combination with PR. Different saturated forms of clinoptilolite zeolites (Ca2+, K+, and NH4 +-saturated) were combined with PR at the ratios of 3.5:1 and 7:1. The P uptake in these treatments was lower than the treatment with soluble KH2PO4. However, the result clearly showed a great enhancement in plant uptakes of P from PR, when it was applied in a combination with NH4 +-zeolite (Pickering et al. 2002). In another study, untreated and treated (with NH4 +, H+, Na+) clinoptilolite were mixed with carbonate apatite at a ratio of 5:1 to examine the possibility of achieving a slow-release fertilization. The addition of all treated forms of clinoptilolite considerably increased the solution P concentration, in comparison to apatite without zeolite addition (Lai and Eberl 1986). Phillipsite and chabazite have also been used in induced dissolution-ion exchange systems as an SRF. In a study, phillipsite zeolite was combined with reactive and unreactive PRs at the ratios of 1:1, 1:10, and 1:100 and added to a sandy clay loam soil to evaluate its effectiveness to enhance the dissolution of PRs. Addition of zeolite had an insignificant effect on the P uptake from the unreactive PR. However, it approximately doubled in the soil containing phillipsite and reactive PR at the ratio of 1:100 (Mnkeni et al. 1994). Another research study reported that chabazite induced insoluble PO4 3− dissolution, like phillipsite and clinoptilolite, when a mixture of natural and NH4 + exchanged chabazite zeolite and animal bone ashes (as a P source) was used in comparison to animal bone ash without chabazite (Lancellotti et al. 2014).
The concentration of P compounds and their ratio to other nutrients in solutions are controlled by several factors including the ratio of zeolite to PR, type of zeolite and PR, type and equivalent fraction of cations such as K+ and NH4 + on zeolite exchange sites, and pH. Increasing the zeolite to PR ratio would promote P solubilization and hence enhance plant growth. In this condition, more exchange sites are available for Ca2+ in the solution, thereby driving the PR dissolution (Pickering et al. 2002). For example, in an experiment that used an NH4 + saturated clinoptilolite zeolite to induce the dissolution of PR (apatite), the results clearly revealed that both the rate and the quantity of the released P are increased with increasing zeolite/PR ratio (Fig. 10). The curves shown in Fig. 10 demonstrate that the zeolite systems have a more gradual release compared to mono-Ca PO4 3− system, in which most of the P was released in the first few extractions (Lai and Eberl 1986). A similar trend of the P release was observed in a system containing different ratios of chabazite zeolite to animal bone ash (Lancellotti et al. 2014).
Cumulative P released from zeolite systems (open circles) with different NH4+-zeolite to PR ratio (numbers next to the curve), compared to compared to a mono-Ca PO4 3− system (solid circles) having an equivalent amount of P (Lai and Eberl 1986)
PR dissolution is enhanced by saturating zeolites with monovalent cations, such as NH4 + and Na+, compared with zeolites saturated with Ca2+ or untreated natural zeolites which initially contain some exchangeable Ca2+. The results of an experimental study clearly revealed that P release rate from animal bone ash increased when NH4 + pre-loaded chabazite was used, in comparison to natural chabazite (Fig. 11). Furthermore, in Fig. 11, it is obvious that the presence of NH4 + in chabazite is more effective in promoting P release than the increase of the zeolite/PR ratio (Lancellotti et al. 2014). Changes in the relative charge fractions of cations such as NH4 + and K+ affect the P release rate due to the ion selectivity of natural zeolites. For example, it was observed that NH4 +-loaded clinoptilolite increased the availability of P from reactive PR, while Ca2+- and K+-loaded zeolites did not increase the P availability of the PR. This observation can be attributed to more selectivity of clinoptilolite for K+ than for NH4 + (Allen et al. 1993). P release is also increased in acidic soil media with lower pH. In a treatment containing the combination of the H+-saturated clinoptilolite and PR, P release was much higher than the other treatments (Lai and Eberl 1986). Furthermore, proton production through the nitrification process or root H+ efflux can promote PR dissolution (Pickering et al. 2002). Finally, an induced dissolution-ion exchange system is much more effective when reactive PRs are used than the non-reactive materials (Pickering et al. 2002).
P concentration in each sample (left) and cumulative P released (right) after leaching treatment of specific solid mixtures (Lancellotti et al. 2014)
3.4 Other Ions Retention and Release in Soil
3.4.1 Effect of Zeolite on Potassium Retention and Release in Soil
Zeolite minerals have a pronounced selectivity for K+ over other ions such as NH4 +, Na+, Ca2+, and Mg2+. Hence, it is difficult to remove K+ from zeolite exchange sites by these less selective cations. In addition, the reported CEC of zeolite obtained using 1 M KCl and 1 M NH4Cl was considerably higher than the values obtained using 0.1 M BaCl2 and 0.1 M BaCl2/NH4Cl solutions (Phillips 1998). It is because of the preferred exchange of the natural zeolites with lower field strengths for cations having a low charge density such as K+ (Barrer et al. 1968; Colella 1996). The hydration spheres of high field strength cations prevent their close approach to the seat of charge in the zeolite framework. Therefore, cations of low field strength such as K+ are generally more tightly held and selectively exchanged by the natural zeolite than other ions (Barrer and Klinowski 1972; Chelishehev et al. 1988; Mumpton 1999).
Because of abovementioned characteristics of zeolites, numerous researches have been conducted to load zeolite sites with K+ and use it as SRFs. The plants can take the K+ from the loaded zeolite by an ion exchange reaction between the plant root hairs and the zeolite (Gonzales and Fuentes 1988). Using zeolite as K+ SRF leads to reduce the potential of K+ leaching to surface runoff and groundwater (Ming and Allen 2001). For example, the effect of zeolite application on K+ release in sandy soils amended with municipal compost was investigated. The results from column tests revealed that zeolite application reduced total K+ leaching up to six times, due to high K+ affinity for the mixture of soil-compost-zeolite (Moraetis et al. 2015). In another study, it was revealed that the use of clinoptilolite zeolite increased K+ contents in plant tissues and reduced its leaching. This increase in K+ uptake might be attributed to the high zeolite CEC, which leads to holding K+ in zeolite structure for slow release to the rhizosphere (Gül et al. 2005). A similar result was observed in a study, in which a soilless potting medium was fertilized with either K+-loaded clinoptilolite or KNO3. About 10% of total K+ on zeolite exchange sites was leached out of the potting medium after collecting 3 L of leachate, while over 90% of total K+ was leached from the KNO3 source (Hershey et al. 1980). Similarly, the addition of K+-saturated clinoptilolite to a soilless medium resulted in 23% less leaching of K+, compared with control substrate (Williams and Nelson 1997).
3.4.2 Effect of Zeolite on Sulfur Retention and Release in Soil
S is considered as the fourth major nutrient after N, P, and K. S is known for its role in the synthesis of proteins, vitamins, chlorophyll, oils, amino acids and flavored compounds in plants. Although both organic and inorganic forms of S can be found in soils, the organic form constituting more than 95% of the total S (Thirunavukkarasu and Subramanian 2014). However, many plants assimilate S in SO4 2− form (Hawkesford and De Kok 2006; Li and Zhang 2010). The SO4 2− fertilizers are readily available for plant uptake, following their dissolution. However, as they are mobile relatively large concentration of SO4 2− in soils over a short period are likely to be lost by leaching or surface runoff.
Because SO4 2− is a negatively charged species, zeolite minerals do not have high affinity to this ion. Nevertheless, it is reported that surfactant-modified clinoptilolite zeolites, using HDTMAB, possess the required properties to be used as carriers for SRF. The results of a study revealed that after leaching with 50 pore volumes, 70 and 85% of the pre-loaded SO4 2− still remained on the zeolites modified to 150 and 200% ECEC, respectively. In addition, the initial SO4 2− concentration in the leachate of SO4 2−-loaded SMZ was decreased by a factor of three, in comparison with the mixtures of zeolite and same loading of soluble SO4 2− (Li and Zhang 2010). In addition to clinoptilolite, Epistilbite zeolite was modified by HDTMAB to develop nano-zeolite based nano-S fertilizer. The results exhibited that SO4 2− supply from SO4 2−-loaded surface modified nano-zeolite was available even after 912 h of continuous percolation, while SO4 2− from (NH4)2SO4 was depleted within 384 h. This slow release of SO4 2− from surface modified nano-zeolite might be attributed to the huge number of pores, cages and channels in the zeolite structure, which hold SO4 2− (Thirunavukkarasu and Subramanian 2014).
4 Conclusion
Rapid population growth and urbanization have restricted the agricultural area to have more crop yield and food production. On the other hand, more efficient agricultural activities need higher nutrient application rate and more efficient water irrigation systems. However, if a high rate of nutrients is applied to soils, a noticeable percentage of it might be washed out, which pollutes water resources as well as undermines product yields. Thus, an inexpensive, pervasive and green solution is essentially required to improve the crop yielding. Zeolites have been widely used in different investigations to enhance agricultural efficiencies.
Zeolites have some impacts on soil physical behaviors. Decreasing soil bulk density and increasing soil porosity are the outstanding impacts. These effects along with high internal pore volume within their structure can efficiently improve water holding capacity. Furthermore, the open network of the zeolites structure can lead to formation of new routes for water movement, which can consequently improve infiltration rate and saturated hydraulic conductivity.
The exceptional chemical and physical properties of natural zeolites, especially high cation exchange capacity and strong affinity for NH4 + and K+, can be exploited to maximize the nitrogen use efficiency in agricultural applications. Although almost all of naturally occurring zeolites and most of the artificial zeolites do not have high affinity for anionic fertilizers such as NO3 −, PO4 3−, and SO4 2−, by modifying their surface chemistries using cationic surfactant, multifunctional adsorbents with capability to trap anions and non-polar organics can be obtained. However, further studies should be conducted prior to any commercial applications to evaluate the risk of leaching toxic surfactants that are loosely attached to the zeolite surface.
The ion exchange properties of natural, synthetic, and modified zeolites can be exploited in the preparation of slow-release fertilizers for nitrogen, potassium, and sulfur. In addition, controlled and gradual release of phosphorous are achievable by a combination of zeolite ion exchange and mineral dissolution (like phosphate rock).
Generally, the impacts of zeolite application on soil physical and chemical properties depend on different experimental conditions, most importantly including zeolite type and application rate, method of the application, soil texture and structure, zeolite particle size and density, as well as water salinity. Zeolite amendments may influence coarse-textured soils to a greater extent than fine-textured soils.
Abbreviations
- BTEX:
-
Benzene, toluene, ethyl benzene, and xylene
- BTC:
-
Breakthrough curve
- CEC/ECEC:
-
Cation exchange capacity/external cation exchange capacity
- DODMA/DODMAB:
-
Dioctadecyldimethylammonium/ dioctadecyldimethylammonium bromide
- ECiw :
-
Electrical conductivity of irrigation water
- ECw :
-
Electrical conductivity
- HDTMA/HDTMAB:
-
Hexadecyltrimethylammonium/ hexadecyltrimethylammonium bromide
- K fs :
-
Field saturated hydraulic conductivity
- K s :
-
Saturated hydraulic conductivity
- MCL:
-
Maximum contaminant level
- NUE:
-
Nitrogen/nutrient use efficiency
- PR:
-
Phosphate rock
- s :
-
Sorptivity
- SARiw :
-
Sodium adsorption ratio of irrigation water
- SMZ:
-
Surfactant-modified zeolite
- SRF:
-
Slow-release fertilizer
- t :
-
Time
- VOC:
-
Volatile organic compounds
- v v −1 :
-
Volume fraction
- WHC:
-
Water holding capacity
- WHO:
-
World Health Organization
- WUE:
-
Water use efficiency
- w w −1 :
-
Mass fraction
- XRD:
-
X-ray diffraction
- z :
-
Cumulative infiltration
- Z :
-
Zeolite application rate
- σ g :
-
Mean soil particle diameter standard deviation
- Al:
-
Aluminum
- AsO4 −3 :
-
Arsenate
- Ca:
-
Calcium
- Cd:
-
Cadmium
- Cr:
-
Chrome
- CrO4 2− :
-
Chromate
- Cs:
-
Cesium
- Cu:
-
Copper
- Fe:
-
Iron
- K:
-
Potassium
- Mn:
-
Manganese
- N:
-
Nitrogen
- Na:
-
Sodium
- Ni:
-
Nickel
- NH4 + :
-
Ammonium
- NO3 − :
-
Nitrate
- N2O:
-
Nitrous oxide
- P:
-
Phosphorous
- Pb:
-
Lead
- PO4 3− :
-
Phosphate
- S:
-
Sulfur
- Si:
-
Silicon
- SO4 2− :
-
Sulfate
- Sr:
-
Strontium
- Zn:
-
Zinc
References
Abbasi, M. K., Tahir, M. M., Sabir, N., & Khurshid, M. (2015). Impact of the addition of different plant residues on nitrogen mineralization-immobilization turnover and carbon content of a soil incubated under laboratory conditions. Solid Earth, 6(1), 197.
Aghaalikhani, M., Gholamhoseini, M., Dolatabadian, A., Khodaei-Joghan, A., & Sadat Asilan, K. (2012). Zeolite influences on nitrate leaching, nitrogen-use efficiency, yield and yield components of canola in sandy soil. Archives of Agronomy and Soil Science, 58(10), 1149–1169.
Ahmed, O. H., Braine Yap, C., & Nik Muhamad, A. (2010). Minimizing ammonia loss from urea through mixing with zeolite and acid sulphate soil. International Journal of the Physical Sciences, 5(14), 2198–2202.
Aiyuk, S., Xu, H., & Van Haandel, A. (2004). Removal of ammonium nitrogen from pretreated domestic sewage using a natural ion exchanger. Environmental Technology, 25(11), 1321–1330.
Akhtar, M., Hassan, F., Ahmed, M., Hayat, R., & Stöckle, C. O. (2016). Is rainwater harvesting an option for designing sustainable cropping patterns for rainfed agriculture? Land Degradation & Development, 27(3), 630–640.
Al-Busaidi, A., Yamamoto, T., Inoue, M., Eneji, A. E., Mori, Y., & Irshad, M. (2008). Effects of zeolite on soil nutrients and growth of barley following irrigation with saline water. Journal of Plant Nutrition, 31(7), 1159–1173.
Allen, E., & Ming, D. (1995). Recent progress in the use of natural zeolites in agronomy and horticulture. Natural Zeolites, 93, 477–490.
Allen, E., Hossner, L., Ming, D., & Henninger, D. (1993). Solubility and cation exchange in phosphate rock and saturated clinoptilolite mixtures. Soil Science Society of America Journal, 57(5), 1368–1374.
Anderson, S. H., Udawatta, R. P., Seobi, T., & Garrett, H. E. (2009). Soil water content and infiltration in agroforestry buffer strips. Agroforestry Systems, 75(1), 5–16.
Aschebrook-Kilfoy, B., Shu, X., Gao, Y., Ji, B., Yang, G., Li, H. L., Rothman, N., Chow, W., Zheng, W., & Ward, M. H. (2013). Thyroid cancer risk and dietary nitrate and nitrite intake in the Shanghai women’s health study. International Journal of Cancer, 132(4), 897–904.
Asilian, H., Mortazavi, S., Kazemian, H., Phaghiehzadeh, S., Shahtaheri, S., & Salem, M. (2004). Removal of ammonia from air, using three Iranian natural zeolites. Iranian Journal of Public Health, 33(1), 45–51.
Azooz, R., & Arshad, M. (1996). Soil infiltration and hydraulic conductivity under long-term no-tillage and conventional tillage systems. Canadian Journal of Soil Science, 76(2), 143–152.
Baek, K., Shin, H., Lee, H., Jun, Y., & Yang, J. (2002). Statistical modeling of electrochemical removal of sodium in fermented food composts. Korean Journal of Chemical Engineering, 19(4), 627–631.
Baerlocher, C., McCusker, L. B., & Olson, D. H. (2007). Atlas of zeolite framework types. Elsevier.
Bagarello, V., Iovino, M., Palazzolo, E., Panno, M., & Reynolds, W. (2006). Field and laboratory approaches for determining sodicity effects on saturated soil hydraulic conductivity. Geoderma, 130(1), 1–13.
Bakhshayesh, B. E., Delkash, M., & Scholz, M. (2014). Response of vegetables to cadmium-enriched soil. Water, 6(5), 1246–1256.
Bansiwal, A. K., Rayalu, S. S., Labhasetwar, N. K., Juwarkar, A. A., & Devotta, S. (2006). Surfactant-modified zeolite as a slow release fertilizer for phosphorus. Journal of Agricultural and Food Chemistry, 54(13), 4773–4779.
Barrer, R., & Klinowski, J. (1972). Influence of framework charge density on ion-exchange properties of zeolites. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 68, 1956–1963.
Barrer, R., Davies, J., & Rees, L. (1968). Thermodynamics and thermochemistry of cation exchange in zeolite Y. Journal of Inorganic and Nuclear Chemistry, 30(12), 3333–3349.
Barros, M., Zola, A., Arroyo, P., Sousa-Aguiar, E., & Tavares, C. (2003). Binary ion exchange of metal ions in Y and X zeolites. Brazilian Journal of Chemical Engineering, 20(4), 413–421.
Bautista, J. M., Kim, H., Ahn, D., Zhang, R., & Oh, Y. (2011). Changes in physicochemical properties and gaseous emissions of composting swine manure amended with alum and zeolite. Korean Journal of Chemical Engineering, 28(1), 189–194.
Behin, J., & Sadeghi, N. (2016). Utilization of waste lignin to prepare controlled-slow release urea. International Journal of Recycling of Organic Waste in Agriculture, 5(4), 289–299.
Bernardi, A. C., Oliviera, P. P. A., De Melo Monte, M. B., & Souza-Barros, F. (2013). Brazilian sedimentary zeolite use in agriculture. Microporous and Mesoporous Materials, 167, 16–21.
Bernardi, A. D. C., Mendonça, F., Haim, P., Werneck, C., & Monte, M. D. M. (2009). Water availability and rice yield due to levels of zeolitic concentrate. Irriga, 14(2), 123–134.
Bhardwaj, D., Sharma, M., Sharma, P., & Tomar, R. (2012). Synthesis and surfactant modification of clinoptilolite and montmorillonite for the removal of nitrate and preparation of slow release nitrogen fertilizer. Journal of Hazardous Materials, 227, 292–300.
Bigelow, C. A., Bowman, D. C., Cassel, D. K., & Rufty, T. W. (2001). Creeping bentgrass response to inorganic soil amendments and mechanically induced subsurface drainage and aeration. Crop Science, 41(3), 797–805.
Bittelli, M., Campbell, G. S., & Tomei, F. (2015). Soil physics with Python: transport in the soil-plant-atmosphere system. OUP Oxford.
Bowman, R. S. (2003). Applications of surfactant-modified zeolites to environmental remediation. Microporous and Mesoporous Materials, 61(1), 43–56.
Bugbee, G., & Elliott, G. (1998). Leaching of nitrogen and phosphorus from potting media containing biosolids compost as affected by organic and clay amendments. Bulletin of Environmental Contamination and Toxicology, 60(5), 716–723.
Cahn, M., Bouldin, D., & Cravo, M. (1992). Nitrate sorption in the profile of an acid soil. Plant and Soil, 143(2), 179–183.
Chelishehev, N., Volodin, N., & Kryukov, V. (1988). Ion exchange properties of natural high silica zeolites
Chesworth, W., Van Straaten, P., Smith, P., & Sadura, S. (1987). Solubility of apatite in clay and zeolite bearing systems: application to agriculture. Applied Clay Science, 2(3), 291–297.
Chmielewska, E. (2014a). Designing clinoptiloliterich tuff columns for adsorptive filtration of water with enhanced ammonium concentration. Fresenius Environmental Bulletin, 23(5).
Chmielewska, E. (2014b). Zeolitic adsorption in course of pollutants mitigation and environmental control. Journal of Radioanalytical and Nuclear Chemistry, 299(1), 255–260.
Chmielewska, E., & Lesný, J. (2012). Selective ion exchange onto Slovakian natural zeolites in aqueous solutions. Journal of Radioanalytical and Nuclear Chemistry, 293(2), 535–543.
Coblentz, W., Daniels, M., Gunsaulis, J., Turner, J., Scarbrough, D., Humphry, J., Coffey, K., Moore, P., Teague, K., & Speight, J. (2004). Effects of nitrogen fertilization on phosphorus uptake in bermudagrass forage grown on high soil-test phosphorus sites. The Professional Animal Scientists, 20(2), 146–154.
Cobzaru, C., & Inglezakis, V. (2012). Mathematical modeling of sorption process of Cu 2 ions on analcime and clinoptilolite. Environmental Engineering and Management Journal, 11(11).
Colella, C. (1996). Ion exchange equilibria in zeolite minerals. Mineralium Deposita, 31(6), 554–562.
Colombani, N., Mastrocicco, M., Di Giuseppe, D., Faccini, B., & Coltorti, M. (2015). Batch and column experiments on nutrient leaching in soils amended with Italian natural zeolitites. Catena, 127, 64–71.
Cordoves, A. P., Valdés, M. G., Fernández, J. T., Luis, G. P., García-Calzón, J., & García, M. D. (2008). Characterization of the binding site affinity distribution of a surfactant-modified clinoptilolite. Microporous and Mesoporous Materials, 109(1), 38–48.
Delkash, M., Bakhshayesh, B. E., & Kazemian, H. (2015). Using zeolitic adsorbents to cleanup special wastewater streams: A review. Microporous and Mesoporous Materials, 214, 224–241.
Delkash, M., Al-Faraj, F. A., & Scholz, M. (2014). Comparing the export coefficient approach with the soil and water assessment tool to predict phosphorous pollution: the Kan watershed case study. Water, Air, & Soil Pollution, 225(10), 1–17.
Demir, A., Gunay, A., & Debik, E. (2002). Ammonium removal from aqueous solution by ion-exchange using packed bed natural zeolite. Water SA, 28(3), 329–336.
Dolley, T. (2014). US Geological Survey Mineral Commodity Summaries 2014. US Geological Survey, 138–139.
Doublet, J., Francou, C., Poitrenaud, M., & Houot, S. (2010). Sewage sludge composting: Influence of initial mixtures on organic matter evolution and N availability in the final composts. Waste Management, 30(10), 1922–1930.
Dwairi, I. (1998). Renewable, controlled and environmentally safe phosphorus release in soils from mixtures of NH4 -phillipsite tuff and phosphate rocks. Environmental Geology, 34(4), 293–296.
Ebrazi, B., & Banihabib, M. E. (2015). Simulation of Ca2 and Mg2 removal process in fixed-bed column of natural zeolite. Desalination and Water Treatment, 55(4), 1116–1124.
Eick, M. J., Brady, W. D., & Lynch, C. K. (1999). Charge properties and nitrate adsorption of some acid southeastern soils. Journal of Environmental Quality, 28(1), 138–144.
El Kenawy, A., McCabe, M. F., Vicente-Serrano, S., López-Moreno, J., & Robaa, S. (2016). Changes in the frequency and severity of hydrological droughts over Ethiopia from 1960 to 2013. Cuadernos de Investigación Geográfica, 42(1), 145–166.
Elliot, A. D., & Zhang, D. (2005). Controlled release zeolite fertilisers: a value added product produced from fly ash.
Enamorado-Horrutiner, Y., Villanueva-Tagle, M., Behar, M., Rodríguez-Fuentes, G., Dias, J. F., & Pomares-Alfonso, M. (2016). Cuban zeolite for lead sorption: application for water decontamination and metal quantification in water using nondestructive techniques. International journal of Environmental Science and Technology, 13(5), 1245–1256.
Englert, A., & Rubio, J. (2005). Characterization and environmental application of a Chilean natural zeolite. International Journal of Mineral Processing, 75(1), 21–29.
Faghihian, H., Kazemian, H., & Maragheh, M. (1999a). Iranian clinoptilolite-rich tuffs for radionuclide removal from water. Journal of Radioanalytical and Nuclear Chemistry, 242(2), 491–495.
Faghihian, H., Marageh, M. G., & Kazemian, H. (1999b). The use of clinoptilolite and its sodium form for removal of radioactive cesium, and strontium from nuclear wastewater and Pb 2 , Ni 2 , Cd 2 , Ba 2 from municipal wastewater. Applied Radiation and Isotopes, 50(4), 655–660.
Feigin, A., Ravina, I., & Shalhevet, J. (2012). Irrigation with treated sewage effluent: management for environmental protection. Springer Science & Business Media.
Filippidis, A. (2010). Environmental, industrial and agricultural applications of Hellenic natural zeolite. Hellenic Journal of Geosciences, 45(20109), 91.
Franzluebbers, A. (2002). Water infiltration and soil structure related to organic matter and its stratification with depth. Soil and Tillage Research, 66(2), 197–205.
Frenkel, H., Goertzen, J., & Rhoades, J. (1978). Effects of clay type and content, exchangeable sodium percentage, and electrolyte concentration on clay dispersion and soil hydraulic conductivity. Soil Science Society of America Journal, 42(1), 32–39.
Ghadiri, S., Nabizadeh, R., Mahvi, A., Nasseri, S., Kazemian, H., Mesdaghinia, A., & Nazmara, S. (2010). Methyl tert-butyl ether adsorption on surfactant modified natural zeolites. Iranian Journal of Environmental Health Science & Engineering, 7(3), 241.
Ghasemi, Z., Sourinejad, I., Kazemian, H., & Rohani, S. (2016). Application of zeolites in aquaculture industry: a review. Reviews in Aquaculture.
Gholamhoseini, M., Ghalavand, A., Khodaei-Joghan, A., Dolatabadian, A., Zakikhani, H., & Farmanbar, E. (2013). Zeolite-amended cattle manure effects on sunflower yield, seed quality, water use efficiency and nutrient leaching. Soil and Tillage Research, 126, 193–202.
Gholamhoseini, M., AghaAlikhani, M., Dolatabadian, A., Khodaei-Joghan, A., & Zakikhani, H. (2012). Decreasing nitrogen leaching and increasing canola forage yield in a sandy soil by application of natural zeolite. Agronomy Journal, 104(5), 1467–1475.
Gholizadeh-Sarabi, S., & Sepaskhah, A. R. (2013). Effect of zeolite and saline water application on saturated hydraulic conductivity and infiltration in different soil textures. Archives of Agronomy and Soil Science, 59(5), 753–764.
Githinji, L. J., Dane, J. H., & Walker, R. H. (2011). Physical and hydraulic properties of inorganic amendments and modeling their effects on water movement in sand-based root zones. Irrigation Science, 29(1), 65–77.
Gonzales, R., & Fuentes, R. (1988). Cuban experience with the use of natural zeolite subsrates in soilless culture, 13–21.
Greer, F. R., & Shannon, M. (2005). Infant methemoglobinemia: the role of dietary nitrate in food and water. Pediatrics, 116(3), 784–786.
Gruener, J. E., Ming, D. W., Henderson, K. E., & Galindo, C. (2003). Common ion effects in zeoponic substrates: wheat plant growth experiment. Microporous and Mesoporous Materials, 61(1), 223–230.
Gu, B., Ge, Y., Chang, S. X., Luo, W., & Chang, J. (2013). Nitrate in groundwater of China: sources and driving forces. Global Environmental Change, 23(5), 1112–1121.
Gül, A., Eroğul, D., & Ongun, A. R. (2005). Comparison of the use of zeolite and perlite as substrate for crisp-head lettuce. Scientia Horticulturae, 106(4), 464–471.
Guo, M., Liu, M., Liang, R., & Niu, A. (2006). Granular urea-formaldehyde slow-release fertilizer with superabsorbent and moisture preservation. Journal of Applied Polymer Science, 99(6), 3230–3235.
Haruna Ahmed, O., Husin, A., & Husni Mohd Hanif, A. (2008). Ammonia volatilization and ammonium accumulation from urea mixed with zeolite and triple superphosphate. Acta Agriculturae Scandinavica Section B Soil and Plant Science, 58(2), 182–186.
Hawkesford, M. J., & De Kok, L. J. (2006). Managing sulphur metabolism in plants. Plant, Cell & Environment, 29(3), 382–395.
He, Z., Calvert, D., Alva, A., Li, Y., & Banks, D. (2002). Clinoptilolite zeolite and cellulose amendments to reduce ammonia volatilization in a calcareous sandy soil. Plant and Soil, 247(2), 253–260.
Heckrath, G., Brookes, P., Poulton, P., & Goulding, K. (1995). Phosphorus leaching from soils containing different phosphorus concentrations in the Broadbalk experiment. Journal of Environmental Quality, 24(5), 904–910.
Hershey, D., Paul, J., & Carlson, R. (1980). Evaluation of potassium-enriched clinoptilolite as a potassium source for potting media [Chrysanthemum morifolium]. HortScience (USA).
Hong, J., & Park, K. (2005). Compost biofiltration of ammonia gas from bin composting. Bioresource Technology, 96(6), 741–745.
Hosono, T., Tokunaga, T., Kagabu, M., Nakata, H., Orishikida, T., Lin, I., & Shimada, J. (2013). The use of δ 15 N and δ 18 O tracers with an understanding of groundwater flow dynamics for evaluating the origins and attenuation mechanisms of nitrate pollution. Water Research, 47(8), 2661–2675.
Huang, H., Xiao, X., Yan, B., & Yang, L. (2010). Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. Journal of Hazardous Materials, 175(1), 247–252.
Huang, Z., & Petrovic, A. (1994). Clinoptilolite zeolite influence on nitrate leaching and nitrogen use efficiency in simulated sand based golf greens. Journal of Environmental Quality, 23(6), 1190–1194.
Ibrahim-Saeedi, F., & Sepaskhah, A. R. (2013). Effects of a bentonite–water mixture on soil-saturated hydraulic conductivity. Archives of Agronomy and Soil Science, 59(3), 377–392.
Inglezakis, V., Stylianou, M., Loizidou, M., & Zorpas, A. (2015). Experimental studies and modeling of clinoptilolite and vermiculite fixed beds for Mn2 , Zn2 , and Cr3 removal. Desalination and Water Treatment, 1–13.
Inglezakis, V., Elaiopoulos, K., Aggelatou, V., & Zorpas, A. A. (2012). Treatment of underground water in open flow and closed-loop fixed bed systems by utilizing the natural minerals clinoptilolite and vermiculite. Desalination and Water Treatment, 39(1–3), 215–227.
Inglezakis, V. J. (2005). The concept of “capacity” in zeolite ion-exchange systems. Journal of Colloid and Interface Science, 281(1), 68–79.
Inglezakis, V. J., & Grigoropoulou, H. P. (2003). Modeling of ion exchange of Pb 2 in fixed beds of clinoptilolite. Microporous and Mesoporous Materials, 61(1), 273–282.
Ippolito, J. A., Tarkalson, D. D., & Lehrsch, G. A. (2011). Zeolite soil application method affects inorganic nitrogen, moisture, and corn growth. Soil Science, 176(3), 136–142.
Jabro, J. (1992). Estimation of saturated hydraulic conductivity of soils from particle size distribution and bulk density data. Transactions of ASAE, 35(2), 557–560.
Jalali, M., & Kolahchi, Z. (2009). Effect of irrigation water quality on the leaching and desorption of phosphorous from soil. Soil and Sediment Contamination, 18(5), 576–589.
Jalali, M. (2009). Phosphorous concentration, solubility and species in the groundwater in a semi-arid basin, southern Malayer, western Iran. Environmental Geology, 57(5), 1011–1020.
Jewell, S., & Kimball, S. (2016). US Geological Survey 2016: Mineral Commodity Summaries. US Govt. Printing Office: Reston.
Jha, B., & Singh, D. N. (2016). Fly ash zeolites: innovations, applications, and directions. Springer.
Jha, V. K., & Hayashi, S. (2009). Modification on natural clinoptilolite zeolite for its NH 4+ retention capacity. Journal of Hazardous Materials, 169(1), 29–35.
Jiang, J., Huang, Y., Liu, X., & Huang, H. (2014). The effects of apple pomace, bentonite and calcium superphosphate on swine manure aerobic composting. Waste Management, 34(9), 1595–1602.
Jlassi, W., Nadal-Romero, E., & García-Ruiz, J. (2016). Modernization of new irrigated lands in a scenario of increasing water scarcity: from large reservoirs to small ponds.
Kazemian, H., Gedik, K., & Imamoglu, I. (2012). Environmental applications of natural zeolites. Handbook of natural zeolites, 473.
Kazemian, H., & Mallah, M. (2008). Removal of chromate ion from contaminated synthetic water using MCM-41/ZSM-5 composite. Journal of Environmental Health Science & Engineering, 5(1), 73–77.
Kazemian, H., & Mallah, M. H. (2006). Elimination of Cd2 and Mn2 from wastewaters using natural clinoptilolite and synthetic zeolite P. Iranian Journal of Chemistry and Chemical Engineering (IJCCE), 25(4), 91–94.
Kazemian, H. (2002). Zeolite science in Iran: a brief review.
Kazemian, H., Rajec, P., Macasek, F., & Kufacakoa, J. (2001). Investigation of lead removal from wastewater by Iranian natural zeolites using 212Pb as a radiotracer. Studies in Surface Science and Catalysis, 135, 6–31.
Khatri, K. L., & Smith, R. (2006). Real-time prediction of soil infiltration characteristics for the management of furrow irrigation. Irrigation Science, 25(1), 33–43.
Klučáková, M. (2010). Adsorption of nitrate on humic acids studied by flow-through coulometry. Environmental Chemistry Letters, 8(2), 145–148.
Kuroda, K., Hanajima, D., Fukumoto, Y., Suzuki, K., Kawamoto, S., Shima, J., & Haga, K. (2004). Isolation of thermophilic ammonium-tolerant bacterium and its application to reduce ammonia emission during composting of animal wastes. Bioscience, Biotechnology, and Biochemistry, 68(2), 286–292.
Lai, T., & Eberl, D. D. (1986). Controlled and renewable release of phosphorous in soils from mixtures of phosphate rock and NH4-exchanged clinoptilolite. Zeolites, 6(2), 129–132.
Lal, R., & Shukla, M. K. (2004). Principles of soil physics. CRC Press.
Lancellotti, I., Toschi, T., Passaglia, E., & Barbieri, L. (2014). Release of agronomical nutrient from zeolitite substrate containing phosphatic waste. Environmental Science and Pollution Research, 21(23), 13237–13242.
Leggo, P. J. (2015). The efficacy of the organo-zeolitic bio-fertilizer. Agrotechnology, 2015.
Leij, F. J., & van Genuchten, M. T. (2001). 6 Solute Transport. Soil physics companion, 189.
Levy, G., Goldstein, D., & Mamedov, A. (2005). Saturated hydraulic conductivity of semiarid soils: combined effects of salinity, sodicity, and rate of wetting. Soil Science Society of America Journal, 69(3), 653–662.
Li, C., Farahbakhshazad, N., Jaynes, D. B., Dinnes, D. L., Salas, W., & McLaughlin, D. (2006). Modeling nitrate leaching with a biogeochemical model modified based on observations in a row-crop field in Iowa. Ecological Modelling, 196(1), 116–130.
Li, H., Xin, H., Liang, Y., & Burns, R. (2008). Reduction of ammonia emissions from stored laying hen manure through topical application of zeolite, Al Clear, Ferix-3, or poultry litter treatment. The Journal of Applied Poultry Research, 17(4), 421–431.
Li, X., Hu, C., Delgado, J. A., Zhang, Y., & Ouyang, Z. (2007). Increased nitrogen use efficiencies as a key mitigation alternative to reduce nitrate leaching in north china plain. Agricultural Water Management, 89(1), 137–147.
Li, Z., & Zhang, Y. (2010). Use of surfactant-modified zeolite to carry and slowly release sulfate. Desalination and Water Treatment, 21(1–3), 73–78.
Li, Z. (2003). Use of surfactant-modified zeolite as fertilizer carriers to control nitrate release. Microporous and Mesoporous Materials, 61(1), 181–188.
Lim, S., Lee, D., Kwak, J., Park, H., Kim, H., & Choi, W. (2015). Fly ash and zeolite amendments increase soil nutrient retention but decrease paddy rice growth in a low fertility soil. Journal of Soils and Sediments, 1–11.
Lin, C., Lo, S., Lin, H., & Lee, Y. (1998). Stabilization of cadmium contaminated soils using synthesized zeolite. Journal of Hazardous Materials, 60(3), 217–226.
Mackinnon, I., Barr, K., Miller, E., Hunter, S., & Pinel, T. (2003). Nutrient removal from wastewaters using high performance materials, 47(11), 101–107.
MacKown, C., & Tucker, T. (1985). Ammonium nitrogen movement in a coarse-textured soil amended with zeolite. Soil Science Society of America Journal, 49(1), 235–238.
MacKown, C. T. (1978). Role of mineral zeolites as soil amendments.
Mahabadi, A. A., Hajabbasi, M., Khademi, H., & Kazemian, H. (2007). Soil cadmium stabilization using an Iranian natural zeolite. Geoderma, 137(3), 388–393.
Malekian, R., Abedi-Koupai, J., & Eslamian, S. S. (2011a). Influences of clinoptilolite and surfactant-modified clinoptilolite zeolite on nitrate leaching and plant growth. Journal of Hazardous Materials, 185(2), 970–976.
Malekian, R., Abedi-Koupai, J., Eslamian, S. S., Mousavi, S. F., Abbaspour, K. C., & Afyuni, M. (2011b). Ion-exchange process for ammonium removal and release using natural Iranian zeolite. Applied Clay Science, 51(3), 323–329.
Mamedov, A. I., Bar-Yosef, B., Levkovich, I., Rosenberg, R., Silber, A., Fine, P., & Levy, G. J. (2016). Amending soil with sludge, manure, humic acid, orthophosphate and phytic acid: effects on infiltration, runoff and sediment loss. Land Degradation & Development, 27(6), 1629–1639.
McGilloway, R., Weaver, R., Ming, D., & Gruener, J. (2003). Nitrification in a zeoponic substrate. Plant and Soil, 256(2), 371–378.
McNeal, B., & Coleman, N. (1966). Effect of solution composition on soil hydraulic conductivity. Soil Science Society of America Journal, 30(3), 308–312.
Meisinger, J., & Jokela, W. (2000). Ammonia volatilization from dairy and poultry manure. Managing nutrients and pathogens from animal agriculture. NRAES-130. Natural Resource, Agriculture, and Engineering Service, Ithaca, NY, 334–354.
Menhaje-Bena, R., Kazemian, H., Shahtaheri, S., Ghazi-Khansari, M., & Hosseini, M. (2004). Evaluation of iron modified zeolites for removal of arsenic from drinking water. Studies in Surface Science and Catalysis, 154, 1892–1899.
Miller, G. L. (2000). Physiological response of Bermuda grass grown in soil amendments during drought stress. Hortscience, 35(2), 213–216.
Ming, D. W., & Allen, E. R. (2001). Use of natural zeolites in agronomy, horticulture and environmental soil remediation. Reviews in Mineralogy and Geochemistry, 45(1), 619–654.
Ming, D. W., & Mumpton, F. A. (1989). Zeolites in soils. Minerals in soil environments (mineralsinsoile), 873–911.
Mnkeni, P., Semoka, J., & Kaitaba, E. (1994). Effects of Mapogoro phillipsite on availability of phosphorus in phosphate rocks. Tropical Agriculture, 71(4), 249–253.
Moharami, S., & Jalali, M. (2014). Phosphorus leaching from a sandy soil in the presence of modified and un-modified adsorbents. Environmental Monitoring and Assessment, 186(10), 6565–6576.
Molla, A., Ioannou, Z., Dimirkou, A., & Skordas, K. (2014). Surfactant modified zeolites with iron oxide for the removal of ammonium and nitrate ions from waters and soils. Topics in Chemistry and Materials Science, Innoslab Ltd, 7, 38–49.
Moosavi, A. A., & Sepaskhah, A. R. (2012). Determination of unsaturated soil hydraulic properties at different applied tensions and water qualities. Archives of Agronomy and Soil Science, 58(1), 11–38.
Moraetis, D., Papagiannidou, S., Pratikakis, A., Pentari, D., & Komnitsas, K. (2015). Effect of zeolite application on potassium release in sandy soils amended with municipal compost. Desalination and Water Treatment, 1–12.
Mumpton, F. A. (1999). La roca magica: uses of natural zeolites in agriculture and industry. Proceedings of the National Academy of Sciences of the United States of America, 96(7), 3463–3470.
Mumpton, F. A. (1985). Using zeolites in agriculture.
Mumpton, F. A. (1980). Zeolites and mesothelioma. American Scientist, 68(3), 244–244.
Mumpton, F. (1972). Clinoptilolite redefined, Ameralogist Mineralogist, 45 (1960) 351-369.[137] JR Boles, Composition, optical properties, cell dimensions, and thermal stability of some heulandites group zeolites. American Mineralogist, 57, 1463–1493.
Nagaraja, M. S., Reddy, G. P., & Srinivasamurthy, C. A. (2016). Estimations of soil fertility in physically degraded agricultural soils through selective accounting of fine earth and gravel fractions. Solid Earth, 7(3), 897.
Nakhli, S. A., Ahmadizadeh, K., Fereshtehnejad, M., Rostami, M. H., Safari, M., & Borghei, S. M. (2014). Biological removal of phenol from saline wastewater using a moving bed biofilm reactor containing acclimated mixed consortia. SpringerPlus, 3, 112–1801–3-112. eCollection 2014.
Ni, B., Liu, M., Lü, S., Xie, L., & Wang, Y. (2010a). Multifunctional slow-release organic–inorganic compound fertilizer. Journal of Agricultural and Food Chemistry, 58(23), 12373–12378.
Ni, B., Liu, M., Lü, S., Xie, L., Zhang, X., & Wang, Y. (2010b). Novel slow-release multielement compound fertilizer with hydroscopicity and moisture preservation. Industrial & Engineering Chemistry Research, 49(10), 4546–4552.
Nus, J., & Brauen, S. (1991). Clinoptilolitic zeolite as an amendment for establishment of creeping bentgrass on sandy media. Hortscience, 26(2), 117–119.
Nyssen, J., Frankl, A., Zenebe, A., Poesen, J., & Deckers, J. (2015). Environmental conservation for food production and sustainable livelihood in tropical Africa. Land Degradation and Development, 26(7), 629–631.
Ober, J. A. (2017). Mineral commodity summaries 2017.
Omar, O. L., Ahmed, O. H., & Muhamad, A. N. (2010). Minimizing ammonia volatilization in waterlogged soils through mixing of urea with zeolite and sago waste water. International Journal of Physical Sciences, 5(14), 2193–2197.
Pal, D., Bhattacharyya, T., Ray, S., Chandran, P., Srivastava, P., Durge, S., & Bhuse, S. (2006). Significance of soil modifiers (Ca-zeolites and gypsum) in naturally degraded Vertisols of the Peninsular India in redefining the sodic soils. Geoderma, 136(1), 210–228.
Peña-Haro, S., Llopis-Albert, C., Pulido-Velazquez, M., & Pulido-Velazquez, D. (2010). Fertilizer standards for controlling groundwater nitrate pollution from agriculture: El Salobral-Los Llanos case study, Spain. Journal of Hydrology, 392(3), 174–187.
Pepper, I., Ferguson, G., & Kneebone, W. (1982). Clinoptilolite zeolite: a new medium for turfgrass growth. Proceedings of ASA: Agronomy Abstracts, 145.
Perrin, T. S., Drost, D. T., Boettinger, J. L., & Norton, J. M. (1998a). Ammonium-loaded clinoptilolite: a slow-release nitrogen fertilizer for sweet corn. Journal of Plant Nutrition, 21(3), 515–530.
Perrin, T., Boettinger, J., Drost, D., & Norton, J. M. (1998b). Decreasing nitrogen leaching from sandy soil with ammonium-loaded clinoptilolite. Journal of Environmental Quality, 27(3), 656–663.
Phillips, I. (1998). Use of soil amendments to reduce nitrogen, phosphorus and heavy metal availability. Journal of Soil Contamination, 7(2), 191–212.
Pickering, H. W., Menzies, N. W., & Hunter, M. N. (2002). Zeolite/rock phosphate—a novel slow release phosphorus fertiliser for potted plant production. Scientia Horticulturae, 94(3), 333–343.
Piñón-Villarreal, A. R., Bawazir, A. S., Shukla, M. K., & Hanson, A. T. (2013). Retention and transport of nitrate and ammonium in loamy sand amended with clinoptilolite zeolite. Journal of Irrigation and Drainage Engineering, 139(9), 755–765.
Polat, E., Karaca, M., Demir, H., & Naci-Onus, A. (2004). Use of natural zeolite (clinoptilolite) in agriculture. Journal of Fruit and Ornamental Plant Research, 12(1), 183–189.
Qian, J. H., Doran, J. W., Weier, K. L., Mosier, A. R., Peterson, T. A., & Power, J. F. (1997). Soil denitrification and nitrous oxide losses under corn irrigated with high-nitrate groundwater. Journal of Environmental Quality, 26(2), 348–360.
Rabai, K. A., Ahmed, O. H., & Kasim, S. (2013). Use of formulated nitrogen, phosphorus, and potassium compound fertilizer using clinoptilolite zeolite in maize (Zea mays L.) cultivation. Emirates Journal of Food and Agriculture, 25(9), 713.
Ramesh, K., Reddy, K. S., Rashmi, I., Biswas, A., & Islam, K. (2015a). Does particle size of clinoptilolite zeolite have a role in textural properties? Insight through differential pore-volume distribution of Barret, Joyner, and Halenda Model. Communications in Soil Science and Plant Analysis, 46(16), 2070–2078.
Ramesh, K., Biswas, A. K., & Patra, A. K. (2015b). Zeolitic farming. Indian Journal of Agronomy, 60(2), 50–56.
Ramesh, K., Damodar Reddy, D., Kumar Biswas, A., & Subba Rao, A. (2011). 4 zeolites and their potential uses in agriculture. Advances in Agronomy, 113, 215.
Ravishankara, A. R., Daniel, J. S., & Portmann, R. W. (2009). Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science (New York, N.Y.), 326(5949), 123–125.
Razmi, Z., & Sepaskhah, A. R. (2012). Effect of zeolite on saturated hydraulic conductivity and crack behavior of silty clay paddled soil. Archives of Agronomy and Soil Science, 58(7), 805–816.
Rehakova, M., Čuvanová, S., Dzivak, M., Rimár, J., & Gaval’Ova, Z. (2004). Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Current Opinion in Solid State and Materials Science, 8(6), 397–404.
Rivero, L., & Rodríguez-Fuentes, G. (1989). Cuban experience with the use of natural zeolite substrates in soilless culture. Notes, 405, 405–416.
Roghani, M., Nakhli, S. A. A., Aghajani, M., Rostami, M. H., & Borghei, S. M. (2016). Adsorption and oxidation study on arsenite removal from aqueous solutions by polyaniline/polyvinyl alcohol composite. Journal of Water Process Engineering, 14, 101–107.
Sadeghi, L. A., Moazed, H., Hooshmand, A., & Chorom, M. (2010). Effects of Na-zeolite application on nitrate and ammonium retention in a silty loam soil under saturated conditions.
Sarkar, B., & Naidu, R. (2015). Nutrient and water use efficiency in soil: the influence of geological mineral amendments. In Nutrient use efficiency: from basics to advances (pp. 29–44). Springer.
Schwen, A., Bodner, G., Scholl, P., Buchan, G. D., & Loiskandl, W. (2011). Temporal dynamics of soil hydraulic properties and the water-conducting porosity under different tillage. Soil and Tillage Research, 113(2), 89–98.
Seifi, L., Torabian, A., Kazemian, H., Bidhendi, G. N., Azimi, A. A., & Charkhi, A. (2011a). Adsorption of petroleum monoaromatics from aqueous solutions using granulated surface modified natural nanozeolites: systematic study of equilibrium isotherms. Water, Air, & Soil Pollution, 217(1–4), 611–625.
Seifi, L., Torabian, A., Kazemian, H., Bidhendi, G. N., Azimi, A. A., Farhadi, F., & Nazmara, S. (2011b). Kinetic study of BTEX removal using granulated surfactant-modified natural zeolites nanoparticles. Water, Air, & Soil Pollution, 219(1–4), 443–457.
Seifi, L., Torabian, A., Kazemian, H., Bidhendi, G. N., Azimi, A. A., Nazmara, S., & AliMohammadi, M. (2011c). Adsorption of BTEX on surfactant modified granulated natural zeolite nanoparticles: parameters optimizing by applying Taguchi experimental design method. Clean: Soil, Air, Water, 39(10), 939–948.
Sepaskhah, A., & Barzegar, M. (2010). Yield, water and nitrogen-use response of rice to zeolite and nitrogen fertilization in a semi-arid environment. Agricultural Water Management, 98(1), 38–44.
Sepaskhah, A., & Yousefi, F. (2007). Effects of zeolite application on nitrate and ammonium retention of a loamy soil under saturated conditions. Soil Research, 45(5), 368–373.
Sfechis, S., Vidican, R., Şandor, M., Stoian, V., Şandor, V., & Muste, B. (2015). Using assessment of zeolite amendments in agriculture. ProEnvironment/ProMediu, 8(21).
Sharpley, A. N., Herron, S., & Daniel, T. (2007). Overcoming the challenges of phosphorus-based management in poultry farming. Journal of Soil and Water Conservation, 62(6), 375–389.
Sharpley, A. N., Chapra, S., Wedepohl, R., Sims, J., Daniel, T. C., & Reddy, K. (1994). Managing agricultural phosphorus for protection of surface waters: issues and options. Journal of Environmental Quality, 23(3), 437–451.
Stylianou, M., Inglezakis, V., & Loizidou, M. (2015). Comparison of Mn, Zn, and Cr removal in fluidized-and fixed-bed reactors by using clinoptilolite. Desalination and Water Treatment, 53(12), 3355–3362.
Suarez, D. L., Wood, J. D., & Lesch, S. M. (2006). Effect of SAR on water infiltration under a sequential rain–irrigation management system. Agricultural Water Management, 86(1), 150–164.
Summaries, M. C. (2015). US Department of the Interior and US Geological Survey. Washington DC.
Sutherland, A., Daroub, S., & Ognevich, I. (2004). Addition of clinoptilolite zeolite to a simulated sandy medium to reduce nitrogen leaching.
Szerement, J., Ambrożewicz-Nita, A., Kędziora, K., & Piasek, J. (2014). Use of zeolite in agriculture and environmental protection. A short review. Вісник Національного університету (781), 172–177.
Taheri-Sodejani, H., Ghobadinia, M., Tabatabaei, S., & Kazemian, H. (2015). Using natural zeolite for contamination reduction of agricultural soil irrigated with treated urban wastewater. Desalination and Water Treatment, 54(10), 2723–2730.
Talebnezhad, R., & Sepaskhah, A. R. (2013). Effects of bentonite on water infiltration in a loamy sand soil. Archives of Agronomy and Soil Science, 59(10), 1409–1418.
Tarkalson, D. D., & Ippolito, J. A. (2010). Clinoptilolite zeolite influence on inorganic nitrogen in silt loam and sandy agricultural soils. Soil Science, 175(7), 357–362.
Thierfelder, C., & Wall, P. C. (2009). Effects of conservation agriculture techniques on infiltration and soil water content in Zambia and Zimbabwe. Soil and Tillage Research, 105(2), 217–227.
Thirunavukkarasu, M., & Subramanian, K. (2014). Surface modified nano-zeolite used as carrier for slow release of sulphur. Journal of Applied and Natural Science, 6(1), 19–26.
Thornton, A., Pearce, P., & Parsons, S. (2007a). Ammonium removal from digested sludge liquors using ion exchange. Water Research, 41(2), 433–439.
Thornton, A., Pearce, P., & Parsons, S. (2007b). Ammonium removal from solution using ion exchange on to MesoLite, an equilibrium study. Journal of Hazardous Materials, 147(3), 883–889.
Tiquia, S., & Tam, N. (2000). Co-composting of spent pig litter and sludge with forced-aeration. Bioresource Technology, 72(1), 1–7.
Torma, S., Vilcek, J., Adamisin, P., Huttmanova, E., & Hronec, O. (2014). Influence of natural zeolite on nitrogen dynamics in soil. Turkish Journal of Agriculture and Forestry, 38(5), 739–744.
Vervoort, R., & Cattle, S. (2003). Linking hydraulic conductivity and tortuosity parameters to pore space geometry and pore-size distribution. Journal of Hydrology, 272(1), 36–49.
Vilcek, J., Torma, S., Adamisin, P., & Hronec, O. (2013). Nitrogen sorption and its release in the soil after zeolite application. Bulgarian Journal of Agricultural Science, 9(2), 228–234.
Virta, R. L. (2002). Zeolites. US Geological Survey Minerals Yearbook-2002, 84.1–84.4.
Waltz, F. C., Quisenberry, V. L., & McCarty, L. B. (2003). Physical and hydraulic properties of rootzone mixes amended with inorganics for golf putting greens. Agronomy Journal, 95(2), 395–404.
Wang, F., & Alva, A. (1996). Leaching of nitrogen from slow-release urea sources in sandy soils. Soil Science Society of America Journal, 60(5), 1454–1458.
Wang, J., Huang, J., Zhao, X., Wu, P., Horwath, W. R., Li, H., Jing, Z., & Chen, X. (2016a). Simulated study on effects of ground managements on soil water and available nutrients in jujube orchards. Land Degradation & Development, 27(1), 35–42.
Wang, K., Li, X., He, C., Chen, C., Bai, J., Ren, N., & Wang, J. (2014). Transformation of dissolved organic matters in swine, cow and chicken manures during composting. Bioresource Technology, 168, 222–228.
Wang, Y., Fan, J., Cao, L., & Liang, Y. (2016b). Infiltration and runoff generation under various cropping patterns in the red soil region of China. Land Degradation & Development, 27(1), 83–91.
Watanabe, Y., Yamada, H., Tanaka, J., Komatsu, Y., & Moriyoshi, Y. (2005). Ammonium ion exchange of synthetic zeolites: the effect of their open-window sizes, pore structures, and cation exchange capacities. Separation Science and Technology, 39(9), 2091–2104.
Weber, M., Barbarick, K., & Westfall, D. (1983). Ammonium adsorption by a zeolite in a static and a dynamic system. Journal of Environmental Quality, 12(4), 549–552.
Wei, Y., Fan, Y., Wang, M., & Wang, J. (2000). Composting and compost application in China. Resources, Conservation and Recycling, 30(4), 277–300.
Williams, K. A., & Nelson, P. V. (1997). Using precharged zeolite as a source of potassium and phosphate in a soilless container medium during potted chrysanthemum production. Journal of the American Society for Horticultural Science, 122(5), 703–708.
Xiubin, H., & Zhanbin, H. (2001). Zeolite application for enhancing water infiltration and retention in loess soil. Resources, Conservation and Recycling, 34(1), 45–52.
Youssefi, S., & Waring, M. S. (2015). Indoor transient SOA formation from ozone α-pinene reactions: impacts of air exchange and initial product concentrations, and comparison to limonene ozonolysis. Atmospheric Environment, 112, 106–115.
Youssefi, S., & Waring, M. S. (2014). Transient secondary organic aerosol formation from limonene ozonolysis in indoor environments: impacts of air exchange rates and initial concentration ratios. Environmental Science & Technology, 48(14), 7899–7908.
Youssefi, S., & Waring, M. (2012). Predicting secondary organic aerosol formation from terpenoid ozonolysis with varying yields in indoor environments. Indoor Air, 22(5), 415–426.
Zhan, Y., Zhu, Z., Lin, J., Qiu, Y., & Zhao, J. (2010). Removal of humic acid from aqueous solution by cetylpyridinium bromide modified zeolite. Journal of Environmental Sciences, 22(9), 1327–1334.
Zhang, P., Avudzega, D. M., & Bowman, R. S. (2007). Removal of perchlorate from contaminated waters using surfactant-modified zeolite. Journal of Environmental Quality, 36(4), 1069–1075.
Zhang, Q., Tongamp, W., & Saito, F. (2010). Mechanochemical route for synthesizing KMgPO4 and NH4MgPO4 for application as slow-release fertilizers. Industrial & Engineering Chemistry Research, 49(5), 2213–2216.
Zhang, Q., & Saito, F. (2009). Mechanochemical synthesis of slow-release fertilizers through incorporation of alumina composition into potassium/ammonium phosphates. Journal of the American Ceramic Society, 92(12), 3070–3073.
Zheng, T., Huang, Y., Zhang, C., & Liu, C. (2006). Reducing non-point source pollution with enhancing infiltration. Journal of Environmental Sciences, 18(1), 115–119.
Zwingmann, N., Singh, B., Mackinnon, I. D., & Gilkes, R. J. (2009). Zeolite from alkali modified kaolin increases NH 4 retention by sandy soil: column experiments. Applied Clay Science, 46(1), 7–12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakhli, S.A.A., Delkash, M., Bakhshayesh, B.E. et al. Application of Zeolites for Sustainable Agriculture: a Review on Water and Nutrient Retention. Water Air Soil Pollut 228, 464 (2017). https://doi.org/10.1007/s11270-017-3649-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3649-1