Abstract
The review focuses on the adsorption and selectivity properties of the inland natural zeolites clinoptilolite and mordenite towards a broad spectrum of environmental pollutants incl. radioactive and compare some of them partially to selected foreign samples. A series of elution experiments into a broad spectrum of individual isomolar metal solutions by means of NH4 +—exchanged clinoptilolite—and mordenite-rich tuffs was done to prove the affinity sequence of both minerals untill the steady-state approaching. Among the all recorded breakthrough profiles onto mordente-rich tuff the ammonium exchanged tuff yielded the best uptake performance towards Cs. Three various clinoptilolite-rich tuff samples (Hungarian, American and Slovakian) were compared to each other in order to evaluate the iodide removal in dynamic regime by using the Ag exchanged tuff.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adsorption science has a very long history and first practical adoption of adsorption was noted already in ancient time. The current adsorption theory and relevant applications initiated by Langmuir’s fundamental work have been developed extensively more or less during the last 80 years. Today they comprise many advanced approaches including a wide spectrum of modern surface chemistry sciences. The autonomous existence of adsorption is based on the enormous complexity that is inherent to adsorption phenomena at various interfaces and the widespread, general occurrence and importance of adsorption and related domains in nature, including everyday life’s product as well as industrial and environmental branches [1–3].
The prospective demands towards adsorption and related domains are based on a growing concern for environmental control and for increase of life quality, respectively. Many of the aspects aimed to this concept require both the use of new adsorbents or adsorbent-like materials and the development of the environmental friendly, low energy adsorption technologies as well [1–5].
Crucial progress in theoretical description of the adsorption has been achieved, mainly through the development of new theoretical approaches formulated on a molecular level, by means of computer simulation methods and owing to some new techniques which examine surface layers or interfacial regions. Moreover, during the last 20 years new classes of solid adsorbents have been developed, such as activated carbon fibres and carbon molecular sieves, fullerenes and heterofullerenes, microporous glasses and nanoporous—both carbonaceous and inorganic—materials as well [4–10].
Adsorption technologies comprise most important techniques and play a significant role in both environmental and human health control as well as in prevention of global warming and ozone layer depletion. Adsorption can also be expected to play a significant role in the environmental control and life supporting system on planetary basis, where adsorbents used to process the habitat air or to recover useful substances from the local environment. Adsorption processes were good candidates for separation and purification in space, by virtue of such characteristics as are gravity independence, high reliability, relatively high energy efficiency, design flexibility, technological maturity and recovery [11, 12].
For this reason, the adsorption has historically played a key role by life support on piloted U.S. and Russian spacecrafts. Thus, the environmental control and life support system on the spacecraft provided the safe and comfortable environment, in which the crew lived and worked by supplying oxygen, water and by removing carbon dioxide, water wapour and trace contaminants from cabin air. The design, ultimately selected for the use on international space station ISS, included an activated charcoal for high molecular weight compounds and ammonia removal, for which the impregnation with 10 % phosphoric acid was used (beside alternatively used natural zeolite). A catalytic oxidiser for removal of destroyed low molecular weight compounds and a postsorbent of LiOH for acid gas removal were sequenced in the series thereafter [11–20].
This review focus on the adsorption and selectivity properties of the inland natural zeolites clinoptilolite and mordenite towards a broad spectrum of environmental pollutants incl. radioactive and compare some of them partially to selected foreign samples. The goal of experimental work was to support or refute former published results dealing with selectivity properties of Slovakian zeolite-rich tuffs by using the both static and dynamic laboratory setup.
Experimental
Characterization of inland natural clinoptilolite and mordenite
Mineralogical and chemical composition of the clinoptilolite-rich tuff from the open quarry nearby Nižný Hrabovec and of the mordenite-rich tuff from the vicinity of Bartošova Lehôtka-Jastrabá presents the paper numbered in References section with [13]. As generally known, the mordenite-rich tuff from Central Slovakian deposit belongs only to the low grade, not valuable zeolitic rock, but the clinoptilolite-rich tuff situated in Eastern Slovakian municipality nearby Nižný Hrabovec contains significant percentage of the active mineral clinoptilolite (cca 85 %) and is consequently classified as an excellent one. Scanning electron micrograph at Fig. 1 vizualizes a characteristic surface morfology of the clinoptilolite-rich tuff incl. basic topology and framework structure with the mobile extraframework cations.
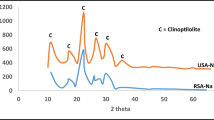
The foreign natural zeolites, i.e. Hungarian and American ones, have been obtained from the Share Holding Company KERKO Košice and originate from the deposit in south-eastern part of the Tokaj Mountains, at the vicinity of the Mád village in Hungary and from the Wyoming deposit in USA (their XRD spectra illustrates Fig. 2).
Pretreatment of zeolite samples
Native zeolite samples were preconditioned into near homoionic sodium, calcium or ammonium forms by equilibrating them by means of periodic shaking machine in 10 % aqueous chloride solutions (10 g in 1 L) in a series of batchwise steps during the 3 days everyone, then washed them in distilled water thoroughly untill any precipitate of AgCl with the AgNO3 solution appeared and finally dried at 105 °C for 2 h in laboratory dry machine. The raw tuffs, crushed and grinded into the fraction of 0.4–1 mm (35–16 mesh) has been supplied for laboratory experiments from Zeocem Bystré and Geological Survey Banská Bystrica.
Analytical procedures
Chemicals necessary for the stock solution preparation were purchased mostly from Lachema Brno (made in Czech Republic) with analytical grade quality. Aqueous model solutions of the pollutants examined in adsorption experiments (mostly as chlorides of metallic cations except the iodide labelled with the adequate radioisotopes of 125I, 137Cs, 133Ba were determined by means of radioindicator method (spectrometrical set of EG & G Berthold Ortec, USA with a scintillation detector NaI (Tl)). Ammonium ions in the solution have been determined using the Nessler reagent at the wavelength 420 nm on the Diode Array HP Spectrometer 8452A and all other elements except Cs, Ba and I by means of AAS Spectrometer using Varian SpectrAA10 Apparatus with the flame technique.
Laboratory setup
In order to determine the equilibrium distribution of the cations between the solid and aqueous phases, static and dynamic laboratory arrangements were proposed. Equilibrium adsorption at the laboratory was done with aqueous model solutions of above labelled elements versus native and pretreated clinoptilolite-rich as well as mordenite-rich tuffs with the solid-to-liquid ratio 1 g/100 mL, at T = 23 ± 0.1 °C.
The equilibrium uptake capacity a (in mg/g) for each sample was calculated according to following mass balance equation (1):
where Ci and Ceq were initial and equilibrium concentrations of cation examined (in mg/L), m was the mass of zeolite sample (in g) and V was volume of the solution in liters (L).
Results and discussion
Discharge of potentially noxious substances into the environment involves some risk, which may or may not be measurable. The nuclear industry is usually providing some information on the probable consequences of contamination, extended over a lifetime, when some radiation into the environment was exposed. In context of pollutants removal especially out of the enormous volume of very low level waste effluents, coming usually from numerous sources within the nuclear power operation, different decontamination procedures are proposed according to published literature [20–27].
When the adsorption process of one or several ionic species is accompanied by the simultaneous desorption of an equivalent amount of ionic species, the process is considered for an ion exchange, and in general may be covered under adsorption, too [1–3]. That was the case of several cationic radionuclides dissolved in aqueous media, studied bellow, which mostly exchanged onto zeolitic tuffs. On the other hand, when the native zeolite was pretreated and exchanged with Ag—cations, then the removal of iodide radionuclide from aqueous solutions was carried out only by adsorption (AgI surface precipitation).
Figure 2 indicates fairly close quantitative and qualitative compositions of the studied Slovakian and American clinoptilolite-rich tuffs, however a different one from the Hungarian clinoptilolite-rich tuff, which were compared to each other by column experiments. Content of the active mineral clinoptilolite in the Hungarian zeolitic tuff was estimated for about 10–15 % lower then was the content of the Slovakian clinoptilolite-rich tuff (~80–85 %).
In order to achieve the highest capacity of zeolites towards the studied radionuclides and to prevent the hydrolysis of solution, the capacity dependence upon the pH, expressed as pKD (KD = a/c), was examined as next step. As can be observed in Fig. 3, Ba radionuclide shows the most pronounced capacity lowering within the pH decreasing. By the Cs radionuclide this dependence is rather shallow, in the pH range between 4 and 5 mostly without any change, however in the range of pH 3–6, a slight capacity difference of both clinoptilolite and mordenite-rich tuffs are visible.
A series of elution experiments into a broad spectrum of individual isomolar metal solutions by means of NH4 +—exchanged clinoptilolite—and mordenite-rich tuffs was done to prove the affinity sequence of both minerals untill the steady-state approaching. These trials were chosen on the base of the enhanced selectivity of both tuffs towards the ammonium ion and its easier and simple analytics. Following sequence for clinoptilolite-rich tuff: Ba > Pb > Cu > Cs > Cd > Co > K > Mg > Ni > Na > Hg > Ca and following for the mordenite-rich tuff was determined: Pb > Cs > K > Ba > Cu > Na > Cd > Ni > Mg > Ca > Co > Hg.
The presented results (Figs. 4, 5) are in good agreement to the published literature [13], according to which the Ba and Pb ions were also preffered or situated before the Cs ion in the measured selectivity sequence, however of raw clinoptilolite-rich tuff (Table 1). On the other hand, Na-exchanged clinoptilolite-rich tuff easier converted into ammonium form than its parent tuff in native form, what simultaneously supports current results and position of K and Mg situated before Na ion. From this point of view, the K and NH4 + ions may compete for the affinity by the raw clinoptilolite-rich tuff, which is higher then for Na ion. It is important to point that the native form of clinoptilolite-rich tuff contains as extraframework cations predominantly Ca, K, Mg and small amount of Na ions. Lower quality mordenite-rich tuff in ammonium form preffered especially Pb, Cs and K ions and its capacity was estimated for ~20 % lower, as visible more or less from the Fig. 5.
On the base of rather considerable amount of the accompanied clay minerals in the inland natural mordenite, which may compensate its low percentage of zeolitic component and thus potentially enhance the capacity [13, 17, 22], the uptake performance for Cs and Ba radionuclides in dynamic regime was examined furthermore. The experimental apparatus consisted of glass tubes with 5 mm diameter and 100 mm length closely packed with 1 g of 0.250–0.315 mm grain-sized zeolite. Downflow operating columns were loaded with Cs and Ba solutions at a rate of 10 BV (bed volume) per hour using a peristaltic pump. Figs.6, 7, and 8 illustrate the breakthrough curves measured using the moderatelly pretreated homoionic mordenite-rich tuff incl. its native form versus Cs and Ba solutions. As can be seen from the plotts (Fig. 6), mostly Cs removal in dynamic regime proved the characteristic and symmetric S-shaped curves, by which despite using the higher initial Cs concentration regarding to Ba solution, also some adsorption zones within the 10–30 V/Vo were observed, while among the breakthrough curves measured with Ba solution only the curve numbered 10 (Figs. 7, 8), i.e. Na-exchanged mordenite-rich tuff was of acceptable shape. However, among the all recorded breakthrough profiles the ammonium exchanged mordenite-rich tuff (curve numbered 7) yielded the best uptake performance.
Typical breakthrough curves measured by means of the laboratory model columns: (1) native mordenite-rich tuff versus 20 mmol/L of CsNO3, (3) Na-mordenite-rich tuff versus 20 mmol/L of CsNO3, (5) Ca-mordenite-rich tuff versus 20 mmol/L of CsNO3, (7) NH4 +—mordenite-rich tuff versus 20 mmol/L of CsNO3
As mentioned above, regarding the Fig. 2, three various clinoptilolite-rich tuff samples were compared with each other finally in order to evaluate the iodide removal in dynamic regime by using the Ag exchanged tuff. As the Fig. 9 indicates, all the plotted breakthrough curves yield typical S-shaped profiles, even with sufficient long adsorption zone, while in agreement to XRD quality determination, only the Hungarian sample proved by these measurements not such a sharp, oppositivelly the most shallow mass transfer within the recorded curves.
Conclusions
Based upon the experimental results, following conclusions can be stated:
-
(i)
Ba, Cs and Pb ions were preffered or situated before the other tested ions in the measured selectivity sequence, in this specific case by the ammonium exchanged clinoptilolite- and mordenite-rich tuffs.
-
(ii)
Among the all recorded breakthrough profiles the ammonium exchanged mordenite-rich tuff yielded the best uptake performance towards Cs.
-
(iii)
Three various clinoptilolite-rich tuff samples (Hungarian, American and Slovakian) were compared with each other in order to evaluate the iodide removal in dynamic regime by using the Ag exchanged tuff. All the plotted breakthrough curves yielded typical S-shaped profiles, even with sufficient long adsorption zone, however the Hungarian zeolite did not prove such a sharp curve’s front like the others, oppositivelly the most shallow one.
References
Dabrowski A (2001) Adsorption—from theory to practice. Adv Colloid Interface Sci 93:135–224
Podkoscielny P, Dabrowski A (2001) Adsorption contribution to the protection of the human environment. Ann Univ Marie Curie Sklodowska Chemia 3:29
Mojumdar SC, Varshney KG, Agrawal A (2006) Hybrid fibrous ion exchange materials: past, present and future. Res J Chem Environ 10(1):89–97
Drexler KE (1986) Engines of creation—the coming era of nanotechnology. Anchor Press/Doubleday, New York. www.foresight.org/EOC/
Zukal A (2007) Moderní trendy syntézy nanoporéznich materiálu. Chem Listy 101:208–216 (in Czech)
Legendre A (2001) Uhlíkové materiály (Od černé keramiky k uhlíkovým vláknam). Informatorium, Praha, p 171 (in Czech)
Shawabkeh RA (2004) Synthesis and characterization of activated carbo-alumino-silicate material from oil shale. Microporous Mesoporous Mater 75:107–114
Park HG, Kim TW, Chae MY, Yoo IK (2007) Activated carbon-containing alginate adsorbent for the simultaneous removal of heavy metals and toxic organics. Process Biochem 42:1371–1377
Lindoy LF, Atkinson IM (2000) Self assembly in supramolecular systems. Royal Society of Chemistry, Cambridge
Lehn JM (1995) Supramolecular chemistry: concepts and perspectives. WCH, New York
Galindo CH Jr, Ming DW, Carr MJ, Morgan A, Pickering KD (2000) Use of Ca-exchanged clinoptilolite for ammonium removal from NASA’s advanced life support wastewater systém. In: Colella C, Mumpton FA (eds) Natural zeolites for the third millennium. Napoli, Italy, pp 363–371
Inglezakis VJ, Zorpas AA (eds) (2012) Handbook of natural zeolites. Bentham Science Publ, New York, pp 399–409. ISBN: 978-1-60805-446-6
Chmielewská E, Lesný J (2012) Selective ion exchange onto Slovakian natural zeolites in aqueous solutions. J Radioanal Nucl Chem 293:535–543
Rosabal BC, Era JB, Fuentes GR (2000) Characterization of Fe2+—containing natural clinoptilolite and its interaction with saccharides. Mesoporous Microporous Mater 38:161–166
Dyer A (1988) An introduction to zeolite molecular sieves. Wiley, Chichester, p 144
Fiala J, Kraus I (2009) Povrchy a rozhraní. ČVUT, Praha, p 299 in Czech
Galamboš M, Rosskopfová O, Kufčáková J, Rajec P (2011) Utilization of Slovak bentonites in deposition of high-level radioactive waste and spent nuclear fuel. J Radioanal Nucl Chem 288(3):765–777
Faghihian H, Malekpour A, Maragheh MG (2003) Removal of radioactive iodide by surfactant-modified zeolites. Adsorpt Sci Technol 21(4):373–381
Ming DW (1992) Application for special-purpose minerals at the lunar base. In: Mendel WW (ed) The second conference on lunar bases and space activities of the 21st century, vol 1. NASA Conference Publ. 3166, Huston, Texas, 5–7 April 1988, pp 385–391
Pepe F, de Gennaro B, Aprea P, Caputo D (2013) Natural zeolites for heavy metals removal from aqueous solutions: modeling of the fixed bed Ba/Na ion-exchange process using a mixed phillipsite/chabazite-rich tuff. Chem Eng J 2019:37–42
Chmielewská-Horváthová E, Lesný J (1996) Sorption of iodide on the surface of modified zeolite. Pol J Environ Stud 5(2):13–19
Galamboš M, Suchánek P, Rosskopfová O (2012) Sorption of anthropogenic radionuclides on natural and synthetic inorganic sorbents. J Radioanal Nucl Chem 293:613–633
Olguín MT, Solache M, Asomoza M, Acosta D, Bosch P, Bulbalian S (1994) UO2 2+ sorption in natural Mexican erionite and Y zeolite. Sep Sci Technol 29(16):2161–2178
Bosch P, Caputo D, Liguori B, Colella C (2004) Safe trapping of Cs in heat-treated zeolite matrices. J Nucl Mater 324:183–188
Novgorodtzev PG, Nikashina VA, Alexandrov AR (2010) Geochemical barrier based on natural zeolite in the path of radionuclide migration from the underground nuclear explosion “Kraton-3” Zeolite 2010, In: Petrov O, Tzvetanova Y (eds) Book of abstracts, Sofia, 10–18 July 2010, pp 70–71
Šeršeň F, Čík G, Havránek E, Sýkorová M (2005) Bio-remediation by natural zeolite on plants cultivated in a heavy metal-contaminated medium. Fresenius Environ Bull 14(1):13–17
Cotton FA, Wilkinson G, Murillo CA, Bochmann M (1995) Advanced inorganic chemistry, 6th edn. Wiley, New York
Acknowledgments
The natural zeolite utilization for water treatment processes was examined at author’s previous employer Water Research Institute in Bratislava and currently is funded by the Slovak Scientific Council VEGA (Project no. 1/0185/12) and under the bilateral Slovak-Chinese Cooperation SK-CN-0033-12 “Liquid waste minimization and valorization by multi-dimensional hierarchical composites of natural resources”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chmielewská, E. Zeolitic adsorption in course of pollutants mitigation and environmental control. J Radioanal Nucl Chem 299, 255–260 (2014). https://doi.org/10.1007/s10967-013-2721-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2721-6