Abstract
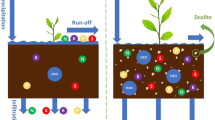
Na+ contamination of irrigation waters represents a global environmental issue for soil structure and plant production. Notwithstanding several techniques for the reduction of Na+ have been proposed in recent years, they generally exhibit disadvantages, including low recyclability and relatively high operational/maintenance costs. In this paper, we propose a natural and eco-friendly solution for the reduction of Na+ risk in coastal agricultural sandy soil (SS), vulnerable to salinity stress. A series of column leaching experiments have been conducted to assess the influence of Italian zeolite-rich tuff (natural zeolites, NZ) addition to soil (NZSS) on Na+ removal, SAR, and CROSS index, under three different salinity scenario. Result showed that the Na+ removal efficiency varied between 46.4 and 54.3% in soil amended with NZ, and analogously SAR index was significantly reduced from 7 to up 13 points. SAR and CROSS indexes resulted better correlated in SS rather than NZSS due to the influence of K+ released by NZ. In conclusion, soil amendment with NZ represents a natural and eco-friendly solution for increasing sandy soil resilience to Na+ risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intensification of agricultural activities caused a constant increment in the use of irrigation water in order to maintain the food production at required levels (Postel et al. 1996; Bouwer 2002; Tilman et al. 2002). In some agricultural areas, especially in coastal and arid/semi-arid regions, primary (natural) or secondary (anthropogenic) salinization phenomena can lead to high sodium (Na+) levels in soils or irrigation water, reflecting an extremely serious risk both for soil structure and plant production (Taiz and Zeiger 2007; Rengasamy and Marchuk 2011). While the presence of high amounts of divalent cations such as calcium (Ca2+) and magnesium (Mg2+) enhance soil aggregation, a high level of Na+ cause soil dispersion, resulting in a lower hydraulic conductivity, lower porosity, and formation of solid crusts (Agassi et al. 1981; Hanson et al. 1999; Miller and Donahue 1995). High Na+ accumulation in plant cells (high Na/K ratio) can lead to toxic effects for the inactivation of enzymes and protein synthesis (Taiz and Zeiger 2007). Additionally, Ca2+ can be replaced by Na+ if this latter is present at high concentration, causing a significant change in plasma membrane permeability, important K+ leaking from the cells (Cramer et al. 1985; Taiz and Zeiger 2007). Na+ can also inhibit the acquisition of essential nutrient (as K+) and photosynthesis if present at high concentration in chloroplasts (Taiz and Zeiger 2007).
In coastal zones, groundwater is the main irrigation resource and salinization is the main cause of its degradation. Groundwater salinization is mainly due to seawater intrusion or mobilization of saline paleo-waters (Han et al. 2011; Ghiglieri et al. 2012; Werner et al. 2013; Sola et al. 2014) induced by the inappropriate management of coastal aquifers. Over-exploitations activities for example favor the intrusion of saltwater or the up rise of brackish paleo-waters into the fresh water zone of the aquifer (Bear et al. 1999; Ferguson and Gleeson 2012; Di Giuseppe et al. 2014; Colombani and Mastrocicco 2017).
Direct root uptake of these compromised waters and their use for irrigation purposes will increase the Na+ content in the upper soil inducing further salinization.
Since many years, natural zeolites are known to be efficient cation exchangers and their use for environmental purposes has become very common (Pond and Mumpton, 1984; Mumpton 1999; Reháková et al. 2004; Misaelides 2011). Zeolites are aluminosilicates with an open three-dimensional framework containing channels and cavities where different kinds of polar and non-polar molecules can be exchanged, including both inorganic and organic compounds (Reháková et al. 2004; Leyva-Ramos et al. 2010). Among natural zeolites, K-rich/Na-poor chabazite zeolites are found in volcanoclastic deposits of the Italian peninsula, where many quarries are actually exploiting this material for the production of construction bricks (Gottardi and Obradovic 1978; Passaglia 2008). According to Galli and Passaglia (2011), these rocks, containing > 50% of zeolite, can be named “zeolitites.” The cutting process for brick production leave a high amount of zeolite-rich material unused, constituting a waste for the quarry but an interesting granular by-product which can be used for many purposes, including soil amendment (Ferretti et al. 2017a, b; Faccini et al. 2018; Ferretti et al. 2018) and filters for reducing SAR in irrigation waters (Passaglia et al. 2005).
Hereafter, we test if the addition of natural Italian K-rich chabazite zeolitite as amendment to a sandy agricultural soil could bring to a mitigation of Na+ levels in soils after the contact with brackish water (e.g., as consequence of recycling of saline irrigation waters or uprising of saline groundwater). The column experiment approach was used in order to determine the removal efficiency (RE %) under different level of Na+ content and to quantify the decrease in the sodium adsorption ration (SAR) of the employed brackish waters.
Material and methods
Soil and natural zeolitite
The sandy soil (SS) used for the experimentation was sampled in an agricultural field located near Lido delle Nazioni (Ferrara, Italy) (44° 45′ 15.44″ N, 12° 14′ 22.84″ E). The soil is defined as Endogleyic Calcaric Arenosols (IUSS 2007) and belong to the paleo-coastal dune sediments developed during Po River Delta progradation (Stefani and Vincenzi 2005; Eslami et al. 2018). According to the Emilia Romagna Region Soil Map (1:50,000), it is part of the cartographic unit CER1 and it is mainly constituted by sandy fractions (more than 90%, of which 13% medium sand, 71% fine sand, and 9% very fine sand) while silt and clay contents represent less than 10% of the grain size distribution. The field is dedicated to cultivation of summer cereals (e.g., maize, wheat, barley, and sorghum) and occasionally to open field horticulture. To improve fertility of the soil, both chemical and especially organic fertilizers (manure, slurry) application is common in this area. The soil was characterized by organic matter (OM) and gravimetric water contents of 2.76% (± 0.61) and 3.57% (± 0.25), respectively, and high carbonate amounts (bivalve shells are very common), sub-alkaline pH (7.67), a bulk density (BD) of 1340 kg m−3, and an electrical conductivity (EC) of 740 μS cm−1.
The zeolite-rich tuff (NZ) used in this study is quarry-waste material obtained from a quarry located near Sorano (Grosseto, Italy). The total zeolite content is 70.9% of the whole rock, of which 68.5% is made of K-rich and Na-poor chabazite, 1.8% of phillipsite, and 0.6% of analcime (Malferrari et al. 2013). NZ grain size was chosen < 2 mm in order to supply finer material to the sandy soil. Whole rock cation exchange capacity (CEC) was 2.17 meq g−1 of which 0.04 due to Mg2+, 0.07 to Na+, 0.60 to K+, and 1.46 to Ca2+ (Malferrari et al. 2013).
Two treatments were tested in this study: (1) SS without zeolitite addition (control) and (2) SS amended with 10 wt% of NZ (NZSS).
Filling solution preparation
In order to reproduce different salinity level of sea-influenced waters, three artificial brackish waters were created mixing seawater from the Adriatic Sea with distilled water (resistivity ~ 18.2 MΩ cm; Millipore USA) and based on the EC or total dissolved salts (TDS):
-
1.
First salinity level (BWa) = 5000 μS cm−1 (TDS 3.2 g L−1);
-
2.
Second salinity level (BWb) = 10,000 μS cm−1 (TDS 6.4 g L−1);
-
3.
Third salinity level (BWc) = 18,000 μS cm−1 (TDS 11.5 g L−1).
Since all BW solutions derives from seawater, Na+ was the main cation, followed by Mg2+ > Ca2+ ≥ K+. Chemical characteristics of each BW solution and SAR indexes are visible in Table 1.
Column experiment
Column test where performed according to the method suggested by Colombani et al. (2015). All the experiments were carried out under laboratory condition (approx. 20 °C) using a polyethylene column (Ø = 20 mm, h = 148 mm); a 50-μm Nitex mesh was applied both at the top and at the bottom of the column, in order to avoid losses of material during the fluxing (Fig. 1). The columns were connected with capillary Teflon tubes to a peristaltic pump with a constant flow rate of 100 ml h−1. The NZSS mixture was slowly inserted into the column until complete filling for a total of 68.8 g (bulk density of approx. 1340 kg m−3). The soil pore-volume (PV) was 20 ml and was calculated by difference between the weight of an unsaturated column and a saturated one. The effective saturation was checked by flushing the column with the appropriate BW solution until the first drop came out from the top side of the column (Fig. 1). Each saturated column was let rest for 24 h in order to allow equilibration with the filling solution; three replicates were performed for each BW solution in order to obtain standard deviation and perform statistical analysis. After 24 h, each column was flushed with MQ-water for a total of 6 pore volume (PV). Each PV was sampled and frozen until analysis.
Analytical techniques
Soil gravimetric water content was measured by oven-drying samples at 105 °C for 24 h while OM content was measured by dry combustion at 550 °C (Tiessen and Moir 1993). Soil pH and EC were measured in 1:5 w/v H2O (resistivity > 18 MOmh cm−1) extracts while PVs, pH, and EC were measured with an Orion 9102BNWP pH meter connected to an Orion 4star pH – ISE benchtop (Thermo Fisher) and a HANNA RS 180–7127 conductivity meter, respectively in the undiluted samples. A conversion factor of 0.64 was used for converting the EC (mS cm−1) as TDS (g L−1) (Marcum 2006).
Major cations (Na+, Ca2+, Mg2+, K+) were analyzed by inductively coupled plasma mass spectrometry (ICP-MS) using a Thermo-Scientific X Series instrument on samples previously diluted 1:20 (or 1:100 for samples having very high EC) by deionized water (resistivity of ~ 18.2 MΩ cm), also introducing known amount of Re and Rh as internal standard. In each analytical session, precision and accuracy were verified by using reference materials EU-L-1 and ES-L1 provided by SCP-Science (www.scpscience.com).
Calculations
The amount of ions retained by the solid phase was calculated by mass-balance Eq. 1 according to Wang et al. (2007):
where Qe (mg g−1) is the amount of ions retained/released by the exchanger solid phase (in this case the NZ mixed in the sandy soil), V (L) is the volume of the solution, M (g) the mass of the exchanger solid phase, C0 (mg L−1) the ion concentration in the filling solution, and Ce (mg L−1) the ion concentration at equilibrium.
The removal efficiency (RE %) was calculated as the % of the referred ion (in this case Na+) removed from the solution with respect to the initial concentration (Eq. 2) as reported by Huang et al. (2010):
C0 and Ce are in this case the starting and final equilibrium concentrations of Na+ ion in solution (mg L−1), respectively.
SAR index was calculated as the amount Na+ ions with respect to Ca2+ and Mg2+, expressed in mmol of charge L−1 following Eq. 3:
SAR index was calculated only for the first drop of the first pore-volume in order to exclude any dilution of the first PV caused by deionized water interaction.
Paired t test were run for evaluating significant differences between the Qe of SS and NZSS treatments at p = 0.05 using SPSS statistic 17.0 software.
Results
Columns filled with BWa solution
The total amounts of Na+, Mg2+, K+, and Ca2+ expressed in mg, introduced in the column by the BWa solution (V = 20 ml), were 14.8 (± 0.6), 1.76 (± 0.07), 0.44 (± 0.02), and 0.50 mg (± 0.02), respectively (calculated from Table 1 as concentration (mg L−1) * PV (L)). The EC and pH of each PVs is showed in Table 2. EC diminished from 4850 to 96 μS cm−1 in SS columns and from 4460 to 102 μS cm−1 in NZSS columns, while leachate pH increased from sub-alkaline values to strong alkaline values from PV one to six.
During the flushing, the concentration in the leached PVs diminished from PV 1 to 6 differently for each investigated cation (Table 3). The final Qe (mg g−1) calculated at the end of the leaching experiment is showed in Fig. 2. The amount of Na adsorbed by NZSS was significantly higher (0.10 mg g−1) with respect to SS column (0.03 mg g−1) (p < 0.05) while the adsorption of Mg decreased from 0.01 mg g−1 in SS to 0.006 mg g−1 in NZSS (p < 0.05). K+ and Ca2+ were both released during the leaching experiment in the SS columns (without zeolitites) (Qe of − 0.01 and − 0.07 mg g−1, respectively). In NZSS treatments, however, a six-fold higher release of K and an almost double release of Ca were observed (Qe of − 0.06 and − 0.12 mg g−1, respectively (Fig. 2). The total RE % of Na measured after 6 PV was thus 14.0% (± 4.99) for SS and 46.4% (± 3) for NZSS (Fig. 3).
Removal efficiency (RE %) with respect to Na+ obtained in columns filled with sandy soil (SS) and sandy soil + natural zeolitite (NZSS) after the contact with each brackish water (BW) solution. Error bars represent standard deviation. The presence of “***” indicate significant differences as result of paired t test at p = 0.05
The SAR index calculated from the first drops of PV1 was 10.7 (± 0.9) for SS and 4.5 (± 0.6) for NZSS (Fig. 4).
Columns filled with BWb solution
The total amount of Na+, Mg2+, K+, and Ca2+ introduced in the column by the BWb solution (20 ml) were 35.5 (± 1.5), 4.22 (± 0.17), 1.02 (± 0.04), and 1.30 (± 0.05) mg, respectively. As for BWa solution, EC drastically decreased from PV1 to PV6 both in NZSS and SS. Significant difference in EC was found only in PV1 where the salinity was lower in NZSS than in SS, while leachate pH tends to increase towards alkaline values from PV1 to PV6 (Table 2). The Qe (mg g−1) calculated from each pore-volume is showed in Fig. 2. Similarly, to BWa columns, Na+ was considerably adsorbed especially in NZSS (0.27 mg g−1) rather than in SS (0.10 mg g−1) (p < 0.05) and Mg2+ retention was higher in SS column (0.027 mg g−1) than in NZSS (0.019 mg g−1) (p < 0.05). In BWb, there was also a significant release of K+, considerably higher in NZSS (Qe = − 0.07 mg g−1) than in SS (− 0.010 mg g−1) (p < 0.05) while the release of Ca2+ was more consistent but always higher in NZSS (− 0.23 mg g−1) with respect to SS (− 0.14 mg g−1) (p < 0.05). The total RE % of Na+ after 6 pore-volume was thus 16.7% (± 4.8) for SS and 51.4% (± 2.7) for NZSS (Fig. 3).
In this scenario, SAR index resulted 17.3 (± 2.9) for SS and 7.3 (± 1.6) for NZSS (Fig. 4).
Columns filled with BWc solution
The total amount of Na+, Mg2+, K+, and Ca2+ introduced in the column by the BWc solution (20 ml) were 67.5 (± 3.1), 7.36 (± 0.34), 2.26 (± 0.10), and 2.17 mg (± 0.10), respectively. As for BWa and BWb solutions, PV1 EC tend to be lower in NZSS than in SS column while a lower increase of pH from PV1 to PV6 was observed (from 8.1–8.3 to 8.4–8.7) (Table 2). The Qe (mg g−1) calculated from each pore-volume is showed in Fig. 2. BWc behaves similarly to BWa and BWb columns: Na+ ions were strongly adsorbed in NZSS (0.53 mg g−1) rather than in SS (0.20 mg g−1), and Mg2+ retention was generally very low but relatively higher in SS column (0.05 mg g−1) than in NZSS (0.03 mg g−1). K+ ions were significantly released in the leachate waters especially by NZSS (− 0.064 mg g−1) rather than SS (0.010 mg g−1). The release of Ca2+ was more consistent but always higher in NZSS (− 0.28 mg g−1) with respect to SS (− 0.16 mg g−1). The total RE % of Na occurred after 6 pore-volume was thus 20.2% (± 4.9) for SS and 54.3% (± 2.8) for NZSS (Fig. 3). In this last case, SAR index resulted 32.8 (± 7.0) for SS and 13.9 (± 3.7) for NZSS (Fig. 4).
Discussions
The Na+ concentration in the three investigated scenarios (BWa, BWb, and BWc) was extremely high and thus Na-risk deriving from the contact of these water (hypothetically because of seawater intrusion or as brackish groundwater uprising) may constitute toxicity for cultures and increase in the dispersion of the already scarce finer fraction (silt and clay) of this sandy soil. For most of the cultivated crops, specific Na+ toxicity can be slight to moderate if the irrigation water SAR varies between 3 and 9, while it can be severe if SAR is > 9 (Ayers and Westcot 1994).
The low natural content of exchangeable Na+ in the employed NZ allowed a good adsorption of this ion once in contact with all the three investigated Na+-rich filling solutions (Fig. 2). Additionally, the high content of exchangeable K+ and Ca2+ in the raw NZ allowed a parallel release of these ions in the leached waters during the process, thus increasing the immediate availability of important nutrients but also increasing pore-water alkalinity, as testified by the marked pH drift from PV1 to PV6. The adsorption capacity (Qe) of Na+ increased at increasing salinity of the filling solution employed (Fig. 2). This was reasonably due to the higher ionic strength BWa < BWb < BWc which facilitated the adsorption in the solid phase at higher salinity (Matschonat and Vogt 1997). The effect of ionic strength on CEC processes was visible also in terms of Na+ RE % since it slightly increased at increasing solution salinity. Coherently, also Mg2+ adsorption and Ca2+ release increased at increasing ionic strength of filling solutions, while K+ adsorption followed a different trend (Fig. 2). SS showed a slight release of K+ in BWa and BWb scenarios while in BWc, a little amount of K+ was adsorbed indicating that at higher ionic strength, little amounts of K+ can be retained by SS soil. On the other hand, the abundant release of K+ was obviously due to the high content of exchangeable K+ in the raw NZ (Malferrari et al. 2013). Both treatments have retained Mg2+(Fig. 2); however, the lower Qe of NZSS can be ascribed to a slight release of Mg2+ by NZ, which is one of the main exchangeable ions of chabazite (Torracca et al. 1998). SS released considerable amount of Ca2+ (Fig. 2, Tables 3, 4, and 5) probably because of the dissolution of CaCO3 (also proved by the alkaline pH of the PVs), as the soil used was an Endogleyic Calcaric Arenosol, whereas the increase in Ca2+ released in NZSS columns for all BW scenario (Fig. 2, Tables 3, 4, and 5) can be the result of the zeolites added as amendment, taking into account that Ca2+ is also one of the main exchangeable cation in the raw NZ (Malferrari et al. 2013).
The RE % (Fig. 3) calculated for Na+ at each scenario was relevant considering that a natural raw material was employed just as soil amendment without any pre-treatment or expensive transformation. The RE % obtained in this study (~ 50%) however are low if compared to that obtained by other studies in which chabazite has been employed for the removal of Na+ from sludge or NH4+ in aqueous solutions. As an example, Ghaly and Verma (2008) used an ion exchange column to remove Na+ from a sludge having Ca/Mg/Na ratios of 1:1.6:36.6 and obtained a Na+ removal of 75.34 and 99.79% employing respectively one or two column filled with chabazite. In our case, however, the amount of chabazite added to soil was only 10% which can reasonably explain the observed lower RE %.
Notwithstanding the debate concerning the effective impact of the different monovalent major cations (exchangeable K+ and Na+) in clay swelling dispersion (El Swaify et al. 1970; Chen et al. 1983), the flocculating power (invers of dispersion power), which depends on ion valence and ionization potential, is 1 for Na+, 1.8 for K+, 27 for Mg2+, and 45 for Ca2+ (Rengasamy and Marchuk 2011). This evidence makes Na+ probably the most dangerous element for soil structure. In this respect, the use of SAR index has been widely identified as a valuable indicator for Na+ risk in soils and irrigation waters. In the present experiment, SAR index strongly decreased after the contact with NZSS because CEC processes occurred within the circulating pore-waters and NZ (Fig. 4). Coherently with RE % results, the higher reduction in SAR index was obtained with the most brackish scenario (BWc), thus considerably decreasing the Na+ risk but not the solution salinity. Na+ can be in fact efficiently adsorbed but other ions are concomitantly released (in this case K+ and Ca2+). Rengasamy and Marchuk (2011) proposed the use of the “cations ratio of soil structural stability (CROSS) to parameterize soil structural effects taking into account the amounts of both monovalent (Na+ and K+) and divalent (Ca2+ and Mg2+) ions in the soil solution and their effects on the basis of the flocculating power (Eq. 4):
where the concentrations of these ions (Na, K, Ca, and Mg) are expressed in mmol of charge L−1.
As visible from Fig. 5, a good correlation was obtained between SAR and CROSS indexes in this experiment, but stronger for soil without the addition of NZ (r2 of 0.962 vs 0.733). The differences between SAR and CROSS indexes are more pronounced in NZSS treatments because of the release of K+ by NZ that clearly had a big influence on CROSS index calculation. Even if K+ contribute to soil dispersion, it is however a fundamental nutrient for plant growth contrary to Na+. In any cases, it is evident the net decrease of both indexes in waters after the contact with NZSS.
The high salinity of all the reproduced BW solutions (TDS ranging from 3.2 to 11.5 g L−1) makes them of course unsuitable for irrigation notwithstanding the strong reduction in SAR and CROSS indexes. However, reducing the Na+ content may help in mitigating at least the immediate negative effects caused by the excessive amount of this monovalent cation once in the soil for an erroneous utilization of saline irrigation waters.
Conclusion
Contamination of irrigation waters with Na+ represents a global environmental issue due to its non-degradable behavior and risk both for soil structure and plant production. Several techniques have been proposed in recent years to remove Na+ from brackish waters. However, majority of the sorbents generally exhibit disadvantages, including relatively high operational/maintenance costs.
The main outcomes of our experiment highlighted that NZ represents a natural and eco-friendly solution for the mitigation of Na+ risk in agricultural areas potentially subjected to the negative effects of seawater intrusion. In the investigated scenarios, the Na+ RE % varied between 46.4 and 54.3; analogously, SAR index was significantly reduced up to 13 points. SAR and CROSS indexes resulted better correlated in SS rather than NZSS due to the influence of K+ released by NZ.
Another important aspect to take into consideration is the use of pre-exchanged zeolitites as amendment. Leyva-Ramos et al. (2010) showed that the exchange capacity (concerning NH4+, a monovalent cation similar to Na+) could be up to 40% higher in Na-exchanged chabazite rather than natural chabazite. However, since we decided to test a natural material (without any pre-treatment), future experiments may consider the use of pre-exchanged zeolites in order to increase the RE %. In this respect, the use of a NH4+-enriched NZ (e.g., pre-saturated with NH4+ deriving from zootechnical effluents) may result in a valuable alternative to the natural material since the adsorption capacity should be increased and important nutrients (e.g., NH4+) can be released in the system after Na+ adsorption.
Abbreviations
- BD:
-
Bulk density
- BW:
-
Brackish water
- BWa:
-
Brackish water having EC = 5 mS cm−1
- BWb:
-
Brackish water having EC = 10 mS cm−1
- BWc:
-
Brackish water having EC = 18 mS cm−1
- CEC:
-
Cation exchange capacity
- Ce :
-
Ions concentration at equilibrium
- C0 :
-
Ions concentration in the filling solution
- CROSS:
-
Cations ratio of soil structural stability
- EC:
-
Electrical conductivity
- NZ:
-
Natural zeolitite
- NZSS:
-
Sandy soil + 10 wt% of natural zeolitite
- OM:
-
Organic matter
- PV:
-
Pore volume
- Qe :
-
Amount of ions retained/released by the exchanger solid phase
- RE %:
-
Removal efficiency (%)
- SAR:
-
Sodium adsorption ratio
- SS:
-
Sandy soil
- TDS:
-
Total dissolved salts
References
Agassi, M., Shainberg, I., & Morin, J. (1981). Effect of electrolyte concentration and soil sodicity on infiltration rate and crust formation. Soil Science Society of America Journal, 48, 848–851.
Ayers, R. S., Westcot, D. W. (1994). Water quality for agriculture. FAO irrigation and frainage paper, 29 Rev. 1. Food and Agriculture Organization of the United Nations Rome, 1985 © FAO. ISBN 92-5-102263-1.
Bear, J., Cheng, A. H. D., Sorek, S., Ouazar, D., & Herrera, I. (1999). In J. Bear, A. H. D. Cheng, S. Sorek, D. Ouazar, & I. Herrera (Eds.), Seawater intrusion in coastal aquifers: concepts, methods and practices (Vol. 14) (p. 627). Springer Science & Business Media.
Bouwer, H. (2002). Integrated water management for the 21st century: problems and solutions. Journal of Irrigation and Drainage Engineering, 128, 193–202.
Chen, Y., Banin, A., & Borochovitch, A. (1983). Effect of potassium on soil structure in relation to hydraulic conductivity. In E. B. A. Bisdom & J. Ducloux (Eds.), Submicroscopic studies of soils (pp. 135–147). Amsterdam: Elsevier.
Colombani, N., & Mastrocicco, M. (2017). Modelling the salinization of a coastal lagoon-aquifer system. IOP Conference Series: Earth and Environmental Science, 82(1), 012003.
Colombani, N., Mastrocicco, M., Di Giuseppe, D., Faccini, B., & Coltorti, M. (2015). Batch and column experiments on nutrient leaching in soils amended with Italian natural zeolitites. Catena, 127, 64–71.
Cramer, G. R., Läuchli, A., & Polito, V. S. (1985). Displacement of Ca2+ by Na+ from the plasmalemma of root cells. A primary response to salt stress? Plant Physiology, 79, 207–211.
Di Giuseppe, D., Faccini, B., Mastrocicco, M., Colombani, N., & Coltorti, M. (2014). Reclamation influence and background geochemistry of neutral saline soils in the Po River Delta plain (northern Italy). Environment and Earth Science, 72, 2457–2473.
El Swaify, S. A., Ahmed, S., & Swindale, L. D. (1970). Effects of adsorbed cations on physical properties of tropical red and tropical black earths. Journal of Soil Science, 21, 188–198.
Eslami, M., Khorassani, R., Coltorti, M., Malferrari, D., Faccini, B., Ferretti, G., Di Giuseppe, D., Fotovat, A., Halajnia, A. (2018). Leaching behaviour of a sandy soil amended with natural and NH4 + and K+ saturated clinoptilolite and chabazite. Archives of Agronomy and Soil Science, 64, 1142–1151.
Faccini, B., Di Giuseppe, D., Ferretti, G., Coltorti, M., Colombani, N., & Mastrocicco, M. (2018). Natural and NH4 +-enriched zeolitite amendment effects on nitrate leaching from a reclaimed agricultural soil (Ferrara Province, Italy). Nutrient Cycling in Agroecosystems, 110, 327–341.
Ferguson, G., & Gleeson, T. (2012). Vulnerability of coastal aquifers to groundwater use and climate change. Nature Climate Change, 2, 342–345.
Ferretti, G., Di Giuseppe, D., Natali, C., Faccini, B., Bianchini, G., & Coltorti, M. (2017a). C-N elemental and isotopic investigation in agricultural soils: insights on the effects of zeolitite amendments. Chemie der Erde-Geochemistry, 77(1), 45–52.
Ferretti, G., Keiblinger, M. K., Zimmermann, M., Di Giuseppe, D., Faccinni, B., Colombani, N., Mentler, A., Zechmeister-Boltenstern, S., Coltorti, M., & Mastrocicco, M. (2017b). High resolution short-term investigation of soil CO2, N2O, NOx and NH3 emissions after different chabazite zeolite amendments. Applied Soil Ecology, 119, 138–144.
Ferretti, G., Keiblinger, M. K., Di Giuseppe, D., Faccini, B., Colombani, N., Zechmeister-Boltenstern, S., Coltorti, M., & Mastrocicco, M. (2018). Short-term response of soil microbial biomass to different chabazite zeolite amendments. Pedosphere, 28, 277–287.
Galli, E., & Passaglia, E. (2011). Natural zeolites in environmental engineering. In H. Holzapfel (Ed.), Zeolites in chemical engineering (pp. 392–416). Vienna: Process Eng EngineeringGmbH.
Ghaly, A. E., Verma, M. (2008). Desalination of saline sludges using ion-exchange column with zeolite. American Journal of Environmental Sciences, 4(4), 388–396.
Ghiglieri, G., Carletti, A., & Pittalis, D. (2012). Analysis of salinization processes in the coastal carbonate aquifer of Porto Torres (NW Sardinia, Italy). Journal of Hydrology, 432(43–51), 432–433.
Gottardi, G., & Obradovic, J. (1978). Sedimentary zeolites in Europe. Fortschritte der Mineralogie, 56, 316–366.
Han, D., Kohfahl, C., Song, X., Xiao, G., & Yang, J. (2011). Geochemical and isotopic evidence for palaeo-seawater intrusion into the south coast aquifer of Laizhou Bay, China. Applied Geochemistry, 26, 863–883.
Hanson, B., Grattan, S. R., & Fulton, A. (1999). Agricultural salinity and drainage. Davis: University of California Irrigation Program. University of California.
Huang, H., Xiao, X., Yan, B., & Yang, L. (2010). Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. Journal of Hazardous Materials, 175, 247–252.
IUSS Working Group WRB (2007). World Reference Base for soil resources 2006, First update 2007. World Soil Resources Reports. (FAO publication, no. 103).
Leyva-Ramos, R., Monsivais-Rocha, J. E., Aragon-Piña, A., Berber-Mendoza, M. S., Guerrero-Coronado, R. M., Alonso-Davila, P., & Mendoza-Barron, J. (2010). Removal of ammonium from aqueous solution by ion exchange on natural and modified Chabazite. Journal of Environmental Management, 91, 2662–2668.
Malferrari, D., Laurora, A., Brigatti, F., Coltorti, M., Di Giuseppe, D., Faccini, B., & Vezzalini, M. (2013). Open-field experimentation of an innovative and integrated zeolitite cycle: project definition and material characterization. Rend Fis acc Lincei, 24, 141–150.
Marcum, K. B. (2006). Use of saline and non-potable water in the turfgrass industry: constraints and developments. Agricultural Water Management, 80, 132–146.
Matschonat, G., & Vogt, R. (1997). Effects of changes in pH, ionic strength, and sulphate concentration on the CEC of temperate acid forest soils. European Journal of Soil Science, 48, 163–171.
Miller, R. W., & Donahue, R. L. (1995). Soils in our environment, Seventh Edition (p. 323). Englewood Cliffs: Prudence Hall.
Misaelides, P. (2011). Application of natural zeolites in environmental remediation: a short review. Microporous and Mesoporous Materials, 144, 15–18.
Mumpton, A. (1999). La roca magica: uses of natural zeolites in agriculture and industry. PNAS, 96, 3463–3470.
Passaglia, E. (2008). Zeoliti naturali, zeolititi e loro applicazioni. Padova: Arvan (in Italian).
Passaglia, E., Poppi, S., Azzolini, P., & Gualtieri, A. F. (2005). Reduction of the Na content of irrigation waters using chabazite rich tuff. Studies in Surface Science and Catalysis, 158, 2097–2104.
Pond, W. G., & Mumpton, F. A. (1984). Zeo-agriculture: use of natural zeolite in agriculture. Boulder: Westview Press.
Postel, S. L., Daily, G. C., & Ehrlich, P. R. (1996). Human appropriation of renewable fresh water. Science, 271, 785–788.
Reháková, M., Čuvanová, S., Dzivák, M., Rimár, J., & Gaval’ová, Z. (2004). Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Current Opinion in Solid State and Materials Science, 8, 397–404.
Rengasamy, P., & Marchuk, A. (2011). Cation ratio of soil structural stability (CROSS). Australian Journal of Soil Research, 49, 280–285.
Sola, F., Vallejos, A., Daniele, L., & Pulido-Bosch, A. (2014). Identification of a Holocene aquifer–lagoon system using hydrogeochemical data. Quat. Res, 82(1), 121–131.
Stefani, M., & Vincenzi, S. (2005). The interplay of eustasy, climate and human activity in the late Quaternary depositional evolution and sedimentary architecture of the Po Delta system. Marine Geology, 222, 19–48.
Taiz, L., & Zeiger, E. (2007). Plant physiology. Spektrum Akademischer Verlag.
Tiessen, H., & Moir, J. O. (1993). Total organic carbon. In M. E. Cater (Ed.), Soil sampling and methods analysis (pp. 187–200). Ann Arbor: Lewis Publisher.
Tilman, D., Cassman, K. G., Matson, P. A., Naylor, R., & Polasky, S. (2002). Agricultural sustainability and intensive production practices. Nature, 418(6898), 671–677.
Torracca, E., Galli, P., Pansini, M., & Colella, C. (1998). Cation exchange reaction of a sedimentary chabazite. Microporous and Mesoporous Materials, 20, 119–127.
Wang, Y. F., Ling, F., & Pang, W. Q. (2007). Ammonium exchange in aqueous solution using Chinese natural clinoptilolite and modified zeolite. Journal of Hazardous Material, 142, 160–164.
Werner, A. D., Bakker, M., Post, V. E. A., Vandenbohede, A., Lu, C., Ataie-Ashtiani, B., Simmons, C. T., & Barry, D. A. (2013). Seawater intrusion processes, investigation and management: recent advances and future challenges. Adv. Water Resour, 51, 3–26.
Acknowledgements
The authors are thankful to Renzo Tassinari for laboratory analysis.
Funding
This study has been funded by Bando 2012 per progetti di ricerca finanziati con il contributo della Camera di Commercio, Industria, Artigianato e Agricoltura” grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferretti, G., Di Giuseppe, D., Faccini, B. et al. Mitigation of sodium risk in a sandy agricultural soil by the use of natural zeolites. Environ Monit Assess 190, 646 (2018). https://doi.org/10.1007/s10661-018-7027-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-7027-2