Abstract
The natural estrogen 17β-estradiol (E2) is a major endocrine disruptor, with adverse effects on wildlife and humans. The aim of this study was to isolate microorganisms able to effectively remove E2 from wastewater. Accordingly, five E2-degrading strains of bacteria were isolated from activated sludge collected from a wastewater treatment plant. Based on their 16S RNA gene sequences, these five strains belonged to the genus Bacillus. All five isolates were capable of converting E2 to estrone (E1), greatly reducing total estrogenic activities in wastewater during E2 biodegradation. However, only two strains (strain E2Y1 and E2Y4) were able to further transform E1, whereas it accumulated in the culture medium of the other isolates. Among all isolates, strain E2Y4, with 100% of the 1,400 bp 16S RNA gene matched that of B. subtilis CICC10075, exhibited the highest E2 and E1 degradation capacities, degrading 1 mg E2/l completely within 4 days and further transforming 40% of the metabolite E1. Furthermore, the E2 degradation rates of strain E2Y4 increased with increasing initial concentrations of the steroid, with a high degradation capacity maintained even at initial concentrations up to 50 mg/l. These results demonstrate the potential significance of strain E2Y4 in biological remediation applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endocrine disrupting compounds (EDCs), including estrogens, have received worldwide attention due to their negative effects on the endocrine systems of animals and humans (Falconer et al. 2006; Liu et al. 2009). The prominent EDCs of interest can be separated into two main groups, estrogens and alkyl phenols. The former can be subdivided into natural (including estrone, 17β-estradiol, and estriol; E1, E2, and E3, respectively) and synthetic (17α-ethynylestradiol, or EE2) estrogens, both of which have been reported to be the main sources of estrogen activity in treated wastewater (Sóle et al. 2000; Johnson and Williams 2004). Estrogens are excreted daily in urine and feces by wildlife, humans, and animals and thus accumulate in domestic sewage. However, they are only partially removed during domestic wastewater treatment such that they are continuously discharged into receiving bodies (Ternes et al. 1999; Kolodiej et al. 2003). Maximum measured levels of E2 are 150 ng/l in domestic wastewater (Vethaak et al. 2002) and 64 ng/l in domestic wastewater treatment plant effluent (Ternes et al. 1999). E2, the most important contributor, can cause reproductive disorders in aquatic organisms at concentrations ranging from 0.1 to 1 ng/l (Purdom et al. 1994). Hence, the removal of E2 from wastewater is a problem that very urgently needs to be solved.
Several studies characterized the estrogen degradation potential of various isolated bacterial strains. As early as in 1991, Ojanotko-Harri et al. (1991) tested the ability of oral isolates of Streptococcus mutans, Streptococcus sanguis, Bacillus cereus and Candida albicans to metabolize E2 by thin-layer chromatography using 4-14C-E2 as the substrate, and found that all test microorganisms could metabolize E2, and E1 was the main metabolic product, except for B. cereus. Recently, in studies examining estrogen removal via biodegradation during wastewater treatment (Andersen et al. 2003; Joss et al. 2004), microorganisms capable of E2 transformation have been identified. Fujii et al. (2002) isolated the E2-degrading bacterium (Novosphingobium tardaugens) and found that no toxic products were accumulated in the culture medium during the biodegradation process, as determined by gas chromatography-mass spectrometry (GC/MS) and 1H nuclear magnetic resonance analysis. Yoshimoto et al. (2004) isolated four strains of Rhodococcus, which each biodegraded 100 mg/l E2 into substances without estrogen activity with human-breast-cancer-derived MVLN cells measurement. In addition, 14 phylogenetically diverse E2-degrading bacteria, distributed among eight different genera (Aminobacter, Brevundimonas, Escherichia, Flavobacterium, Microbacterium, Nocardioides, Rhodococcus, and Sphingomonas) and three phyla (Proteobacteria, Actinobacteria, and Bacteroidetes), were isolated by Yu et al. (2007).
In the present study, five 17β-estradiol-degrading Bacillus spp. strains were isolated from activated sludge obtained from a wastewater treatment plant associated with a steroid-contraceptives manufacturing factory. Furthermore, the E2- and E1- degrading abilities of these Bacillus spp. strains were characterized.
Materials and methods
Chemicals
E2 (>99% pure) and E1 (>99% pure) were obtained from Sigma–Aldrich. A mixed working solution containing these two compounds at a concentration of 10 mg/l was prepared by diluting the individual solutions of 1,000 mg/l in methanol. An internal standard, 17β-estradiol-d4, purchased from Cambridge Isotope Laboratories (USA), was used for quantification purposes. All the organic solvents used, including methanol, ethyl acetate, acetone, and hexane, also purchased from Sigma–Aldrich, were of distilled-in-glass grade. BSTFA containing 1% trimethylchlorosilane (TMCS) was supplied by Sigma–Aldrich. The other chemicals and purification materials were obtained from Pharmacia Fine Chemicals.
Enrichment culture of 17β-estradiol-degrading strains
Activated sludge, collected from the wastewater treatment plant of a factory producing oral contraceptives, located in Zhejiang, China, was used as an inoculum. Enrichment culture was performed in a rotary shaker (150 rpm) at 28°C, with the inoculant, consisting of 15 ml of seed sludge, added to sterile medium (50 ml in 250-ml conical flasks) containing E2. The inoculum was grown by successive batch cycles, and the E2 concentration in each batch cycle was increased stepwise from 0.1 to 1 mg/l. In the first cycle, complete degradation of E2 occurred within 5 days. Hence, a batch cycle of the enrichment culture was defined as having a duration of 5 days.
Modified Dominic and Graham’s (MDG) culture medium (Yoshimoto et al. 2004) was used for bacterial enrichment and cultivation. The pH of the MDG medium was adjusted to 7.0. Acetone-free MDG-estrogen medium was prepared as follows: a defined amount of estrogen stock solution was added to the MDG medium, which was then heated to 80°C (the boiling point of acetone is 59°C), purged with air at a flow rate of 120 ml/min for 30 min to remove the acetone, and then cooled to room temperature before use.
Isolation of 17β-estradiol-degrading strains
Enrichment cultures were obtained over a period of 5 months, after which 1 ml of the culture was removed and serial 10-fold dilutions were prepared in physiological saline. Samples of 0.1 ml containing the 101–109 dilutions were plated on MDG agar plates containing 1 mg E2/l. The plates were incubated at 28°C for 5 days. After numerous streakings, morphologically distinct colonies were selected and tested for their ability to degrade E2.
The strains thus obtained were inoculated into 10 ml of MDG medium containing 50 or 0 mg E2/l and shake-incubated. Cultures in which the turbidity (absorbance at 620 nm) increased were selected, while strains that grew in the absence (0 mg E2/l) of estrogen were omitted. The selected strains were then tested for their ability to degrade E2 and E1.
Strain identification
The strains were identified based on their 16S RNA gene sequences. DNA of these five isolates was extracted using a 3S DNA Isolation Kit for Environmental Samples V2.2 (Shanghai Biocolor BioScience & Technology company, China) according to manufacturer’s instructions. The 16S RNA gene was amplified using the forward primer BSF8/20 (5′-AGAGTTTGATCCTGGCTCAG-3′) and the reverse primer BSR1541/20 (5′-AAGGAGGTGATCCAGCCGCA-3′) (Xia et al. 2005) in a thermal cycler (Bio-rad, USA) under the following conditions: 94°C for 4 min, 35 cycles of 60 s at 94°C,60 s at 59°C, 90 s at 72°C, and a final step of 10 min at 72°C, 10 min at 4°C. The PCR products were extracted and purified from the agarose gel using the High-Pure PCR product purification kit (Bio-Rad, USA). The amplified fragment was sequenced by Gene core Biotech Corp. (Shanghai, China). The Blast procedure was used to search sequence homology.
Estrogen degradation tests
The first degradation experiment was conducted to examine whether isolates were capable of: (1) degrading E2 and E1 and (2) converting E2 to non-estrogenic compounds. E2 and E1 were used as the target compounds. The tests were carried out in a series of 250-ml vials containing 50 ml of MDG medium containing 1 mg E2 or E1/l. All isolates were pregrown for 24 h in R2A medium containing 1 mg E2/l before they were harvested by centrifugation for experimental use. The harvested cells were washed with 10 mM phosphate buffered saline and then resuspended in acetone-free MDG-estrogen growth medium to an optical density (OD600) of 1. A predetermined volume of culture was added to obtain an OD600 of 0.1. The vials were then incubated on a rotary shaker at 150 rpm at 30°C. Culture samples removed over time were analyzed for E2 or E1 concentrations by GC/MS analysis and for estrogenic activities by yeast estrogenic screening (YES) assays during the E2 degradation process. All degradation tests were performed in duplicate.
The aim of the second degradation experiment was to examine the degradation kinetics of the selected strain, identified based on its ability to degrade both E2 and E1, by varying the initial E2 concentration from 0.5 to 50 mg/l. The bacterial strain was pre-treated as above and then diluted to yield an optical density (OD600) of around 0.1. The culture vials were incubated on a rotary shaker at 150 rpm at 30°C for a period of 7 days, and the concentration of E2 examined at defined intervals during the incubation.
Estrogen analysis
Bacterial cells were separated from the culture medium by centrifugation at 5,000 g. Estrogens in the supernatant were extracted with methylene chloride, derivatized, and then analyzed by GC/MS (Zhang et al. 2006). Briefly, the extracted liquid samples were spiked with 17β-estradiol-d2 and then derivatized with pyridine and BSTFA. The derivatized samples were injected into a Hewlett-Packard (HP) 6,890 gas chromatograph equipped with a HP 5-MS column (30 m × 0.25 mm i.d.; 0.25-μm film thickness) and an HP 5,975 mass selective detector (MSD) system. Helium carrier gas was maintained at a constant flow rate of 1.0 ml/min in GC. MS was operated in full-scan or selected ion monitoring mode with positive ionization by electron impact. The inlet and MS transfer line temperatures were maintained at 280°C; the ion source temperature was 250°C. Two microliters of the sample was injected in splitless mode. To separate the compounds, the following GC column temperature program was used: 100°C for 1 min, increase to 200°C via a ramp of 10°C/min and to 300°C via a ramp of 3°C/min, and maintenance at 300°C for 10 min.
Determination of estrogenic activity using a recombinant yeast assay
The decrease in estrogenic activities during E2 degradation by the test strain was measured using a yeast two-hybrid system obtained from the Research Center for Eco-Environmental Sciences, Chinese Academy of Science. Measurements were made according to a previously described method (Gaido et al. 1997; Ma et al. 2005; Li et al. 2008).
Yeast strains were grown overnight at 30°C with vigorous shaking at 130 rpm. Each test chemical was serially diluted in dimethyl sulfoxide (DMSO). Five microliters of the serially diluted test sample was combined with 995 μl of medium containing 5 × 103 yeast cells/ml, resulting in a test culture. Two hundred microliters of each test culture was transferred into a well in a 96-well plate, which was subsequently incubated at 30°C with vigorous orbital shaking (800 rpm) for 2 h. The cell density of the culture was measured at a wavelength of 600 nm.
A 50-μl test culture was transferred to a new 96-well plate and 120 μl of Z-buffer (16.1 g/l Na2HPO4·7H2O; 5.5 g/l NaH2PO4·H2O; 0.75 g/l KCl; 0.246 g/l MgSO4·7H2O) and 20 μl chloroform were added; the samples were then carefully mixed (vortex 25 s). The enzymatic reaction was initiated by the addition of 40 μl of o-nitrophenyl-β-d-galactopyranoside (13.3 mmol/l, dissolved in Z-buffer) and the samples incubated at 30°C for 60 min. Following termination of the reactions by the addition of 100 μl Na2CO3 (1 mol/l), the samples were centrifuged at 12,000 g for 15 min, 200 μl of supernatant was transferred into a new 96-well plate, and the absorbance at 420 nm was determined. β-galactosidase activities were calculated according to the method of Ma et al. (2005) and expressed as the estrogenic activities of the E2 degradation solution produced by the isolates.
Results
Isolation and identification of E2-degrading bacteria
Five 17β-estradiol-degrading strains, designated E2Y1–E2Y5, were successfully isolated from the activated sludge by the direct isolation method. The 16S RNA gene sequences of these five strains were determined to identify the bacterial species. The 16S RNA gene sequences (about 1,400 bp) of E2Y1, E2Y2, E2Y3, E2Y4 and E2Y5 were identical to that of Bacillus GJ24, Bacillus amyloliquefaciens BCRC 11601, Bacillus AH-E-1, Bacillus subtilis CICC10075, and Bacillus cereus DS at similarity of >98%, respectively. Figure 1 shows the phylogenetic tree constructed by the neighbor-joining method for these five strains and closely related Bacillus spp.
Phylogenetic tree showing the relationship of 16S RNA gene sequences within E2Y1,E2Y2, E2Y3, E2Y4, E2Y5 and closely related Bacillus sp. The tree was constructed by neighbor-joining analysis based on 16S RNA gene sequences. Bootstrap confidence values obtained on 100 resamplings are given at the branch points. The bar indicates a genetic distance of 0.05
E2-degrading capacity of the isolated strains
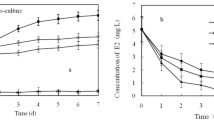
The E2-degrading capacities of the five E2-degrading strains were examined in 8-day cultures incubated in shake flasks containing E2 at an initial concentration of 1 mg/l. Residual E2 concentrations and E1 production were examined as well. Based on the extent of E2 degradation as well as the production of E1 and its further transformation, two degradation patterns (A and B) were identified and are described in the following.
Degradation pattern A
Isolates in this group were able to degrade both E2 and E1 within 8 days (Fig. 2). In strain E2Y4, E1 increased rapidly during E2 degradation, accumulating to 0.70 mg/l before declining. As E2 was degraded completely, E1 began to decline as well, with only 0.27 mg residual E1/l detected after 8 days. In strain E2Y1, the degradation pattern was the same. However, compared with the 0.43 mg E1/l degraded by strain E2Y4, only 0.22 mg E1/l (declining from 0.37 to 0.15 mg/l) was degraded. Based on these results, strains E2Y4 and E2Y1 produced E1 as a metabolite during E2 transformation and both strains were able to degrade E1.
Degradation pattern B
Unlike the isolates behaving according to degradation pattern A, isolates in this group were capable of degrading E2 but not E1 within 8 days (Fig. 3). The transformation of E2 to E1 occurred within 4 (strain E2Y2), 5 (strain E2Y3), and 6 (strain E3Y5) days. However, as the concentrations of E2 decreased, the concentration of E1 increased such that it accumulated over the 8-day test period, reaching approximately 0.46, 0.67, and 0.33 mg E1/l following degradation by strain E2Y2, E2Y3, and E2Y5, respectively.
E1 degradation by the isolated strains
The ability of the isolated strains to degrade E1 was further examined through E1 degradation tests. Figure 4 shows time (9-day)-dependent variations in E1 concentration during batch-experiment cultivation, with an initial concentration of 1 mg E1/l. The E1-degrading capacities of the five isolated strains were consistent with the results obtained in the above-described 8-day E2 degradation tests. More than 40 and 20% of the E1 was degraded by strains E2Y4 and E2Y1, respectively, within 9 days. By contrast, no E1 was degraded by strains E2Y2, E2Y3, and E2Y5.
Estrogenic activity of the E2 degraded by the isolated strains
An important aspect in evaluating bacterial degradation abilities was to determine whether the isolated strains transformed the estrogens to non-estrogenic compounds, resulting in the complete removal of estrogenic activity. Figure 5 shows the estrogenic activities remaining after the degradation of 1 mg E2/l by culture solutions of the five isolated strains after 1, 6, and 15 days. The five strains decreased the estrogenic activities in the culture solution to 1/15 ~ 1/10 on day 1 and to 1/36 ~ 1/18 on day 15, as expressed as a fraction of the initial activity. Since the estrogenic activity of E1 is about 38–50% of that of E2, according to YES assays (Johnson and Sumpter 2001; Aerni et al. 2004), the transformation by the isolates of E2 to E1 has the potential to reduce the estrogen activity of the test solution. Furthermore, the decrease in estrogenic activities obtained with strains E2Y4 and E2Y1 was most likely due to their ability to degrade both E2 and E1.
Degradation kinetics of strain E2Y4
The above results indicated that strain E2Y4 rapidly degraded E2 and E1 into non-estrogenic compounds. Therefore, this strain was selected to examine E2 degradation kinetics. The degradation rates for strain E2Y4 in the exponential growth phase were measured at E2 concentrations of 0.5–50 mg/l (Fig. 6). E2 degradation by strain E2Y4 was achieved at concentrations up to 50 mg/l. The relationship between the different initial substrate concentrations and the initial transformation rates was therefore determined to be linear (R2 > 0.99).
Discussion
In this study, five bacterial strains were isolated from activated sludge based on their ability to effectively degrade E2. All five strains were shown to belong to the genus Bacillus, and none were similar to previously reported estrogen-degrading strains also isolated from activated sludge: one strain of Novosphingobium (Fujii et al. 2002), one strain of Achromobacter, one strain of Ralstonia (Weber et al. 2005), and four strains of Rhodococcus (Yoshimoto et al. 2004), and 14 phylogenetically diverse E2-degrading bacteria (Aminobacter, Brevundimonas, Flavobacterium, Nocardioides, and Sphingomonas) (Yu et al. 2007). In two other studies, high concentrations of E2 (0.1% w/v and 3 mg/l) were used for enrichment and isolation; likewise, a high E2 concentration (1 mg/l) was chosen in our study. The five strains thus obtained were able to degrade E2 completely in far higher concentrations than those were present either in the influent or in the treated wastewater of the wastewater treatment plant. Further studies are required to determine if these isolates will degrade E2 at environmentally relevant concentrations.
The results of the present study are consistent with previous reports that E2 is highly susceptible to biodegradation, with the transformation to E1 as the first step (Yoshimoto et al. 2004; Yu et al. 2007; Pauwels et al. 2008). In this study, all five isolated strains converted E2 to E1 under toxic conditions. E1 was degraded by only two strains (strains E2Y1 and E2Y4), whereas it accumulated in cultures of the other three strains. The transformation of E2 and E1 can be explained by the mechanisms proposed by Yu et al. (2007). The ability to oxidize the secondary alcohol on the C17 position of E2 to a ketone might be a common feature among these five isolates. Furthermore, the degradation of E1, which was the major metabolite of E2 biodegradation, might be the rate-limiting step for the conversion of E2 to non-estrogenic metabolites or end products. These mechanisms were supported by many field studies involving wastewater treatment (Johnson and Sumpter 2001, Baronti et al. 2000), in which good removal of E2 was obtained whereas E1 removal was not satisfactory. Since E1 still has relatively high estrogenic potency, its removal from wastewater is important in order to achieve an overall reduction in total estrogenic activity (Aerni et al. 2004). The two strains, especially strain E2Y4, exhibited high E2- and E1-degrading capacities and thus hold great promise for conditions requiring the complete mineralization of estrogen.
According to the analysis of genetic specificity, strain E2Y4 belongs to B. subtilis. This strain rapidly (<4 days) degraded 1 mg E2/l with a 100% ratio. Although the degradation ability of strain E2Y4 was less than that of Sphingomonas sp. (Yu et al. 2007), its E2 degradation rates increased with increasing initial concentrations, and still showed a high E2 degradation capacity at initial E2 concentrations of up to 50 mg/l. Moreover, the practical significance of strain E2Y4 lies in its ability to achieve maximum E1 degradation rates of 40%. Quan et al. (2005) reported that a B. subtilis strain isolated from soil rapidly degraded di-2-ethylhexyl phthalate (DEHP, an EDC). Hence, it may be that B. subtilis species possess an enzyme system to degrade EDCs and thus to eliminate these compounds as pollutants. Studies examining the ability of B. subtilis strains to degrade other estrogenic compounds in wastewater, the pathways and enzymes by which they do so, and the degradation kinetics of estrogen degradation are needed before strain E2Y4 and similar strains can be fully exploited.
References
Aerni HR, Kobler B, Rutishauser BV, Wettstein FE, Fischer R, Giger W, Hungerbuehler A, Marazuela MD, Peter A, Schoenenberger R, Voegeli AC, Suter MJF, Eggen RIL (2004) Combined biological and chemical assessment of estrogenic activities in wastewater treatment plant effluents. Anal Bioanal Chem 378:688–696
Andersen H, Siegrist HR, Halling-sørensen B, Ternes TA (2003) Fate of estrogens in a municipal sewage treatment plant. Environ Sci Technol 37:4021–4026
Baronti C, Curini R, D′Ascenzo G, Di Corcia A, Gentili A, Samperi R (2000) Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environ Sci Technol 34:5059–5066
Falconer IR, Chapman HF, Moore MR, Ranmuthugala G (2006) Edocrine-disrupting compounds: a review of their challenge to sustainable and safe water supply and water reuse. Environ Toxicol 21:181–191
Fujii K, Kikuchi S, Satomi M, Ushio-Sata N, Morita N (2002) Degradation of 17β-estradiol by a gram-negative bacterium isolated from activated sludge in a sewage treatment plant in Tokyo, Japan. Appl Environ Microbiol 68:2057–2060
Gaido KW, Leonard LS, Lovell S, Gould JC, Babai D, Portier CJ (1997) Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxcol Appl Pharmacol 143:205–212
Johnson AC, Sumpter JP (2001) Removal of endocrine-disrupting chemicals in activated sludge treatment works. Environ Sci Technol 35:4697–4703
Johnson AC, Williams RJ (2004) A model to estimate influent and effluent concentrations of estradiol, estrone and ethinylestradiol at sewage treatment works. Environ Sci Technol 38:3649–3658
Joss A, Andersen H, Ternes TA, Richle PR, Siegrist H (2004) Removal of estrogens in municipal wastewater treatment under aerobic and anaerobic conditions: consequences for plant optimization. Environ Sci Technol 38:3047–3055
Kolodiej EP, Gray JL, Sedlak DL (2003) Quantification of steroid hormones with pheromonal properties in municipal wastewater effluent. Environ Toxicol Chem 22:2622–2629
Li J, Li N, Ma M, Giesy JP, Wang ZJ (2008) In vitro profiling of the endocrine disrupting potency of organochlorine pesticide. Toxicol Lett 183:65–71
Liu ZH, Kanjo Y, Mizutani S (2009) Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment—physical means, biodegradation, and chemical advanced oxidation: a review. Sci Total Environ 407:731–748
Ma M, Li J, Wang ZJ (2005) Assessing the detoxication efficiencies of wastewater treatment processes using a battery of bioassays/biomarkers. Arch Environ Contam Toxicol 49:480–487
Ojanotko-Harri A, Laine M, Tenovuo J (1991) Metabolism of 17 beta-estradiol by oral Streptococcus mutans, Streptococcus sanguis, Bacillus cereus and Candida albicans. Oral Microbiol Immunol 6:126–128
Pauwels B, Wille K, Noppe H, De Brabander H, Van de Wiele T, Verstraete W, Boon N (2008) 17α-ethinylestradiol cometabolism by bacteria degrading estrone, 17β-estradiol and estriol. Biodegradation 19:683–693
Purdom CE, Hardiman PA, Bye VJ, Eno NC, Tyler CR, Sumpter JP (1994) Estrogenic effects of effluents from sewage treatment works. Chem Ecol 8:275–285
Quan CS, Liu Q, Tian WJ, Kikuchi J, Fan SD (2005) Biodegradation of an endocrine-disrupting chemical, di-2-ethylhexyl phthalate, by Bacillus subtilis No. 66. Appl Microbiol Biotech 66:702–710
Sóle M, Lopez de Alda MJ, Castillo M, Porte C, Ladegaard-Pedersen K, Barcelo D (2000) Estrogenicity determination in sewage treatment plants and surface waters from the Catalonian area (NE Spain). Environ Sci Technol 34:5076–5083
Ternes TA, Stumph M, Mueller J, Haberer H, Wilken RD, Servos M (1999) Behavior and occurrence of estrogens in municipal sewage treatment plants—I. Investigations in Germany, Canada and Brazil. Sci Total Environ 225:81–90
Vethaak AD, Lahr J, Kuiper RV (2002) Estrogenic effects in fish in the Netherlands: some preliminary results. Toxicology 181:147–150
Weber S, Leuschner P, Kampfer P, Dott W, Hollender J (2005) Degradation of estradiol and ethinyl estradiol by activated sludge and by activated sludge and by a defined mixed culture. Appl Environ Microbiol 67:106–112
Xia Y, Min H, Rao G, Lv ZM, Liu J, Ye YF, Duan XJ (2005) Isolation and characterization of phenanthrene-degrading Sphingomonas paucimobilis strain ZX4. Biodegradation 16:393–402
Yoshimoto T, Nagai F, Fujimoto J, Watanabe K, Mizukoshi H, Makino T, Kimura K, Saíno H, Sawada H, Omura H (2004) Degradation of estrogen by Rhodococcus zopfii and Rhodococcus equi isolates from activated sludge in wastewater treatment plants. Appl Environ Microbiol 70:5283–5289
Yu CP, Roh H, Chu KH (2007) 17β-estradiol-degrading bacteria isolated from activated sludge. Environ Sci Technol 41:486–492
Zhang ZL, Hibberd A, Zhou JZ (2006) Optimisation of derivatisation for the analysis of estrogenic compounds in water by solid-phase extraction gas chromatography-mass spectrometry. Anal Chem Acta 577:52–61
Acknowledgments
This work was funded by the Natural Science Foundation of Zhejiang Province (Grant No. Y506237), and Analysis and Testing Foundation from Science and Technology Department of Zhejiang Province (Grant No. 2008F70014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, L., Yang, J. & Chen, J. Isolation and characteristics of 17β-estradiol-degrading Bacillus spp. strains from activated sludge. Biodegradation 21, 729–736 (2010). https://doi.org/10.1007/s10532-010-9338-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-010-9338-z