Abstract
We investigated the effects of exposure to molluscicidal metaldehyde (MET) on golden apple snail (GAS) Pomacea canaliculata and Rhinella arenarum tadpoles by assessing mortality and/or other effects via: acute toxicity assays; B-esterase activities (acetilcholinesterase (AChE) and carboxilesterase (CbE)) and oxidative responses (glutathione-S-transferase (GST) and catalase (CAT)). The effect of sublethal concentrations of MET was also analysed by assessing biochemical changes and swimming parameters in tadpoles. The LC50 value in P. canaliculata was as 0.50 mg L−1 and in R. arenarun tadpoles, 229.7 mg L−1 at 48 h. The intestine of MET-exposed P. canaliculata exhibited a significant reduction in CbE and CAT activities, but not in AChE activity; hepatopancreas of GAS showed a decreased GST activity decreased with respect to control individuals. In addition, a significant reduction of CbE activities was detected in R. arenarum tadpoles exposed to MET, and AChE presented lower values than the control but without statistical differences. Antioxidant enzymes (GST and CAT) were significantly reduced in tadpoles exposed to MET compared with the control group. In addition, MET had a significant effect on the swimming behaviour of R. arenarum. Finally, since amphibian tadpoles and P. canaliculata often co-occur, other native amphibian species should be studied to elucidate the ecological risk of MET to amphibian populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The golden apple snail (GAS) Pomacea canaliculata (Lamarck) is a polyphagous and voracious Neotropical freshwater gastropod native to South America. Due to its rapid reproduction, growth and spread (Lach et al. 2000; Boland et al. 2008), the species has become one of the greatest pests in rice-producing countries (Cowie 2002) and one of the 100 most harmful invasive species worldwide (Hayes et al. 2008). This species feeds voraciously on freshwater vegetation, especial rice shoots (Carlsson et al. 2004), and finds in this type of crop suitable conditions for development, mainly due to the high concentrations of nutrients and phytoplankton biomass (Carlsson et al. 2004). The most common method to control this snail has been the use of molluscicides (Joshi et al. 2002), but these are very toxic to non-target organisms and destroy the ecosystem (Wu et al. 2010). One of the most commonly used molluscicides is metaldehyde (Henderson and Triebskorn 2002). The modes of action of this pesticide in snail is well documented, with metaldehyde inducing severe alterations and ultrastructural destruction in mucocytes, leading to dehydration and subsequent death (Triebskorn et al. 1998). Amphibian tadpoles and GAS coexist naturally in agroecosystems, particularly in rice fields (Bambaradeniya et al. 2004). Molluscicides with novel modes of action are extensively used in these cropping systems throughout the world, but few studies have examined their ecotoxicological impacts on amphibians. In the last years, toxicological studies of amphibians as non-target organisms have increased (Sánchez-Bayo 2012), principally because insecticides are one of the main causes of global decline of this animal group (e.g. Sparling and Fellers 2009; Mann et al. 2009). Those studies have identified enzyme activities as important early signals indicating adverse pesticide effects (Ossana et al. 2013; Attademo et al. 2014a, b). Accordingly, B-type esterases participate in the detoxification of different pesticides (Wheelock et al. 2008) and can be used as biomarkers to identify risks from pesticides in amphibian species (Sparling and Fellers 2009; Ferrari et al. 2011; Attademo et al. 2015). Aldridge (1953) classified these hydrolase enzymes as A- and B-esterases, and within the B-esterases, he determined cholinesterases (ChEs) and carboxylesterases (CbEs). ChEs include acetylcholinesterase (AChE; EC 3.1.1.7) and butyrylcholinesterase (BChE; EC 3.1.1.8), whereas CbEs (EC 3.1.1.1) consist of multiple isozymes that vary with both tissue and organism and play significant roles in the metabolism and subsequent detoxification of many agrochemicals (Wheelock et al. 2008). Furthermore, given the potential damage of free radicals and hyperoxides, and the possible direct effect of several oxidants on cell components, the cell defense systems become very important (Halliwell and Gutteridge 2007). Accordingly, there is an antioxidant system composed of a group of enzymes and compounds of low molecular weight, such as glutathione-S-transferase (GST; EC 2.5.1.18) and catalase (CAT; EC 181.11.1.6) (Cnubben et al. 2001). Another indicator of pesticide damage to amphibians is swimming performance of tadpoles, which evidences sublethal stress associated with exposure to toxic chemicals; indeed, immediately after exposure to the contaminant, tadpoles show alterations in swimming activity (Almeida et al. 2010; Denoël et al. 2012). Some behavioural functions, such as foraging, predator avoidance, parental behaviour and migration, may have an indirect negative effect on population dynamics (Cohn and MacPhail 1996).
The aim of the present study was to determine the effects of exposure to molluscicidal metaldehyde (MET) on GAS (P. canaliculata) and Rhinella arenarum tadpoles by assessing mortality and/or effects via: acute toxicity assays, B-esterase (AChE and CbE) and oxidative responses (GST and CAT) activities. Attention has also been focused on the effect of sublethal concentrations of MET by assessing biochemical changes and swimming parameters in amphibian tadpoles. The obtained information may help to assess and characterise potential risks of the application of this insecticide, and therefore contribute with the mitigation of ecological effects on wild amphibian fauna.
2 Materials and Methods
2.1 Species Selection
2.1.1 GAS P. canaliculata
Adult specimens (length = 33. 45 ± 2.68 mm, weight = 16.52 ± 2.43 g) of P. canaliculata were manually collected from a semipermanent pond at the University Ecological Reserve in Santa Fe city (31° 38′ 26.0″ S–60° 40′ 20″ W Santa Fe province, Argentina) in November 2015. The collected animals were maintained in glass aquaria containing de-chlorinated tap water (DTW; pH 7.40 ± 0.05; conductivity, 165 ± 10.5 μmhos cm–1; dissolved oxygen concentration, 6.0 ± 1.5 mgL–1; and hardness, 54.8 mgL–1 of CaCO3) for acclimatisation to laboratory conditions (12/12-h light/dark cycle and 23 ± 1 °C) for at least 2 days before exposure to the insecticide.
2.1.2 Non-target Tadpole’s R. arenarum
Non-target tadpoles of R. arenarum were selected as model organisms in ecotoxicology (Lajmanovich et al. 2013). This common anuran has an extensive Neotropical distribution (Vaira et al. 2012) and is frequently found in wetlands, agricultural land and urban territories (Peltzer et al. 2006). Eggs were collected with dip net from temporary ponds in artificial wetlands (31° 39′ 52.90″ S–60° 42′ 50.20″ W, South Park Lake, Santa Fe, Santa Fe province, Argentina) in October 2015; these sites had not been treated with chemical or biological pesticides, as determined by the laws for wildlife protection. Eggs collection was authorised by the Ministerio de Aguas, Servicios Públicos y Medio Ambiente, Santa Fe province, Argentina. The eggs were transported to the laboratory and maintained in 10-L glass aquaria filled with DTW under natural photoperiod 12 L/12D (light/dark) at 23 ± 1 °C. The eggs were allowed to develop until tadpoles reached Gosner stage 31 (Gosner 1960). Tadpoles (total length = 28.60 ± 0.30 mm, weight = 0.090 ± 0.01 g) were fed boiled lettuce until the start of the experiment.
2.2 Molluscicidal Assay
Short-term static toxicity tests (48 h, Lajmanovich et al. 2013) were conducted using MET insecticide (20 % active ingredient (a.i.), metaldehyde 2,4,6,8-tetramethyl-1,3,5,7-tetraoxycyclo-octane), a commercial liquid formulation of Babosil Super produced by NINIVE SACIFIA (Argentina). Here, the pesticide was tested as a complex commercial mixture, the mode of application to crops. In addition, the contribution of other inert ingredients contained in formulations to amphibian pesticide toxicity has been demonstrated (e.g. Relyea and Jones 2009).
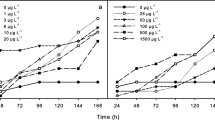
Glass aquaria (12.5 cm in diameter and 13.5 cm in height) containing 1 L of test solution were used in the acute experiments. Each aquarium was covered with a mosquito net to prevent GAS from escaping. Laboratory toxicity tests were conducted at 22 ± 2 °C at a 12/12-h light/dark cycle. Because of the lack of information in the literature about the effects of MET exposure on GAS and amphibians (particularly on native species), the first step was to assess the direct toxicity of the molluscicidal on GAS (P. canaliculata) and R. arenarum tadpoles. Range-finding toxicity tests consisted of exposing GAS and tadpoles to MET to estimate the median lethal concentration (LC50). The nominal concentrations used to test toxicity were: 0.25, 0.50, 1, 2, 4, 8 and 16 mg L−1 for GAS and 0.25, 0.50, 1, 2, 4, 8, 16, 32, 64, 128, 256 and 512 mg L−1 for tadpoles plus a negative control with DTW. Treatments were randomly assigned to the containers and to the sampling order. Both control and test suspensions were carried out in triplicate with six GAS and seven tadpoles per aquarium (n = 90 and 252, respectability). Mortality of GAS and tadpoles was recorded every 24 h, and cumulative mortality in each treatment was calculated at 48 h of exposure. Dead GAS and tadpoles were removed to avoid contamination of aquarium water during the experiment.
2.3 Enzyme Assays
For biomarker assessment, after the end of the experiments each snail (survival rate >85 % at 48 h) was dissected and digestive organs (intestine and hepatopancreas) were removed; these organs were found to be involved in pesticide detoxification (Attademo et al. 2014b). Snail tissues were washed in distilled water and placed on filter paper to remove excess fluids. To examine the vulnerability of tadpoles to MET, effect of exposure was assessed by evaluating changes in enzymatic level and swimming parameters at the sublethal concentrations tested in snails. The tested concentrations were also found at relevant concentrations in the environment (Lazartigues et al. 2012). It has been demonstrated that a chemical at relevant environmental concentrations does not necessarily cause mortality but may significantly disrupt biological functions (e.g. Weis and Candelmo 2012; Peltzer et al. 2013): in addition, these values (0.25 and 0.50 mg L−1) were considered useful in the risk assessment process to identify sublethal effects on non-target organisms in the environment (US EPA 1992). Individuals of both tested were euthanised according to ASIH et al. (2011) criteria and with approval from the animal ethics committee of the Faculty of Biochemistry and Biological Sciences. Whole tissues for GAS (intestine and hepatopancreas) and tadpoles were homogenised (on ice) in 20 % (w/v) buffer containing 0.1 % t-octylphenox-y polyethoxy ethanol (Triton X-100) in 25 mM tris-(hydroxymethyl) amino methane hydrochloride (pH 8.0) using a polytron. The homogenates were centrifuged at 10,000 rpm at 4 °C for 15 min, and the supernatant was collected and frozen at −80 °C until assayed for enzymatic determination.
2.4 B-esterase Response
AChE activity was determined colorimetrically following the procedure of Ellman et al. (1961). The reaction mixture (final volume (F.V.) = 930 μL)) consisted of 25 mM Tris-HCl containing 1 mM CaCl2 (pH = 7.6), 10 μL 20 mM acetylthiocholine iodide (AcSCh) and 50 μL DTNB (3 × 10−4 M, final concentration). Variation in optical density was measured in duplicate at 410 nm at 25 °C for 1 min using a Jenway 6405 UV-VIS spectrophotometer. Total protein concentrations in the supernatants were determined using the Biuret method (Kingbley 1942). AChE activity was expressed in nanomoles of hydrolysed substrate per minute per milligramme of protein using a molar extinction coefficient of 13.6 × 103 M−1 cm−1. CbE was determined using 1-naphthyl acetate (1-NA) substrate, and specific enzyme activity was expressed as nanomoles per minute per milligram of protein. The hydrolysis of 1-NA by CbE was measured as described by Gomori (1953) and adapted by Bunyan and Jennings (1968). The reaction medium (F.V. = 1950 μL) contained 25 mM Tris-HCl, 1 mM CaCl2 (pH = 7.6) and sample. The reaction was initiated by adding 50 μL α-naphthyl acetate (1.04 mg mL−1 in acetone) after a preincubation period of 5 min at 25 °C. The formation of naphthol was stopped after 10 min by adding 500 μL of 2.5 % sodium dodecyl sulphate and subsequently 0.1 % of Fast Red ITR in 2.5 % Triton X-100 in deioniser water (prepared immediately before use). The samples were allowed to develop in the dark for 30 min, and the absorbance of the complex was read at 530 nm (using a molar extinction coefficient of 33.225 × 103 M−1 cm−1).
2.5 Oxidative Response
GST activity was determined spectrophotometrically using the method described by Habig et al. (1974) and adapted by Habdous et al. (2002) for mammal serum GST activity. The enzyme assay was performed at 340 nm in 100 mM Na-phosphate buffer (pH 6.5) (F.V. = 920 μL), 20 μL of 0.2 mM 1-chloro-2,4-dinitrobenzene, 50 μL of 5 mM reduced glutathione and the sample. Enzyme kinetics assays were performed at 25 °C, and whole GST activity was expressed as micromoles per minute per milligram of protein using a molar extinction coefficient of 9.6 × 103 M−1 cm−1.
CAT activity was measured using the method described by Aebi (1984), and was expressed as micromoles H2O2 per minute per milligram of protein using a molar extinction coefficient of H2O2 40.10−3 L mol cm−1. The reaction medium was 50 mM phosphate buffer (pH = 7.2) and 30 mM H2O2 and absorbance was read on the spectrophotometer at a wavelength of 240 nm at 25 °C (quartz cuvette).
2.6 Analysis of Swimming Activity
Behavioural alterations can be measured as endpoints for sublethal MET toxicity. Swimming behaviour was immediately tested after exposure for 2 h. One larva was released at the centre of a Petri dish (15 cm diameter, 2 cm height) filled with 200 mL of DTW. After 30 s of acclimatisation, behavioural variables were recorded during 3 min using a digital video-camera (Motic®, 10.0 mega pixel) placed just above the dishes. Each tadpole was treated as an independent experimental unit (Van Buskirk and McCollum 2000), and ten replicates were performed for each MET concentration, including the control. Videos (avi format) were analysed automatically using Smart 3.0.02 (Panlab Harvard Apparatus). In our sep-up, the immobile threshold was 10 %, and below this value tadpoles were considered immobile. The following four behavioural endpoints were analysed: mean speed (cm s−1), total distance moved (cm), immobility (s) and global activity (cm2).
2.7 Statistical Analysis
Lethal concentration (LC50) values and their respective 95 % confidence limits (95 % CL) were determined using the Trimmed Spearman-Karber method (Hamilton et al. 1977). The criterion of non-overlapping 95 % confidence intervals (APHA 1989) was used to determine significant differences (P ≤ 0.05) between LC50 values. The data of enzymatic activity were expressed as means ± standard error (SEM). Mann-Whitney U was used to compare B-esterases and GST activities between control and exposed P. canaliculata. In tadpoles, Kruskal-Wallis and Dunn post hoc tests were used to compare enzymatic activity and swimming parameters between control and exposed animals (Zar 1999). Normality and homogeneity of variances were verified using the Kolmogorov-Smirnov test and the Levene test, respectively. Statistical analyses were performed using GraphPad InStad®. The criterion for significance was p ˂ 0.05.
3 Results
3.1 Acute Toxicity Tests
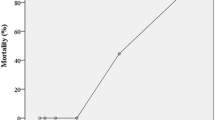
The calculated 48 h acute LC50 value (95 % CL) based on the Trimmed Spearman-Karber of MET on GAS was 0.50 mg L−1 (0.36–0.70) and on R. arenarum tadpoles, 229.7 mg L−1 (184.52–286.04). No mortality was observed in the control treatment in either experiment. The LC50 values were stabilised at 24 h of exposure.
3.2 B-esterase and Oxidative Stress Enzymes in Digestive Tissues in P. canaliculata
3.2.1 Hepatopancreas
The activities of AChE, CbE and CAT (mean ± SEM) in the liver of the control group were 6.08 ± 1.25 nmol min−1 mg−1 protein, 53.35 ± 4.66 nmol min−1 mg−1 protein and 47.11 ± 3.98 μmol min−1 mg−1 protein at 48 h, respectively. AChE, CbE and CAT activities did not vary significantly between treatments (Mann-Whitney U = 68; p ˃ 0.05; U = 69; p ˃ 0.05 and U = 60; p ˃ 0.05, respectively) with respect to control (Fig. 1a, b, and d). The mean value of GST activity in the hepatopancreas of P. canaliculata control individuals was 189.05 ± 18.56 nmol min−1 mg−1at 48 h. GST activity was significantly lower in hepatopancreas of individuals exposed to MET than in the control group (U = 36; p ˂ 0.05; Fig. 1c).
3.2.2 Intestine
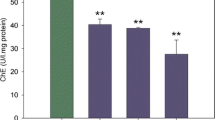
AChE activity in the intestine of the control group was 5.99 ± 0.88 nmol min−1 mg−1 proteins at 48 h. AChE activity in treatments (U = 64; p ˃ 0.05; Fig. 2a) did not vary significantly from control. CbE and GST activities in intestine of P. canaliculata individuals was 12.13 ± 1.63 nmol min−1 mg−1 protein and 178.55 ± 22.08 nmol min−1 mg−1 protein at 48 h. CbE and GST activities increased significantly in MET-exposed individuals compared with control (U = 12; p < 0.05 and U = 20; p < 0.05) (Fig. 2b, c). CAT activity (mean ± SEM) in the intestine of the control group was 12.37 ± 1.67 μmol min−1 mg−1 protein at 48 h. CAT activity was significantly lower in intestine of individuals exposed to MET than in the control group at 48 h (U = 38; p ˂ 0.05 Fig. 2d).
3.3 B-esterase and Oxidative Stress Enzymes in R. arenarum Tadpoles
The mean value of the AChE activity in control tadpoles was 14.74 ± 0.94 nmol min−1 mg−1protein at 48 h. The tested MET concentrations did not affect AChE activities significantly (Kruskal-Wallis (KW) = 5.58; p ˃ 0.05; Fig. 3a). Control CbE activities were 10.90 ± 0.88 nmol min−1 mg 1 protein at 48 h. CBE activities were affected significantly by MET concentrations (KW = 16.08; p < 0.05). CbE inhibitions at each concentration differed significantly from the control (Dunn post hoc test; Fig. 3b). The mean value of GST activity in control tadpoles was 115.11 ± 21.01 nmol min−1 mg−1 proteins at 48 h. The assayed commercial formulation decreased GST activities significantly (KW = 6.44; p < 0.05), differing significantly from the control GST activity at 0.25 and 0.50 mg L−1 (Dunn post hoc test p < 0.05, Fig. 3c). Control CAT activity was 45.51 ± 3.94 μmol min−1 mg−1 protein at 48 h. Likewise, CAT activity was significantly reduced by MET (KW = 6.24; p < 0.05). CAT activity differed significantly from the control at all concentrations (Dunn post hoc test p < 0.05; Fig. 3d).
3.4 Behavioural Endpoints in R. arenarum Tadpoles
Sublethal exposure to MET caused alteration to swimming endpoints (Table 1). The exposure of R. arenarum to the molluscicidal had a significant effect on three of the four swimming endpoints: mean speed (KW = 6.34; p < 0.05), distance moved (KW = 6.18; p < 0.05) and immobility (KW = 5.44; p < 0.05). Post hoc tests showed that tadpoles exposed to MET increased their mean swimming speed and travelled greater distances at 0.25 mg L−1 and were immobile for a shorter period (0.25 and 0.50 mg L−1).
4 Discussion
The use of pesticides in agriculture poses a great threat to ecosystems, with their indiscriminate application having raised global concern (Geiger et al. 2010; Mann et al. 2009). The adverse effects of pesticides lie in the multiplicity, toxicity and persistence, even when small rates are applied. Here, we studied a P. canaliculata and non-target (R. arenarum) species. The results clearly showed that the LC50 value in P. canaliculata was 0.50 mg L−1 at 48 h. This observation is in agreement with findings of Cagauan and Joshi (2002), who determined that this pesticide was extremely toxic (0.84 mgL−1) in P. canaliculata. Joshi et al. (2005) reported an LC50 value of 4.4 mg L−1 in the bioassay with standard commercial molluscicide (MET) in P. canaliculata.
A significant induction of CbE and GST activity was detected in the intestine of P. canaliculata exposed to MET, whereas CAT activity was lower in P. canaliculata than in control individuals. In hepatopancreas, GST exhibited lower values than those of control individuals. Unlike our observations, Triebskorn (1991) observed a significant reduction of CbE activity in the slug Deroceras reticulatum exposed to MET. According to the known role of CbE in detoxification of pesticides, over-expression or induction of CbE may be involved in the resistance mechanism of exposed organisms (Maxwell and Brecht 2001; Wheelock et al. 2008). In addition, in other aquatic invertebrates CbE activities were found to be more sensitive than ChE activities at different pesticides (Basack et al. 1998; Kristoff et al. 2012). Likewise, MET did not have any effects on the AChE activity of the isopod Porcellionides pruinosus, as we observed in our study with snails. A decrease in AChE activity in the nervous tissue of the snail Lymnaea acuminata produced by MET was reported (Tiwari et al. 2008). On the other hand, in the isopod species P. pruinosus inhibition of GST was found after 24 and 32 h of exposure to MET (Drobne et al. 2008). In snails, the induction of GST may be linked to a high detoxification of contaminants (or endogenous compounds), as demonstrated by Lee (1988). In addition, CAT activity decreased in the intestine of P. canaliculata with respect to control. Accordingly, previous studies observed a significant decreased and increase in CAT activity in terrestrial snails after exposure to MET (El-Wakil and Radwan 1991), suggesting a defense mechanism against cell damage in the organism due to the formation of reactive oxygen species. MET formulations are applied to control aquatic snail pests in different countries (Henderson and Triebskorn 2002). Metaldehyde is toxic to the GAS nervous system and has anaesthetic properties (Mills et al. 1989). Cheng (1989) demonstrated that MET is quite selective for Pomacea spp., with no effect on other invertebrates or fish. Thus, we consider that there is no adequate chronic toxicity data available on vertebrate species (e.g. amphibians). Acute toxicity tests are a useful tool to exploring the toxicity of a compound and its effect on a species, and contribute to the assessment of ecotoxicological risk by showing the earliest evidences of damage to exposed animals (Lajmanovich et al. 2015). The 48-h LC50 value of MET obtained in this study in R. arenarun tadpoles (229.7 mg L−1) is considerably high and similar to other non-target organisms, such as the fish Oncorhynchus mykiss, with a 96-h LC50 of 75 mg L−1, and the carp Ciprinus carpio, of 100 mg L−1 (Schnorbach et al. 2006). However, there are controversial results in the literature about the risk of MET on non-target aquatic organisms. Borlogan et al. (1996) found that MET is non-toxic to juvenile milkfish (Chanos chanos) exposed to concentrations of 25 and 175 mg L−1 for 7 days. At these non-toxic MET concentrations, exposed individuals may be affected by multiple sublethal effects (Stark et al. 2007); hence, the use of biomarkers appears particularly suitable to assess impacts of pesticides on these non-target organisms. A significant reduction of CbE activities was detected in R. arenarum exposed to MET, and AChE presented lower values than the control but did not show statistical differences. Inhibition of CbE activity by MET may have a similar toxicological meaning than that by OP pesticides. It is well known that these agrochemicals act as suicide substrates, binding to the active site of CbE (Stenersen 2004; Wheelock et al. 2008). In addition, inhibition of CbE activity by surfactants or pesticide is not surprising because detergents might alter enzyme binding to its catalytic/anionic site (Zhang et al. 2014). Likewise, surfactants may modify enzyme solubility by disrupting cellular membrane or by changing allosteric interaction of the enzyme structure (Cserháti et al. 2002). Recent studies have reported that other classes of substances such as pharmaceuticals inhibit CbE activity in different aquatic organisms (Solé and Sánchez-Hernández 2015). However, no data on the effects of MET on CBE is available, and it would be interesting to learn more about the role of this enzyme in decoding this chemical. The analysis of antioxidant enzymes (GST and CAT) revealed a significant decrease in tadpoles exposed to MET compared with the unexposed control group. Furthermore, MET is known to produce oxidative stress (Drobne et al. 2008). The interruption of the balance between oxidants and antioxidants—either by reduced antioxidant defences or by increased reactive oxygen species– may produce oxidative stress (Valavanidis et al. 2006). Hence, GST and CAT provide valuable information on the adaptive response of amphibians to toxic stress (Martínez-Álvarez et al. 2005; Venturino and Pechen de D’Angelo 2005).
MET appears to have a stimulatory rather than a sedative effect at both concentrations, since tadpoles increased their mean mobility. However, the lower MET concentration significantly increased swimming speed and distances of R. arenarum larvae. Sublethal exposure triggers different responses in tadpoles that may involve a reduction (Brunelli et al. 2009; Denoël et al. 2012) or an increase (Mandrillon and Saglio 2007; Peltzer et al. 2013) of swimming activities and may be linked to hormetic concentration response (Calabrese 2004), whereas lower MET concentrations stimulate swimming behaviour. In addition, the effects of MET on behaviour are typical of other pesticides, such as pyrethroid (Berrill et al. 1993; Scott and Sloman 2004; Robles-Mendoza et al. 2011). An excitatory effect on muscle contraction in the zebra mussel was attributed to MET (Putchakayala and Ram 2000); however, that effect was not due to an enhanced or inhibited effect of acetylcholine, but to other mechanisms probably affecting movement. Moreover, the most common symptoms of MET intoxication are convulsions and coma (Booze and Oehme 1985), which could explain the change in swimming behaviour. Our results are the first to describe MET effect on swimming behaviour of toad larvae.
5 Conclusion
The present study shows the importance of measuring a battery of enzyme biomarkers (such as B-esterases and oxidative stress) in different tissues (hepatopancreas and intestine) to accurately assess the effect of MET on GAS and non-target species, such as amphibian tadpoles. Using this approach, we found that exposure of GAS to MET produced significant induction of CbE and GST, and inhibition of CAT activities in intestine, whereas only GST was inhibited in hepatopancreas. The effects of MET commercial formulation on Neotropical tadpoles showed that sublethal exposure (0.25 and 0.50 mg L−1) affected B-esterase (CbE) activity and oxidative stress (GST and CAT) and swimming parameters. Finally, since amphibian tadpoles and P. canaliculata often co-occur, other native amphibian species should be studied to elucidate the ecological risk of MET to amphibian populations.
References
Aebi, H. (1984). Catalase in vitro. Metods Enzymology, 105, 121–126.
Aldridge, W. N. (1953). Serum esterases. (1) Two types of esterases (A and B) hydrolyzing p- nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochemistry Journal, 52, 110–117.
Almeida, J. R., Oliveira, C., Gravato, C., & Guilhermino, L. (2010). Linking behavioural alterations with biomarkers responses in the European sea bass Dicentrarchus labrax L. exposed to the organophosphate pesticide fenitrothion. Ecotoxicology, 19, 1369–1381.
APHA. (1989). Standard Methods for the Examination of Water and Wastewater (17th ed., p. 1,268). Washington D.C: American Public Health Association.
ASIH, HL, & SSAR. (2011). Guidelines for use of live amphibians and reptiles in field and laboratory research, Herpetological Animal Care and Use Committee (HACC) of the American Society of Ichthyologists and Herpetologists. Washington, DC, USA
Attademo, A. M., Peltzer, P. M., Lajmanovich, R. C., Cabagna-Zenklusen, M., Junges, C. M., Lorenzatti, E., Aró, C., & Grenón, P. (2015). Biochemical changes in certain enzymes of Lysapsus limellium (Anura: Hylidae) exposed to chlorpyrifos. Ecotoxicology and Environmental Safety, 113, 287–294.
Attademo, A. M., Peltzer, P. M., Lajmanovich, R. C., Cabagna-Zenklusen, M. C., Junges, C. M., & Basso, A. (2014a). Biological endpoints, enzyme activities, and blood cell parameters in two anuran tadpole species in rice agroecosystems of mid-eastern Argentina. Environmental Monitoring and Assessment, 186, 635–649.
Attademo, A. M., Peltzer, P. M., Lajmanovich, R. C., Basso, A., & Junges, C. (2014b). Tissue-specific variations of esterase activities in the tadpoles and adults of Pseudis paradoxa (Anura: Hylidae). Water Air Soil and Pollution, 225, 1903.
Bambaradeniya, C. N. B., Edirisinghe, J. P., De Silva, D. N., Gunatilleke, C. V. S., Ranawana, K. B., & Wijekoon, S. (2004). Biodiversity associated with an irrigated rice agroecosystem in Sri Lanka. Biodiversity and Conservation, 13, 1715–1753.
Basack, S. B., Oneto, M. L., Fuchs, J. S., Wood, E. J., & Kestenm, E. M. (1998). Esterases of Corbicula fluminea as biomarkers of exposure to organophosphorous pesticides. Bulletin of Environmental Contamination and Toxicology, 61, 569–576.
Berrill, M., Bertrama, S., Wilson, A., Louis, S., Brighaman, D., & Stromberg, C. (1993). Lethal and sublethal impacts of pyrethroid insecticides on amphibian embryos and tadpoles. Environmental Toxicology and Chemistry, 12, 525–539.
Boland, B. B., Meerhoff, M., Fosalba, C., Mazzeo, N., Barnes, M. A., & Burks, R. L. (2008). Juvenile snails, adult appetites: contrasting resource consumption between two species of applesnails (Pomacea). Journal of Molluscan Studies, 74(1), 47–54.
Booze, T. F., & Oehme, F. W. (1985). Metaldehyde toxicity—a review. Vet. Hum. Tox., 27(I), 11–19.
Borlogan, I. G., Coloso, R. M., & Blum, R. A. (1996). Use of metaldehyde as molluscicide in milkfish ponds. In I. F. Henderson (Ed.), Slug & snail pests in agriculture (pp. 205–212). BCPC Monograph no. 66.
Brunelli, E., Bernabó, I., Berg, C., Lundstedt-Enkel, K., Bonacci, A., & Tripepi, S. (2009). Environmentally relevant concentrations of endosulfan impair development, metamorphosis and behaviour in Bufo bufo tadpoles. Aquatic Toxicology, 91, 135–142.
Bunyan, P. J., & Jennings, D. M. (1968). Organophosphorus poisoning; some properties of avian esterase. Journal of Agricultural and Food Chemistry, 16, 326–331.
Cagauan, A. G., & Joshi, R. C. (2002). Golden Apple Snail Pomacea spp. in the Philippines. 7th ICMAM Special Working Group on Golden Apple Snail, 22 October, 2002
Calabrese, E. J. (2004). Hormesis: from marginalization to mainstream a case for hormesis as the default dose-response model in risk assessment. Toxicology and Applied Pharmacology, 197, 125–136.
Carlsson, N., Kestrup, A., Martensson, M., & Nyström, P. (2004). Lethal and non-lethal effects of multiple indigenous predators on the invasive golden apple snail (Pomacea canaliculata). Freshwater Biology, 49(10), 1269–1279.
Cheng, E. Y. (1989). Control strategy for the introduced snail, Pomacea lineate, in rice paddy. In I. F. Henderson (Ed.), Slugs and snails in world agriculture (pp. 69–75). BCPC Monograph no. 41.
Cnubben, N. H. P., Rietjens, I. M., Wortelboer, H., van Zanden, J., & van Bladeren, P. J. (2001). The interplay of glutathione-related processes in antioxidant defense. Environomental Toxicology and Pharmacology, 10, 141–152.
Cserháti, T., Forgacs, E., & Oros, G. (2002). Biological activity and environmental impact of anionic surfactants. Environment International, 28(5), 337–348.
Cohn, J., & MacPhail, R. C. (1996). Ethological and experimental approaches to behavior analysis: implications for ecotoxicology. Environmental Health Perspectives, 104, 305.
Cowie, R. H. (2002). Apple snails (Ampullariidae) as agricultural pests: their biology, impacts and management. Molluscs as crop pests, , 145–192.
Denoël, M., D’Hooghe, B., Ficetola, G. F., Brasseur, C., De Pauw, E., Thomé, J. P., & Kestemont, P. (2012). Using sets of behavioral biomarkers to assess short-term effects of pesticide: a study case with endosulfan on frog tadpoles. Ecotoxicology, 21, 1240–1250.
Drobne, D., Blazic, M., Van Gestel, C. A. M., Leser, V., Zidar, P., Jemec, A., & Trebse, P. (2008). Toxicity of imidacloprid to the terrestrial isopod Porcellio scaber (Isopoda, Crustacea). Chemosphere, 71, 1326–1334.
El-Wakil, H. B., & Radwan, M. A. (1991). Biochemical studies on the terrestrial snail, Eobania vemiculala (Muller) treated with some pesticides. Journal of Environmental Science and Health, B26(5-6), 479–489.
Ellman, G. L., Courtney, K. D., Andreas, V., Jr., & Featherstone, R. M. (1961). A new and rapid calorimetric determination of cholinesterase activity. Biochemistry Pharmacology, 7, 88–95.
Ferrari, A., Lascano, C., Pechen de D’Angelo, A. M., & Venturino, A. (2011). Effects of azinphos methyl and carbaryl on Rhinella arenarum tadpoles esterases and antioxidant enzymes. Comparative Biochemistry and Physiology, 153, 34–39.
Geiger, F., Bengtssonb, J., Berendse, F., Weisser, W. W., Emmersond, M., & Morales, M. B. (2010). Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic and Applied Ecology, 11, 97–105.
Gomori, G. (1953). Human esterases. Journal of Laboratory and Clinical Medicine, 142, 445–453.
Gosner, K. L. (1960). A simplified table for staging anuran embryos and tadpoles with notes on identification. Herpetologica, 16, 183–190.
Habdous, M., Visvikis, S., & Visvikis, S. (2002). Rapid spectrophotometric method for serum glutathione S-transferases activity. Clinical and Chemistry Acta, 326, 131–142.
Habig, W. H., Pabst, M. J., & Jakoby, W. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249, 7130–7139.
Hamilton, M. A., Russo, R. C., & Thurston, R. V. (1977). Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environmental and Science Technology, 11, 714–719.
Halliwell, B., & Gutteridge, J. M. C. (2007). Free radicals in biology and medicine. New York, USA: Oxford University Press.
Hayes, K. A., Joshi, R. C., Thiengo, S. C., & Cowie, R. H. (2008). Out of South America: multiple origins of non-native apple snails in Asia. Diversity and Distributions, 14(4), 701–712.
Henderson, I., & Triebskorn, R. (2002). Chemical control of terrestrial gastropods. In G. M. Barker (Ed.), Molluscs as crop pests (pp. 1–31). Wallingford, .K: CABI Publishing.
Joshi, R., Meepagala, K., Sturtz, G., Cagauan, A., Mendoza, C., Dayan, F., & Duke, S. (2005). Molluscicidal activity of vulgarone B from Artemisia douglasiana (Besser) against the invasive, alien, mollusk pest, Pomacea canaliculata (Lamarck). International Journal of Pest Management, 51(3), 175–180.
Joshi, R. C., De La Cruz, M. S., & Duca, A. V. (2002). Ovicidal effect of a molluscicide on golden apple snail in the Philippines. International Rice Research Notes, 27, 26–27.
Kingbley, G. R. (1942). The direct Biuret method for the determination of serum proteins as applied to photoelectric and visual colorimetry. Journal of Laboratory and Clinical Medicine, 27, 840–845.
Kristoff, G., Barrionuevo, D. C., Cacciatore, L., Verrengia Guerrero, N. R., & Cochón, A. C. (2012). In vivo studies on inhibition and recovery of B-esterase activities in Biomphalaria glabrata exposed to azinphos-methyl: Analysis of enzyme, substrate and tissue dependence. Aquatic Toxicology, 112–113, 19–26.
Lach, L., Britton, D. K., Rundell, R. J., & Cowie, R. H. (2000). Food preference and reproductive plasticity in an invasive freshwater snail. Biological Invasions, 2, 279–288.
Lajmanovich, R. C., Junges, C. M., Cabagna-Zenklusen, M. C., Attademo, A. M., Peltzer, P. M., Maglianese, M., Márquez, V. E., & Beccaria, A. J. (2015). Toxicity of Bacillus thuringiensis var. israelensis in aqueous suspension on the South American common frog Leptodactylus latrans (Anura: Leptodactylidae) tadpoles. Enviromental Research, 136, 205–212.
Lajmanovich, R. C., Junges, C. M., Attademo, A. M., Peltzer, P. M., Cabagna Zenklusen, M., & Bassó, A. (2013). Individual and mixture toxicity of commercial formulations containing glyphosate, metsulfuron-methyl, bispyribac-sodium, and picloram on Rhinella arenarum tadpoles. Water, Air, & Soil Pollution, 224, 1404.
Lazartigues, A., Banas, D., Feidt, C., Brun-Bellut, J., & Thomas, M. (2012). Pesticide pressure and fish farming in barrage pond in Northeastern France. Part I: site characterization and water quality. Environmental Sciences and Pollution Research, 19(7), 2802–2812.
Lee, R. F. (1988). Glutathione S-transferase in marine invertebrates from Langesundfjord. Marine Ecology, 46, 33–36.
Mandrillon, A., & Saglio, P. (2007). Herbicide exposure affects the chemical recognition of a non native predator in common toad tadpoles (Bufo bufo). Chemoecology, 17, 31–36.
Mann, R., Harding, J. M., & Southworth, M. J. (2009). Reconstructing pre-colonial oyster demographics in the Chesapeake Bay, USA. Estuarine, Coastal and Shelf Science, 85, 217–222.
Martínez-Álvarez, R. M., Morales, A. E., & Sanz, A. (2005). Antioxidant defenses in fish: biotic and abiotic factors. Reviews Fish Biology Fisheries, 15, 75–88.
Maxwell, D. M., & Brecht, K. M. (2001). Carboxylesterase: specific and spontaneous reactivation of an endogenous scavenger for organophosphorus compounds. Journal of Applied Toxicology, 21, 103–107.
Mills, J. D., Bailey, S. E. R., & McCrohan, C. R. (1989). Effects of molluscicides on feeding behavior and neuronal activity. In: I. Henderson (Ed.), British Crop Protection Council Monograph, no. 41. Slugs and snails in world agriculture; Symposium, Surrey, England, UK, April 10–12 (pp. 77–84). Thornton Heath, England, UK.
Ossana, N. A., Castañé, P. M., & Salibián, A. (2013). Use of Lithobates catesbeianus tadpoles in a multiple biomarker approach for the assessment of water quality of the Reconquista river (Argentina). Archives of Environmental Contamination and Toxicology, 65, 486–497.
Peltzer, P. M., Junges, C. M., Attademo, A. M., Bassó, A., Grenón, P., & Lajmanovich, R. C. (2013). Cholinesterase activities and behavioral changes in Hypsiboas pulchellus (Anura: Hylidae) tadpoles exposed to glufosinate ammonium herbicide. Ecotoxicology, 22, 1165–1173.
Peltzer, P. M., Lajmanovich, R. C., Attademo, A. M., & Beltzer, A. H. (2006). Diversity of anurans across agricultural ponds in Argentina. Biodiversity and Conservation, 15, 3499–3513.
Putchakayala, S. M., & Ram, J. L. (2000). Toxic and excitatory effects of the molluscicide metaldehyde on the biofouling bivalve Dreissena polymorpha Pallas. Pest Management Science, 56, 39–42.
Relyea, R. A., & Jones, D. K. (2009). The toxicity of Roundup Original Max to 13 species of larval amphibians. Environmental Toxicology and Chemistry. doi:10.1897/09-021.1.
Robles-Mendoza, C., Zúñiga-Lagunes, S. R., Ponce de León-Hill, C. A., Hernández-Soto, J., & Vanegas-Pérez, C. (2011). Esterases activity in the axolotl Ambystoma mexicanum exposed to chlorpyrifos and its implication to motor activity. Aquatic Toxicology, 105, 728–734.
Sánchez-Bayo, F. (2012). Insecticides mode of action in relation to their toxicity to non-target organisms. Journal of Environmental and Analytical Toxicology, S4, 002.
Schnorbach, H. J., Rauen, H. W., Bieri, M., Joshi, R., & Sebastian, L. (2006). Chemical control of the golden apple snail, Pomacea canaliculata. In R. C. Joshi & L. S. Sebastian (Eds.), Global advances in ecology and management of golden apple snails (pp. 419–438). Nueva Ecija: Philippine Rice Research Institute.
Scott, G., & Sloman, K. (2004). The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquatic Toxicology, 68, 369–392.
Solé, M., & Sanchez-Hernandez, J. C. (2015). An in vitro screening with emerging contaminants reveals inhibition of carboxylesterase activity in aquatic organisms. Aquatic Toxicology, 169, 215–222.
Sparling, D. W., & Fellers, G. M. (2009). Toxicity of two insecticides to California, USA, anurans and its relevance to declining amphibian populations. Environmental and Toxicology Chemistry, 28, 1696–1703.
Stark, J. D., Vargas, R., & Banks, J. E. (2007). Incorporating ecologically relevant measures of pesticide effect for estimating the compatibility of pesticides and biocontrol agents. Journal of Economic Entomology, 100, 1027–1032.
Stenersen, J. (2004). Chemical pesticides: mode of action and toxicology. Boca Raton, FL: CRC Press LLC.
Tiwari, F., Singh, K., & Singh, D. K. (2008). Enzyme inhibition by different bait formulations in the nervous system of the snail Lymnaea acuminate. In B. D. Joshi, P. C. Joshi, & N. Joshi (Eds.), Environmental Pollution and Toxicology (pp. 115–128). New Dehli, India
Triebskorn, R. (1991). The impact of molluscicides on enzyme activities in the hepatopancreas of Deroceras reticulatum (Miiller). Malacologia, 33, 255–272.
Triebskorn, R., Christensen, K., & Heim, I. (1998). Effects of orally and dermally applied metaldehyde on mucus cells of slugs (Deroceras reticulatum) depending on temperature and duration of exposure. Journal of Molluscan Studies, 64, 467–487.
US EPA. (1992). EPA probit analysis program, version 1.5 (Ecological Monitoring Research). Cincinnati, OH: Division Environmental Monitoring Systems Laboratory.
Vaira, M., Akmentins, M., Attademo, A., Baldo, D., Barrasso, D., et al. (2012). Categorización del estado de conservación de los Anfibios de la República Argentina. Cuadernos de Herpetologia, 26, 131–159.
Valavanidis, A., Vlahogianni, T., Dassenakis, M., & Scoullos, M. (2006). Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicology and Environmental Safety, 64, 178–189.
Van Buskirk, J., & McCollum, S. A. (2000). Influence of tail shape on tadpole swimming performance. Journal of Experimental Biology, 203, 2149–2158.
Venturino, A., & Pechen de D’Angelo, A. M. (2005). Biochemical targets of xenobiotics: biomarkers in amphibian ecotoxicology. Applied Herpetology, 2, 335–353.
Weis, J. S., & Candelmo, A. (2012). Pollutants and fish predator/prey behaviour: a review of laboratory and field approaches. Current Zoology, 58, 9–20.
Wheelock, C. E., Phillips, B. M., Anderson, B. S., Miller, J. L., Miller, M. J., & Hammock, B. D. (2008). Applications of carboxylesterase activity in environmental monitoring and toxicity identification evaluations (TIEs). Reviews of Environmental Contamination and Toxicology, 195, 117–178.
Wu, J. Y., Meng, P. J., Liu, M. Y., Chiu, Y. W., & Liu, L. L. (2010). A high incidence of imposex in Pomacea apple snails in Taiwan: a decade after triphenyltin was banned. Zoological Studies, 49, 85–93.
Zhang, J., Li, D., Ge, P., Guo, Y., Zhu, K. Y., Ma, E., & Zhang, J. (2014). Molecular and functional characterization of cDNAs putatively encoding carboxylesterases from the migratory locust, Locusta migratoria. PlosOne, 9, e94809.
Zar, J. H. (1999). Biostatistical analysis (4th ed.). New Jersey: Prentice Hall.
Acknowledgements
This study was supported in part by Secretaría de Ciencia y Tecnología (SECYT), Curso de Acción para la Investigación y Desarrollo (Programa de I+D Orientado a Problemas Sociales y Productivos, UNL) and Agencia Santafesina de Ciencia, Tecnología e Innovación (ASaCTeI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animals used in this research have been treated according to ASIH et al. (2011) criteria and with approval from the animal ethics committee of the Faculty of Biochemistry and Biological Sciences. http://www.fbcb.unl.edu.ar/pages/investigacion/comite-deetica.php.
Conflict of Interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Attademo, A.M., Lajmanovich, R.C., Peltzer, P.M. et al. Acute Toxicity of Metaldehyde in the Invasive Rice Snail Pomacea canaliculata and Sublethal Effects on Tadpoles of a Non-target Species (Rhinella arenarum). Water Air Soil Pollut 227, 400 (2016). https://doi.org/10.1007/s11270-016-3083-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3083-9