Abstract
Declines of amphibian populations have been a worldwide issue of concern for the scientific community during the last several decades. Efforts are being carried out to elucidate factors related to this phenomenon. Among these factors, pathogens, climate change, and environmental pollution have been suggested as possible causes. Regarding environmental pollutants, some pesticides are persistent in the environment and capable of being transported long distances from their release point. In Costa Rica, some pesticides have been detected in protected areas, at locations where amphibian populations have declined. Information about toxicity of pesticides used in Costa Rican agriculture to amphibians is still scarce, particularly for native species.
Toxicity tests with chlorothalonil, a fungicide intensively used in Costa Rica, were carried out exposing tadpoles of three Costa Rican native species: Agalychnis callidryas, Isthmohyla pseudopuma, and Smilisca baudinii in order to evaluate acute and chronic toxicity as well as the biomarkers cholinesterase activity (ChE), glutathione-S transferase activity (GST), and lipid peroxidation (LPO).
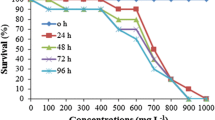
96-h LC50: 26.6 (18.9–35.8) μg/L to A. callidryas, 25.5 (21.3–29.7) μg/L to I pseudopuma and 32.3 (26.3–39.7) μg/L to S. baudinii were determined for chlorothalonil. These three species of anurans are among the most sensitive to chlorothalonil according to the literature. Besides, GST was induced in S. baudinii after exposure to sub-lethal concentrations of chlorothalonil while evisceration occurred in S. baudinii and A. callidryas tadpoles exposed to lethal concentrations of the fungicide. Chronic exposure to sub-lethal concentrations accelerated development in S. baudinii and caused lesions in tail of S. baudinii and I. pseudopuma tadpoles. Our results demonstrate that chlorothalonil is highly toxic to native amphibian species and that low concentrations can cause biochemical responses related to phase II of biotransformation and effects on development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Declines of amphibian populations have been a worldwide issue of concern for the scientific community during the last several decades (Pounds and Crump 1994; Alford and Richards 1999; Lips et al. 2005; Mendelson et al. 2006; Whitfield et al. 2007; Blaustein et al. 2011). Several factors have been suggested as possible causes for these population declines; among them pathogens, specifically the infection caused by Batrachochytrium dendrobatidis (Bd) (Berger et al. 1998; Lips et al. 2006; Gillespie et al. 2015), climate change (Pounds 2001; Li et al. 2013), habitat loss and fragmentation (Becker et al. 2008), invasive species (Adams 1999; Kats and Ferrer 2003), environmental pollution (Sparling et al. 2001) and the interaction of these factors (Collins and Storfer 2003; Rohr and Palmer 2013).
Costa Rica, with only 51,100 km2 of terrestrial and 568,000 km2 marine territories, is among the 20 most biodiverse countries in the word, almost 100 thousand species have been described for the country and it represents 4.75 % of world’s biodiversity (SINAC 2009). As of 2009, 192 amphibian species have been described in Costa Rica, making this among the top 13 most species-rich countries for amphibians (Bolaños 2009). Of those amphibian species described, 63 (33 %) are considered from vulnerable to extinct (2) according to IUCN red list. One of the extinct species is the emblematic golden toad (Incilius periglenes) which was endemic in Monteverde’s cloud forest, Costa Rica (IUCN 2015).
Despite of the rich biodiversity, Costa Rican ecosystems face threats caused by human activities like other regions. Regarding amphibian populations, these pressures include global climate change, habitat loss due to land use conversion to productive or urbanistic activities, pollution, invasive species and diseases (Lips et al. 2005; Whitfield et al. 2009). In a country where agriculture is the main economic activity, pesticides are one of the major risks regarding chemical pollution. Furthermore, the use of pesticides in local agriculture is high compared to developed and even other developing countries and some compounds forbidden in many other places are used here (de la Cruz et al. 2014).
Pesticides are considered among the environmental pollutants related to amphibian declines. Pesticides can cause organism mortalities but also, even at low environmental concentrations they might have negative effects on amphibian’s growth and development (Hayes et al. 2006). Besides, some of these compounds are persistent in the environment and capable of being transported long distances from their point of release. In Costa Rica, some pesticides used in agriculture have been detected in air and fog samples collected from protected areas, far from their site of use and at places where amphibian populations have declined (Daly et al. 2007; Shunthirasingham et al. 2011). Information about effects of pesticides and other environmental pollutants on neotropical amphibian species is scarce and necessary. Especially as atmospheric drift might transport pollutants from croplands to protected areas.
The risk posed by fungicides to amphibian populations should be considered as it is one of the most imported and used groups of pesticides in Costa Rica (de la Cruz et al. 2014). Specifically, chlorothalonil (2,4,5,6-tetrachloroisophthalonitrile) is applied in banana, melon, coffee, chayote and other fruit and vegetable crops, along the country (Bravo et al. 2013). Chlorothalonil is a multi-site contact fungicide which causes toxicity by complexing with sulphydryl containing proteins, affecting glutathione reserves. Aside from fungi, this thiol-related mechanism has also been described in fish and aquatic invertebrates (Elskus 2012). Chlorothalonil’s toxicity is considered high for amphibians, fish, and aquatic invertebrates (de la Cruz et al. 2014; Van Scoy and Tjeerdema 2014); acute effects are expected on fish and invertebrates with environmental concentrations above 5.25 and 1.8 μg/L respectively while chronic effects are expected above 3 and 0.6 μg/L respectively for those groups (Elskus 2012). Chlorothalonil’s half-life in aquatic environments goes from 2 h to 8 days (Grabuski et al. 2004). In Costa Rica, concentrations as high as 11 μg/L have been documented in surface water samples (Castillo et al. 2000). Daly et al. (2007) reported presence of chlorothalonil in air and soil samples from different sites in Costa Rica, the higher concentrations measured were 17.3 ng/m3 in air and 1.06 ng/g in soil, both in samples collected at the central area of the country.
Evaluation of the effects of this pollutant on amphibians is necessary in order to suggest and eventually establish protective limits to its use. For toxicity assays with amphibians, early life stages (embryo and larvae) are frequently used; several endpoints can be evaluated in these tests, among them, survival, growth, development, behavior, deformities and several biochemical biomarkers (Sparling et al. 2010). Information on acute and chronic toxicity as well as early warning physiological responses can be obtained. Typically, ecotoxicological information for amphibians is obtained using a few surrogates, well known and laboratory “adapted” species. This might pose an inconvenience as life history and other important ecological traits vary greatly among amphibians and it might affect the way they are exposed to pollutants (Sparling et al. 2010). In this regard, evaluating the toxicity of chlorothalonil on tropical species would contribute to build up information regarding the effects of this relevant pesticide.
In this work, we aimed to undertake the evaluation of one of the factors suspected to interact in the complex causing amphibian population declines: the toxicity and other effects caused by environmental pollutants. Specifically, we evaluated effects caused by chlorothalonil, a fungicide used in Costa Rican agriculture, to tadpoles of three native anurans species.
Material and methods
Toxicity of chlorothalonil was evaluated on tadpoles of three Costa Rican native species: Agalychnis callidryas, Isthmohyla pseudopuma, and Smilisca baudinii. The three species are listed as Least Concern by the IUCN (IUCN 2015). All the tests were carried out at the Laboratory for Ecotoxicology Studies (ECOTOX) of the Central American Institute of Studies on Toxic Substances (IRET) at the Universidad Nacional (UNA) in Heredia.
Tadpole collection and maintenance
All tadpoles were acclimatized to laboratory conditions during at least 1 week before any assay, they were fed ad libitum, three times per week with TetraVeggie spirulina-enhanced flakes (Tetra®) and water was 50 % renewed weekly. Collection of eggs or tadpoles of each species was carried out according to their reproductive habits.
A. callidryas
Eight egg masses were collected from vegetation surrounding a pond located outside Tirimbina Rainforest Center in Sarapiqui. Egg masses were transported in plastic containers with water from the pond and at the ECOTOX laboratory they were placed in aquariums with a shallow amount of carbon filtered, UV treated tapwater (UV water). Leaves containing the eggs were held with adhesive tape to aquarium walls just above the water surface, until they hatched and the tadpoles fell into the water. All A. callidryas tests were started using tadpoles on stages 26–27 (Gosner 1960).
I. pseudopuma
Four egg masses were collected from temporary or permanent natural ponds located in mountain area of San Rafael in Heredia. Organisms were transported in plastic container with site water to the laboratory where they were treated as described for A. callidryas. Toxicity tests were started with tadpoles on stages 25–27 (Gosner 1960).
S. baudinii
Two hundred recently hatched tadpoles were collected from the Palo Verde wetland, at Palo Verde National Park in Guanacaste, transported to laboratory in water from the site and then transferred to clean UV treated tapwater. Toxicity tests using S. baudinii started with tadpoles on stages 28–34 (Gosner 1960).
Acute test
Acute, static, 96-h toxicity tests were carried out to find chlorothalonil’s LC50 for each species. A minimum of six pesticide concentrations were tested in every assay (Table 1), a negative (UV treated tapwater) and a solvent control (methanol added to UV water in a volume equal to the one added to the highest concentration of chlorothalonil stock in the corresponding assay) were also set for every test. Tadpoles were exposed individually in 1 L glass containers with 500 mL of exposure solution. For every treatment, nine replicates were set and tadpoles were randomly distributed among treatments and replicates. Animals were not fed during the assay and mortality was recorded every 24 h.
Chronic test
Chronic effects (growth and development) were evaluated in tadpoles exposed to sub-lethal concentrations (Table 1) (defined according to the outcome of acute tests) of chlorothalonil in a semi-static assay. At least three concentrations of fungicide plus controls were tested; with nine replicates per treatment and 500 mL of exposure solution. During the test, tadpoles were fed three times per week with approximately 15 mg of tetraVeggie/individual and once a week transferred into containers with freshly prepared solutions. Weight, total length, and developmental stage were registered weekly until tadpoles reached stage 42 or died (Gosner 1960).
For each experiment, exposure solutions were prepared by adding an aliquot of a stock solution to the exposure medium (UV water). Stock solution (1162 μg/mL) was prepared from 97.5 % pure chlorothalonil standard (Dr. Ehrenstorfer, Germany) dissolved in HPLC-grade, 99.97 % methanol (J.T. Baker) and kept at 4 °C. Aliquots were taken using a micro volume syringe (SGE Analytical science).
Concentration of chlorothalonil was evaluated at the beginning and at the end of acute and chronic tests: a 20 mL sample of each exposure solution was extracted (liquid/liquid extraction) with 98 % pure n-hexane (SupraSolv®) and re-suspended in a final volume of 99.8 % pure isooctane (SupraSolv®). Determination of chlorothalonil in the extracts was performed using gas chromatography coupled to mass spectrometry (GC-MS) and electron capture detection (GC-ECD). For determination, an external calibration curve of chlorothalonil was processed. Initial concentrations were confirmed with a regression to nominals of R 2 = 0.86; no chlorothalonil was detected in samples collected at the end of assays, either acute (96-h) or chronic (7 days). For this reason, acute tests should be interpreted as static 96-h exposure with an initial pulse of the nominal concentrations and the chronic tests, the exposure to a weekly pulse of the fungicide during the time the assay lasted. A 10 % error associated with the analytical methods should be considered for the quantification.
Four physical-chemical parameters: pH (Corning pH meter 220), dissolved oxygen (WTW Oxi 325), temperature and conductivity (WTW Cond 315i) were monitored in exposure solutions during the tests. Average and standard deviation of these parameters kept during the assays were pH = 6.6 ± 0.25; dissolved oxygen = 5.3 ± 1.35 mg/L; conductivity = 108.8 ± 4.5 μS/cm and temperature = 25.1 ± 0.7 °C.
Biomarkers
Muscle (tail) cholinesterase activity (ChE), liver glutathione-S transferase activity (GST), and liver lipid peroxidation (LPO) were evaluated in tadpoles after 96 h of exposure to sub-lethal concentrations of chlorothalonil. Samples were homogenized in an appropriate buffer and processed as described in Mena et al. (2014) for biomarker determinations. Briefly, protein content in sample homogenates was determined by the method of Bradford (1976) using γ-globulin as standard. ChE activity was measured using the method of Ellman et al. (1961), using 1 mM acetylthiocholine and 0.1 mM 5,5′ dithiobis-2-dinitrobenzoic acid (DTNB) as substrate and conjugate; reaction was measured at 415 nm during 15 min and expressed as nmol/min/mg protein. GST activity was determined as described by Habig et al. (1974), exposing samples to 1 mM CDNB and 1 mM GSH and monitoring the reaction at 340 nm during 3 min.; activity reported as nmol/min/mg protein. Lipid peroxidation was measured by the thiobarbituric reactive species (TBARS) (Oakes and Van Der Kraak 2003) and expressed as nmol TBARS per mg of protein.
Data analysis
Toxicity parameters: (LC50) and 95 % confidence limits were calculated using IBM® SPSS® Statistics 22 (trial version). Concentration data were log-transformed and effect data were Probit-transformed.
To determine the effects of clorothalonil on total length, weight and development stage of tadpoles, we used linear mixed-effect models (LMMs) as suggested by Cox (2010). The total length, weight and development stage of tadpoles were specified as response variables. We used specified treatment as fixed factor and the number of individuals as random effect to account for repeated measures in each tadpole. During the analysis, the variables were classified as significant if the 95 % confidence intervals (CI) did not overlap zero (which represents the negative control).
We constructed linear models for each species to assess the effect of clorothalonil for each biomarker (LPO level, ChE and GST activities). Models were compared with null models (hypothesizes a difference of 0) and ranked according to their Akaike Information Criterion adjusted for small sample sizes (AICc). When a treatment effect was detected, we conducted model-averaged effect sizes (Mazerolle 2006); this is an information-theoretic alternative to multiple comparisons (e.g., Burnham et al. 2011). We log-transformed response variables if they showed non-normality.
The analyses of chronic effects and biomarkers were conducted in R version 3.1.2 (R Development Core Team 2014) using the package “lme4” (Bates et al. 2014) to conduct LMMs, and the package “AICcmodavg” to calculate AICc and model-averaged effect sizes (Mazerolle 2006).
Results
Acute toxicity
The tadpoles of the three native species tested showed a high and very similar sensitivity to chlorothalonil (Table 2, Fig. 1). Additionally, in A. callidryas and S. baudinii acute assays, it was observed that tadpoles which died during the first 24 h exposed to lethal concentrations of chlorothalonil, a spontaneous rupture of linea alba and posterior evisceration was observed (Fig. 2). In A callidryas, after 15 h of exposure, this effect was observed in one out of nine tadpoles exposed to 40 μg/L, two out of nine tadpoles exposed to 50 μg/L and three out of nine individuals exposed to 60 μg/L. During acute tests, we had a hundred percent survival of controls.
Chronic effects
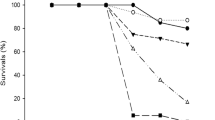
Exposure to chlorothalonil did not affect the variables associated with growth and development measured in I. pseudopuma individuals compared to controls (Fig. 3a–c). In contrast, development of S. baudinii tadpoles exposed to 20 μg/L of the fungicide showed a positive difference compared to controls (95 % CI 0.69, 3.43) (Fig. 3f); while total length and body weight were not affected in this species (Fig. 3d, e). In the case of S. baudinii, the solvent control affected significantly the body weight of the tadpoles, therefore, for animals exposed to chlorothalonil, this variable was tested against the solvent control (Fig. 3e).
As an additional observation, during chronic exposure, lesions were observed on tails of S. baudinii and I. pseudopuma tadpoles exposed to the fungicide but not in the controls (Fig. 4). In S. baudinii, this effect was observed in five out of nine tadpoles exposed to 5 μg/L, six out of nine exposed to 10 μg/L and six out of nine exposed to 20 μg/L.
During chronic tests with I. pseudopuma no mortalities occurred in the negative control. In the case of S. baudinii, three individuals in the control died during the experiment before reaching stage 42, their data was analyzed for the period they lived. Chronic tests with A. callidryas were ended because of mortality of more than four replicates in controls during the first week of exposure. In this case, the batch of organisms used to start chronic test came from different egg masses than the ones used for acute assays.
Biomarkers
The analysis of model selection identified an effect of chlorothalonil treatments for I. pseudopuma ChE activity and S. baudinii GST activity (Table 3).
ChE activity was not affected in tadpoles of A. callidryas and S. baudinii exposed to chlorothalonil (Fig. 5a, c). In the case of I. pseudopuma, tadpoles exposed to 3 μg/L had a lower ChE activity compared to tadpoles exposed to higher concentrations of the pesticide (Fig. 5b).
Biomarkers measured in tadpoles exposed to sub-lethal concentrations of chlorothalonil: muscle ChE activity (a–c), liver GST activity (d–f) and liver LPO (g–i). Bars represent mean ± 95 % CI of the biomarker measured for each treatment. Differences among treatments in an experiment are indicated with lowercase letters. C = negative control, SC = solvent control.
A significant increase in GST activity was observed in livers of S. baudinii tadpoles exposed to 10 and 20 μg/L of chlorothalonil compared to controls and lower concentrations of the pesticide (Fig. 5f). A positive dose-response relationship was observed in this species (Fig. 5f), indicating an induction of phase II biotransformation process caused by the fungicide.
We measured LPO liver levels as a possible sign of oxidative stress induced by exposure of tadpoles to the fungicide; however, no significant increase of this biomarker was observed in any of the three species tested (Fig. 5g–i).
Discussion
The three species tested showed a similar sensitivity to chlorothalonil and this level of sensitivity agrees with observations made on other amphibian species (Yu et al. 2013). Also, this data confirms that larval stages of amphibians are more sensitive to chlorothalonil than other aquatic organisms (invertebrates and fish) (Yu et al. 2013). Compared to databases reports and literature, the three species tested are among the most sensitive to chlorothalonil (US-EPA 2015; Kegley et al. 2015). Previous works developed in Costa Rica found also a high toxicity of chlorothalonil to A. callidryas tadpoles (Johnson et al. 2013; Ghose et al. 2014). The studies mentioned, used formulations of the fungicide and evaluated acute and chronic endpoints also. The acute 96-h LC50 reported by them is higher than the one reported in this study, but still agrees with the fact that compared to other species, A. callidryas is very sensitive to chlorothalonil. According to our data, this species is more sensitive to the pure compound than to the formulation. Regarding the toxicokinetics of pesticides, some research shows that through dermal exposure, the process of absorption of chemicals in amphibians is two orders faster than mammals (Brühl et al. 2013) which could be related with the higher sensitivity observed.
Considering other anurans, a great variation of sensitivity among species has been reported. For instance, McMahon et al. (2011) observed significant mortality in tadpoles of two frog species exposed to chlorothalonil at a concentration below the range tested in this work (16.4 ng/L) while the exposure of Osteopilus septentrionalis tadpoles to the a concentration thousand times higher (17.6 μg/L) did not cause mortality (McMahon et al. 2013). This last report agrees with our results regarding sub-lethal concentrations. In other example, McMahon et al. (2012) applied chlorothalonil in concentrations above 164 μg/L to a mesocosm where Osteopilus septentrionalis and Rana sphenocephala tadpoles among aquatic organisms were present. The treatment with the fungicide in this case increased mortality of the tadpoles and other groups. For the species we tested, this concentration would be lethal to a hundred percent of the animals.
Chlorothalonil is a multi-site contact fungicide, which in aquatic organisms is known to affect immune responses and interfere with glutathione-related and oxidase-like enzymes (Elskus 2012; Van Scoy and Tjeerdema 2014). In humans, the occurrence of dermatitis has been reported in individuals exposed to this compound (Penagos 2002) and this effect has been attributed to irritant properties of the substance (Lensen et al. 2007). During acute assays with A. callidryas and S. baudinii, evisceration was observed in tadpoles exposed to lethal concentrations and we hypothesize that this effect might be attributed to a rupture of the linea alba because of the pesticide irritant properties. It would be necessary to confirm our findings with histological analysis. In this case, due to overnight mortality, some decomposition had already occurred in the samples and it was not possible to properly process them.
During chronic exposure, damage in tails was observed in S. baudinii and I. pseudopuma tadpoles. Yu et al. (2013) reported a similar effect of chlorothalonil on amphibians and they suggest that it might be related with an apoptotic process. It has been observed that xenobiotics have the capacity to produce skin lesions: recently it was found that after a short exposure to endosulfan, morphology and structure of the skin of Bufo bufo tadpoles suffered alterations and degenerative processes (Bernabò et al. 2013). Other pesticides may produce similar effects, in Sri Lanka, tadpoles of an endemic species were exposed chronically to chlorpyrifos, dimethoate, glyphosate and propanil. They developed malformations at high frequencies, mainly kyphosis, scoliosis, skin ulcers and edema (Jayawardena et al. 2010).
Regarding sub-lethal chronic effects, in most of the cases reported so far, exposure to pesticides has been observed to affect negatively the growth and development of tadpoles (Brunelli et al. 2009; Sparling and Fellers 2009; Baker et al. 2013). In the specific case of chlorothalonil, its sub lethal toxicity to A. callidryas has been studied and it resulted in a reduction of biomass in tadpoles exposed to the chemical for periods of 8 or 12 days (Johnson et al. 2013; Ghose et al. 2014; Alza et al. 2016). Also, Yu et al. (2013) found that 96-h exposure to chlorothalonil reduced length of Xenopus leavis tadpoles.
The study we conducted maintained a prolonged exposure of tadpoles to the fungicide. In these conditions, we had no effect on I. pseudopuma growth or development; but in S. baudinii, development was positively affected by the exposure. This response has already been observed with the herbicide acetochlor in the presence of an exogenous dose of 3, 3′, 5-triiodothyronine (T3), which is the active form of thyroxine (T4) hormone, both involved in the metamorphosis activation. It produced an accelerated development of Rana pipiens forelimbs and this was attributed to an enhancement of the hormone action caused by the presence of the pesticide (Cheek et al. 1999). In X. laevis, when exposed to acetochlor and T3, the metamorphosis was accelerated. The authors relate the course of action of acetochlor with gene transcription during metamorphosis (Crump et al. 2002).
Acceleration of development associated with pollutants interacting with thyroid hormones is an endpoint already considered in toxicity testing (OECD 2009). In this regard, Grabuski et al. (2004) suggested the potential of chlorothalonil as an endocrine dusruptor as it can potentially interfere with endogenous hormones. In mammals, it has been reported that this fungicide can be an agonist of the aryl hydrocarbon receptor (AhR) (Elskus 2012), which can be a molecular pathway to endocrine disrupting effects. Considering the outcome of our test with S. baudinii, we suggest that further investigation should be aimed to elucidate this issue. Based on our observations, we recommend the evaluation of malformations and behavioral effects as well as the continuation of the assays post-metamorphosis in order to identify possible alterations caused by the compound during juvenile and adult stages. This would be especially valuable considering that I. pseudopuma inhabits areas where declines have occurred (Abarca 2012) and the life cycle stage and parameters we evaluated did not reveal significant chronic effects on this species.
No significant ChE inhibition was observed in muscle samples of A. callidryas or S. baudinii, this is reasonable as this fungicide is not associated with a mechanism of ChE inhibition. It is still important to consider and measure ChE activity as its inhibition has been related to several pollutants others than organophosphates and carbamates (Lionetto et al. 2011). Tadpoles of I. pseudopuma exposed to 3 μg/L of chlorothalonil had a lower ChE activity compared to individuals exposed to higher concentrations of the pesticide but not lower compared to controls. We consider this difference unrelated with a possible inhibition caused by the pesticide as ChE inhibitors are known to show a clear dose-response relationship (Thomson 1999; Pathiratne et al. 2008; Mena et al. 2012). However, nonmonotonic responses like this have been observed in the dose-mortality relationship of three amphibian species exposed to chlorothalonil (McMahon et al. 2011). Also, immunological parameters of fish exposed to chlorothalonil have shown nonmonotonic responses (Shelley et al. 2009).
Chlorothalonil’s mode of action is related to interference with glutathione and cellular sulfhydryl enzymes (Van Scoy and Tjeerdema 2014), which are involved in phase II of biotransformation. In this work, we found evidence of biotransformation phase II induction in tadpoles of S. baudinii exposed to sub-lethal concentrations of chlorothalonil. The role of GST in biotransformation of chlorothalonil has been broadly described in bacteria (Wang et al. 2011) but also in aquatic vertebrates (fish) (Davies 1985). Ezemonye and Tongo (2010) reported the induction of GST in amphibians (adult toads) exposed to endosulfan and diazinon, furthermore they found that the liver is a good tissue to measure this activity. On the other hand, we did not find signs of oxidative stress evidenced as lipid peroxidation. This absence of oxidative stress has been observed in amphibians exposed to pesticides (Venturino et al. 2003), even when phase II responses are evident, like in our case. This might be attributed to the reported low activity of the mixed-function oxidases belonging to the cytochrome P450 family in amphibians (Huang et al. 1998; Venturino et al. 2003; Jung et al. 2004), being xenobiotics metabolized via phase II without a significant induction of phase I reactions and a lower oxidative process.
This work represents one of the few efforts in order to characterize the sensitivity of tropical amphibian species to pesticides. Two studies carried out in Costa Rica (Daly et al. 2007; Shunthirasingham et al. 2011) evidenced that pesticides used in agriculture in the lowlands of the country (including chlorothalonil) are atmospherically transported and found at places where habitat degradation is not evident. Although the three species tested in this work are listed as Least Concern by the IUCN (IUCN 2015), there is already evidence of the presence of pesticides, including chlorothalonil, in areas they inhabit: Cordillera Volcanica Central (Daly et al. 2007), the Caribbean Region (Castillo et al. 2000) and the Pacific Region (Mena et al. 2014). Accordingly, these and other amphibians are exposed to pollution and consequences as those shown in this work, during their life cycle (aquatic and terrestrial). Declines of some amphibian populations have been documented in pristine areas where Bd and temperature variability have been identified as causes for those events (Lips et al. 2008; Rohr and Raffel 2010). At the same time, La Marca et al. (2005) warn about the lack of information regarding environmental pollutants released by human activities and their effects on neotropical amphibian species. In this regard, efforts should be undertaken in order to elucidate the role of pollutants and hopefully their interaction with other environmental stressors as part of the factors influencing amphibian declines.
Conclusions
In the present study, we report that amphibian species that inhabit pristine areas of Costa Rica are sensitive to chlorothalonil, a pesticide that has been found to be transported atmospherically and that is intensively used in the country. Thus, efforts should be made in order to reduce the use of pesticides in the country and also focus on the protection of amphibian populations that are currently affected by declines.
Induction of phase II biotransformation indicates that relevant environmental concentration of chlorothalonil can be inducing biological responses that are related to its mode of action.
This study evaluated the chronic effects of a prolonged exposure to chlorothalonil on anurans. Because the tadpoles of S. baudinii showed impaired growth and development, it is recommended to investigate the possible role of this pesticide as an endocrine disruptor. It is also suggested to assess effects on juveniles and adults of the species tested.
Spontaneous rupture of linea alba and posterior evisceration was observed in the tadpoles exposed to lethal concentrations of chlorothalonil as well as lesions in tail at sub-lethal concentrations, these effects could be related to histological alterations of the skin, but further studies are needed to confirm this.
References
Adams MJ (1999) Correlated factors in amphibian decline: exotic species and habitat change in Western Washington. J Wildl Manag 63(4):1162–1171
Abarca JG (2012) Cambios en la estructura de la comunidad de anuros (Amphibia: Anura) en el Cerro Chompipe, Costa Rica. Cuadernos de Investigación UNED 4(1):9–15
Alford RA, Richards SJ (1999) Global amphibian declines: a problem in applied ecology. Annu Rev Ecol Syst 30:133–165
Alza CM, Donnelly MA, Whitfield SM (2016) Additive effects of mean temperature, temperature variability, and chlorothalonil to red-eyed treefrog (Agalychnis callidryas) larvae. Environ Toxicol Chem in press
Baker NJ, Bancroft BA, Garcia TS (2013) A meta-analysis of the effects of pesticides and fertilizers on survival and growth of amphibians. STOTEN 449:150–156
Bates D, Mächler M, Bolker BM, Walker SC (2014) Fitting linear mixed-effects models using lme4. J Stat Softw 51 p
Becker CG, Fonseca CR, Baptista Haddad CF, Fernandes Batista R, Prado PI (2008) Habitat split and the global decline of amphibians. Science 318(14):1775–1777
Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, Mcdonald KR, Hines HB, Lips KR, Marantelli G, Parkes H (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci 95:9031–9036
Bernabò I, Guardia A, La Russa D, Madeo G, Tripepi S, Brunelli E (2013) Exposure and post-exposure effects of endosulfan on Bufo bufo tadpoles: Morpho-histological and ultrastructural study on epidermis and iNOS localization. Aquat Toxicol 142–143:164–175
Blaustein AR, Han BA, Relyea RA, Johnson PTJ, Buck JC, Gervasi SS, Kats LB (2011) The complexity of amphibian population declines: understanding the role of cofactors in driving amphibian losses. Ann. N.Y. Acad Sci 1223:108–119
Bolaños F (2009) Situación de los anfibios de Costa Rica. Biocenosis 22(1–2):95–108
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dyebinding. Anal Biochem 72:248–254
Bravo V, De la Cruz E, Herrera G, Ramírez F (2013) Uso de plaguicidas en cultivos agrícolas como herramienta para el monitoreo de peligros en salud. Uniciencia 27(1):351–376
Brunelli E, Bernabò I, Berg C, Lundstedt-Enkel K, Bonaccia A, Tripepi S (2009) Environmentally relevant concentrations of endosulfan impair development, metamorphosis and behaviour in Bufo bufo tadpoles. Aquat Toxicol 9:135–142
Brühl C, Schmidt T, Pieper S, Alscher A (2013) Terrestrial pesticide exposure of amphibians: an underestimated caused of global decline? Sci Rep 3:1135
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations and comparisons. Behav Ecol Sociobiol 65:23–25
Castillo LE, Ruepert C, Solis E (2000) Pesticide residues in the aquatic environment of banana plantation areas in the north atlantic zone of Costa Rica. Environ Toxicol Chem 19(8):1942–1950
Cheek A, Ide C, Bollinger J, Rider C, McLachlan (1999) Alteration of leopard frog (Rana pipiens) metamorphosis by the herbicide acetochlor. Arch. Environ Contam Toxicol 37:70–77
Collins JP, Storfer A (2003) Global amphibian declines: sorting the hypotheses. Divers Distrib 9(2):89–98
Cox SB (2010) Statistical models in wildlife toxicology. In: Kendall RJ, Lacher TE, Cobb GP, Cox JP (eds) Wildlife toxicology: emerging contaminant and biodiversity issues. CRC Press, Florida, USA, pp. 173–195
Crump D, Werry K, Veldhoen N, Van Aggelen G, Helbing C (2002) Exposure to the herbicide acetochlor alters thyroid hormone-dependent gene expression and metamorphosis in Xenopus laevis. Environ Health Perspect 110:1190–1205
Daly GL, Lei YD, Teixeira C, Muir DCG, Castillo LE, Wania F (2007) Accumulation of current-use pesticides in neotropical montane forests. Environ Sci Technol 41:1118–1123
Davies PE (1985) The toxicology and metabolism of chlorothalonil in fish. Metabolism, enzymatic and detoxication in Salmo spp. and Galaxias spp. Aquat Toxicol 7:277–299
de la Cruz E, Bravo-Durán V, Ramírez F, Castillo LE (2014) Environmental hazards associated with pesticide import into Costa Rica, 1977-2009. J Environ Biol 35(1):43–55
Ellman GL, Courtney KD, Andres VJ, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Elskus AA (2012) Toxicity, sublethal effects, and potential modes of action of select fungicides on freshwater fish and invertebrates: U.S. Geological Survey Open-File Report 2012–1213, 42 p., at http://pubs.usgs.gov/of/2012/1213/.
Ezemonye L, Tongo I (2010) Sublethal effects of endosulfan and diazinon pesticides on glutathione-S-transferase (GST) in various tissues of adult amphibians (Bufo regularis). Chemosphere 81:214–217
Ghose SL, Donnelly MA, Kerby J, Whitfield SM (2014) Acute toxicity tests and meta-analysis identify gaps in tropical ecotoxicology for amphibians. Environ Toxicol Chem 33(9):2114–2119
Gillespie GR, Hunter D, Berger L, Marantelli G (2015) Rapid decline and extinction of a montane frog population in southern Australia follows detection of the amphibian pathogen Batrachochytrium dendrobatidis. Anim Conserv 18(3):295–302
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16(3):183–190
Grabuski J, Martin PA, Struger J (2004) Pesticides in Ontario: A Critical Assessment of Potential Toxicity of Urban Use Products to Wildlife, with Consideration for Endocrine Disruption. Volume 3: Phenoxy herbicides, chlorothalonil and chlorpyrifos. Technical Report Series No. 410. Canadian Wildlife Service, Ontario Region, Burlington, Ontario, Canada. http://dsp-psd.pwgsc.gc.ca/Collection/CW69-5-410E.pdf. Accessed 14 June 2016
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. Biol Chem 249:7130–7139
Hayes TB, Case P, Chui S, Chung D, Haeffele C, Haston K, Lee M, Mai VP, Marjuoa Y, Parker J, Tsui M (2006) Pesticide mixtures, endocrine disruption, and amphibian declines: are we underestimating the impact? Environ Health Perspect 114(1):40–50
Huang YW, Melancon MJ, Jung RE, Karasov WH (1998) Induction of cytochrome P450-associated monooxigenases in northern leopard frogs, Rana pipiens, by 3,3′4,4′,5-Pentachlorobiphenyl. Environ Toxicol Chem 17(8):1564–1569
Jayawardena UA, Rajakaruna RS, Navaratne AN, Amerasinghe PH (2010) Toxicity of agrochemicals to common hourglass tree frog (Polypedates cruciger) in acute and chronic exposure. Int J Agric Biol 12(5):641–648
Johnson LA, Welch B, Whitfield SM (2013) Interactive effects of pesticides mixtures, predators, and environmental regimes on the toxicity of two pesticides to red-eyed tree frog larvae. Environ Toxicol Chem 32(10):2379–2386
Jung RE, Karasov WH, Melancon MJ (2004) Cytochrome P450 activity in green frogs (Rana clamitans melanota) exposed to water and sediments in the Fox River and Green Bay, Wiscounsin, USA. Bull Environ Contam Toxicol 73:955–962
Kats LB, Ferrer LP (2003) Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers Distrib 9:99–110
Kegley S. E, Hill B. R, Orme S, Choi A. H (2015) PAN: Pesticides Database, Pesticide Action Network. http://www.pesticideinfo.org/Search_Chemicals.jsp. Accessed 8 Dec 2015
La Marca E, Lips KR, Lotters S, Puschendorf R, Ibáñez R, Rueda-Almonacid JV, Schulte R, Marty C, Castro F, Manzanilla-Puppo J, García-Pérez JE, Bolaños F, Chaves G, Pounds JA, Toral E, Young BE (2005) CatastrophicPopulation declines and extinctions in neotropical harlequin frogs (Bufonidae: Atelopus). Biotropica 37(2):190–201
Lensen G, Jungbauer F, Goncalo M, Coenraads PJ (2007) Airborne irritant contact dermatitis and conjunctivitis after occupational exposure to chlorothalonil in textiles. Contact Dermatitis 57:181–186
Li Y, Cohen JM, Rohr JR (2013) Review and synthesis of the effects of climate change on amphibians. Integr Zool 8:145–161
Lionetto MG, Caricato R, Calisi A, Schettino T (2011) Acetylcholinesterase inhibition as a relevant biomarker in environmental biomonitoring: new insights and perspectives. In: Ecotoxicology around the globe. Nova Science Publishers, Inc. 88–115
Lips KR, Borrowes PA, Mendelson JR III, Parra-Olea G (2005) Amphibian population declines in Latin America: a synthesis. Biotropica 37(2):222–226
Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, Carey C, Livo L, Pessier AP, Collins JP (2006) Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. P Natl Acad Sci USA 103(9):3165–3170
Lips KR, Diffendorfer JE, Mendelson JR, Sears MW (2008) Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biol 6:441–454
Mazerolle MJ (2006) Improving data analysis in herpetology: using Akaike’s information criterion (AIC) to assess the strength of biological hypotheses. Amphibia-Reptilia 27:169–180
McMahon TA, Halstead NT, Johnson S, Raffel TR, Romansic JM, Crumrine PW, Boughton RK, Martin LB, Rohr JR (2011) The fungicide chlorothalonil is nonlinearly associated with corticosterone levels, immunity, and mortality in amphibians. Environ Health Perspect 119(8):1098–1103
McMahon TA, Halstead NT, Johnson S, Raffel TR, Romansic JM, Crumrine PW, Rohr JR (2012) Fungicide-induced declines of freshwater biodiversity modify ecosystem functions and services. Ecol Lett 15:714–722
McMahon TA, Romansic JM, Rohr JR (2013) Nonmonotonic and monotonic effects of pesticides on the pathogenic fungus Batrachochytrium dendrobatidis in culture and on tadpoles. Environ Sci Technol 47:7958–7964
Mena F, Pfennig S, Arias-Andrés M, Márquez-Couturier G, Sevilla A, Protti M (2012) Acute toxicity and cholinesterase inhibition of the nematicide ethoprophos in larvae of gar Atractosteus tropicus (Semionotiformes: Lepisosteidae). Int J Trop Biol 60(1):361–368
Mena F, Fernández San Juan M, Campos B, Sánchez-Ávila J, Faria M, Pinnock M, de la Cruz E, Lacorte S, Soares AMVM, Barata C (2014) Pesticide residue analyses and biomarker responses of native Costa Rican fish of the Poeciliidae and Cichlidae families to assess environmental impacts of pesticides in Palo Verde National Park. J Environ Biol 35:19–27
Mendelson JR III, Lips KR, Gagliardo RW, Rabb GB, Collins JP, Diffendorfer JE, Daszak P, Ibáñez R, Zippel KC, Lawson DP, Wright KM, Stuart SN, Gascon C, da Silva HR, Burrowes PA, Joglar RL, La Marca E, Lötters S, du Preez LH, Weldon C, Hyatt A, Rodriguez-Mahecha JV, Hunt S, Robertson H, Lock B, Raxworthy CJ, Frost DR, Lacy RC, Alford RA, Campbell JA, Parra-Olea G, Bolaños F, Calvo Domingo JJ, Halliday T, Murphy JB, Wake MH, Coloma LA, Kuzmin SL, Price MS, Howell KM, Lau M, Pethiyagoda R, Boone M, Lannoo MJ, Blaustein AR, Dobson A, Griffiths RA, Crump ML, Wake DB, Brodie ED Jr (2006) Confronting amphibian declines and extinctions. Science 313:48
Oakes FD, Van Der Kraak VD (2003) Utility of TBARS assay in detecting oxidative stress in white sucker (Catotomus commersoni) populations exposed to pulp mill effluent. Aquat Toxicol 63:447–463
OECD (2009) Guideline for Testing of Chemicals, No. 231: The Amphibian Metamorphosis Assay
Pounds JA, Crump ML (1994) Amphibian declines and climate disturbance: the case of the golden toad and the harlequin frog. Conserv Biol 8(1):72–85
Pounds JA (2001) Climate and amphibian declines. Nature 410:639–640
Pathiratne A, Chandrasekera L, De Seram P (2008) Effect of biological and technical factors on brain and muscle cholinesterases in Nile tilapia, Oreochromis niloticus: implications for biomonitoring neurotoxic contamination. Arch Environ Contam Toxicol 54:309–317
Penagos H (2002) Contact dermatitis caused by pesticides among banana plantation workers in Panama. Int J Occup Environ Health 8:14–18
R Development Core Team (2014) R Foundation for Statistical Computing. Version 3.1.2. http://www.rproject.org/
Rohr JR, Raffel TR (2010) Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. P Natl Acad Sci USA 107:8269–8274
Rohr JR, Palmer BD (2013) Climate change, multiple stressors, and the decline of ectotherms. Conserv Biol 27(4):741–751
Shelley LK, Balfry SK, Ross PS, Kennedy CJ (2009) Immunotoxicological effects of a sub-chronic exposure to selected current-use pesticides in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 92:95–103
Shunthirasingham C, Gouin T, Lei YD, Ruepert C, Castillo LE, Wania F (2011) Current-use pesticide transport to Costa Rica’s high-altitude tropical cloud forest. Environ Toxicol Chem 30(12):2709–2717
Sistema Nacional de Areas de Conservación (SINAC) (2009) IV Informe de País al Convenio sobre la Diversidad Biológica. GEF-PNUD. Mimeografiado. 220 p
Sparling D, Fellers G, McConnell L (2001) Pesticides and amphibian population declines in California, USA. Environ Toxicol Chem 20:1591–1595
Sparling DW, Fellers GM (2009) Toxicity of two insecticides to California, USA, anurans and its relevance to declining amphibian populations. Environ Toxicol Chem 28(8):1696–1703
Sparling DW, Linder G, Bishop CA, Krest SK (2010) Ecotoxicology of amphibians and reptiles. SETAC Press, USA
The IUCN Red List of Threatened Species. Version 2015.2. www.iucnredlist.org. Downloaded on 25 August 2015
Thomson H (1999) Esterases as markers of exposure to organophosphates and carbamates. Ecotoxicology 8:369–384
US Environmental Protection Agency (2015) ECOTOX Database. http://www.epa.gov/ecotox. Accessed 8 Dec 2015
Van Scoy AR, Tjeerdema RS (2014) Environmental fate and toxicology of chlorothalonil. Rev Environ Contam T 232:89–105
Venturino A, Rosenbaum E, Caballero de Castro A, Anguiano OL, Gauna L, Fonovich de Schroeder T, de D’Angelo P (2003) Biomarkers of effect in toads and frogs. Biomarkers 8(3–4):167–186
Wang G, Liang B, Li F, Li S (2011) Recent advances in the biodegradation of chlorothalonil. Curr Microbiol 63(5):450–457
Whitfield SM, Bell KE, Philippi T, Sasa M, Bolaños F, Chaves G, Savage JM, Donnelly MA (2007) Amphibian and reptile declines over 35 years at La Selva, Costa Rica. PNAS 104(20):8352–8356
Whitfield SM, Lips KR, Donnelly MA (2009) Decline and conservation of amphibians in central America. In: Heatwole HH, Barrios-Amoros C, Wilkenson JW (eds) Amphibian biology, Vol 9—status of conservation and declines of amphibians: western hemisphere. Surrey Beatty and Sons, Sydney, Australia
Yu S, Wages MR, Cobb GP, Maul JD (2013) Effects of chlorothalonil on development and growth of amphibian embryos and larvae. Environ Pollut 181:329–334
Acknowledgments
This project was supported by the Fondo Especial para la Educación Superior (FEES-CONARE). The authors recognize the contributions of Professor Manuel Spínola, PhD and MSc. Randall Jiménez during the data analysis; Professor David R. S. Lean, PhD for the revision of the English language of the final manuscript and the two anonymous reviewers for their thorough revision of the document. We also want to extent our gratitude to all the friends and colleagues who supported us in the collections of the eggs masses and tadpoles for the experiments.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Thomas Braunbeck
Rights and permissions
About this article
Cite this article
Méndez, M., Obando, P., Pinnock-Branford, M. et al. Acute, chronic and biochemical effects of chlorothalonil on Agalychnis callidryas, Isthmohyla pseudopuma and Smilisca baudinii tadpoles. Environ Sci Pollut Res 23, 21238–21248 (2016). https://doi.org/10.1007/s11356-016-7301-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7301-1