Abstract
Wildlife and livestock are known to visit and interact with tailings dam and other wastewater impoundments at gold mines. When cyanide concentrations within these water bodies exceed a critical toxicity threshold, significant cyanide-related mortality events can occur in wildlife. Highly mobile taxa such as birds are particularly susceptible to cyanide toxicosis. Nocturnally active bats have similar access to uncovered wastewater impoundments as birds; however, cyanide toxicosis risks to bats remain ambiguous. This study investigated activity of bats in the airspace above two water bodies at an Australian gold mine, to assess the extent to which bats use these water bodies and hence are at potential risk of exposure to cyanide. Bat activity was present on most nights sampled during the 16-month survey period, although it was highly variable across nights and months. Therefore, despite the artificial nature of wastewater impoundments at gold mines, these structures present attractive habitats to bats. As tailings slurry and supernatant pooling within the tailings dam were consistently well below the industry protective concentration limit of 50 mg/L weak acid dissociable (WAD) cyanide, wastewater solutions stored within the tailings dam posed a minimal risk of cyanide toxicosis for wildlife, including bats. This study showed that passively recorded bat echolocation call data provides evidence of the presence and relative activity of bats above water bodies at mine sites. Furthermore, echolocation buzz calls recorded in the airspace directly above water provide indirect evidence of foraging and/or drinking. Both echolocation monitoring and systematic sampling of cyanide concentration in open wastewater impoundments can be incorporated into a gold mine risk-assessment model in order to evaluate the risk of bat exposure to cyanide. In relation to risk minimisation management practices, the most effective mechanism for preventing cyanide toxicosis to wildlife, including bats, is capping the concentration of cyanide in tailings discharged to open impoundments at 50 mg/L WAD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanide is the most significant contaminant influencing wildlife mortality in the gold mining industry (Henny et al. 1994; Eisler and Wiemeyer 2004; Donato et al. 2007). A range of vertebrate fauna visit, and interact with, cyanide-bearing solutions stored in open impoundments such as tailings dams and process water ponds at gold mining operations (Donato 1999; Donato et al. 2007). When concentrations of bioavailable cyanide exceed a critical threshold, cyanide toxicosis can occur in wildlife (Creekmore 1999; National Industrial Chemicals Notification and Assessment Scheme 2010). However, documentation of wildlife cyanide toxicosis events at gold mining operations is scarce (Clark and Hothem 1991; Henny et al. 1994; Sinclair et al. 1997; Donato 1999). Defining prescriptive lethal toxicity concentration thresholds for the range of species that interact with cyanide-bearing solutions within tailings impoundments is therefore difficult.
Birds are particularly susceptible to cyanide toxicosis at wastewater impoundments at gold mines (Eisler and Wiemeyer 2004). It is not known if this sensitivity is primarily related to their physiology,or whether flight simply makes open impoundments more accessible to birds than less mobile wildlife (Wiemeyer et al. 1986; Donato et al. 2007). Bats (Chiroptera) also have easy access to cyanide-bearing water bodies, but the risks they present to bats are not well understood (O'Shea et al. 2001), particularly in terms of a critical cyanide concentration toxicity threshold (Clark 1991; Clark et al. 1991). However, research conducted in North America shows conclusively that bats are at risk and significant cyanide-related mortality events can occur. For example, between 1980 and 1989, bats and rodents were the most commonly reported mammal mortalities (34 and 35 % of 519 deaths, respectively) at cyanide-extraction gold mines in California, Nevada and Arizona (Clark and Hothem 1991), and Henny et al. (1994) reported that bats comprised 25 % of 665 cyanide-related mammal mortalities reported at gold mining operations in Nevada between 1986 and 1991.
Despite this evident risk, monitoring interactions between bats and water bodies at gold mines is either inadequate or non-existent (Donato et al. 2007; Griffiths et al. 2009). Limited electronic monitoring of bat echolocation calls conducted at mine sites in the Eastern and Northern Goldfields of Western Australia has shown that bats are common visitors to the airspace above tailings dams and a range of other water bodies at gold mines (Donato and Smith 2007; Smith et al. 2008), but the degree to which bats ingested cyanide-bearing solutions could not be determined.
Bats are commonly recorded in large numbers in the airspace above water bodies from dusk to early evening when they emerge from daytime roosts to forage and drink (Ciechanowski 2002; Korine and Pinshow 2004; Adams and Thibault 2006). Bats drink by swooping down to lap at the water’s surface while in flight (i.e. drinking on the wing) (Cockrum and Cross 1964; Adams and Simmons 2002; Krausman et al. 2006), a mode of drinking similar to that adopted by swifts (Apodidae) and swallows and martins (Hirundinidae) (Higgins et al. 2006). In hot, arid environments bats require daily access to free water to maintain a positive water balance (Webb 1995; Razgour et al. 2010). The water requirements of bats change throughout the year, such as when females require significantly more water during lactation (Kurta et al. 1989a, b; Kurta et al. 1990). Such physiological requirements can exert a strong influence on temporal patterns of bat activity at sources of drinking water (Adams and Hayes 2008; Geluso and Geluso 2012). Factors influencing temporal patterns of bat activity at gold mining water bodies, and thereby patterns in the risk of exposure to cyanide, have not been investigated previously.
Since its inception in May 2000, the International Cyanide Management Code for the Manufacture, Transport and Use of Cyanide in the Production of Gold (the Cyanide Code) has provided impetus for the gold mining industry to develop methods to investigate exposure of wildlife, including bats, to cyanide (and other toxicants) stored in open impoundments (International Cyanide Management Institute (ICMI 2012b). The Cyanide Code is a voluntary programme for gold mining companies, and producers and transporters of cyanide used in the gold mining industry, to develop industry-wide improvements for the responsible management of cyanide (ICMI 2005). To attain certification as a signatory to the Cyanide Code, gold mining operations must undergo an independent third-party audit of all monitoring procedures and practices relating to potential environmental impacts associated with the use of cyanide (ICMI 2007). There was, however, a significant knowledge gap in understanding, monitoring and managing risks of wildlife exposure to cyanide-bearing waste solutions (Smith and Donato 2007). Recent studies in Australia have focused on risks of diurnal wildlife interactions with wastewater impoundments while highlighting the need for further investigation into risks posed to nocturnal wildlife (Donato and Smith 2007; Adams et al. 2008; Donato et al. 2010). While this knowledge gap exists, full certification under the Cyanide Code remains problematic (Donato et al. 2008).
To help address the lack of prescriptive methods for monitoring bat interactions with water bodies at gold mines, we investigated long-term activity of bats in the airspace above two water bodies at a Cyanide Code-certified Australian gold mine. Our aims were:
-
1.
To quantify the activity patterns and species composition of echolocating bats in the airspace above gold mining water bodies; and
-
2.
Based on these findings, develop recommendations for methods to monitor this component of the nocturnal fauna for compliance with the Cyanide Code.
Methods
Study site
This study was conducted at Barrick Gold Corporation’s Cowal Gold Mine (CGM), located in central New South Wales (NSW), Australia (33° 38′ S, 147° 23′ E). The climate of West Wyalong (32 km south of CGM) is characterised by warm summers (average summer maximum, 32 °C), cool winters (average winter maximum, 15 °C) and average rainfall of 481 mm/annum (Australian Bureau of Meteorology 2012).
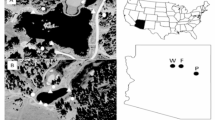
Operation of CGM under Barrick Gold Corporation commenced on 30 April 2006. CGM is an open-cut and underground mining operation, with mill process facilities designed to treat ore by conventional carbon-in-leach and sulphide floatation technologies including the use of sodium cyanide. A two-celled peripheral discharge paddock-style tailings dam covering an area of approximately 170 ha was constructed to treat and recycle mine waste (tailings) (Fig. 1). A small, freshwater farm dam (Hillgrove Dam), with an approximate surface area of 0.9 ha, is located 5 km west of the tailings dam (Fig. 1).
CGM is a signatory to the Cyanide Code and has developed systematic procedures to protect wildlife from waste solutions stored in the tailings dam (ICMI 2012a). In accordance with Consent Condition 5.3(a) of CGM’s NSW Environment Protection License (EPL no. 11912, NSW Department of Environment and Heritage), mineral processing is designed to ensure cyanide levels in the tailings dam are maintained below 20 mg/L weak acid dissociable (WAD) cyanide for 90 % of the time, with an allowance to exceed this level from ≤10 % of the time to ≤30 mg/L WAD. These concentrations are considered to be non-toxic to wildlife (Eisler 2000; Eisler and Wiemeyer 2004) and are well below the protective concentration threshold of 50 mg/L WAD cyanide prescribed by the Cyanide Code (ICMI 2005).
Water samples were collected by CGM personnel at the tailings discharge point (spigot) and in the decant water (supernatant) of the tailings dam twice daily during the course of this study (February 2011 to May 2012). On-site laboratory tests of WAD cyanide concentration were undertaken using an Analytical CNSolutionTM FS 3100 analyser (Orion Instruments, Redwood City, California). Daily cyanide concentrations were consistently well below the 50 mg/L WAD protective concentration limit, and there was extremely low variation in cyanide concentration at the spigot (mean (±standard error (s.e.)) = 4.9 ± 0.09 mg/L WAD) and in the supernatant pond (mean = 1.9 ± 0.05 mg/L WAD). The maximum tailings discharge concentration was 26 mg/L WAD cyanide (28 August 2011), exceeding 10 mg/L WAD on only 21 days (4.6 % of all days). The maximum cyanide concentration recorded in supernatant pooling in the central decant pond (the location where bats are at highest risk of exposure via drinking on the wing) was 10 mg/L WAD (30 August 2011).
CGM has implemented measures designed to minimise the use of the tailings dam by fauna by maintaining the facilities as non-conducive habitats to wildlife (Condition E5.1 of CGM’s NSW Environment Protection License. There is a 2-m high fence around the perimeter of the tailings dam with a mesh gauge of 50 mm in the upper half and 20-mm diameter in the lower half. In addition, fence mesh is buried 500 mm below ground level to deny access to burrowing animals. The fence is designed to keep medium and large terrestrial fauna (such as echidnas, kangaroos, wallabies and stock), as well as amphibians, from entering the tailings dam. Consequently, the only vertebrate wildlife likely to interact with cyanide-bearing tailings at CGM are flying birds and bats. These animals are therefore the focus of monitoring and remedial activities.
Acoustic sampling of bat calls
Bat echolocation calls were used as an index of bat activity in the airspace directly above water bodies. Bat activity was surveyed with ultrasonic bat detectors (Anabat SD1, Titley Scientific, Lawnton, Queensland), calibrated by adjusting sensitivity levels against an ultrasound frequency generator (Anabat Chirper 2, Titley Scientific). Permanent detector stations were established in February 2011 at the tailings dam and farm dam. Echolocation data were collected nightly until May 2012. Detectors were placed within weatherproof plastic boxes, with the microphone protruding through a hole cut in the side of the box. Boxes were secured to a metal stake at a height of approximately 1 m, and the microphone was oriented parallel to the water surface, facing the middle of the water body. The detectors were programmed to start recording 30 min before sunset and end recording at sunrise and were triggered automatically (to record a single file up to 15 s in length) by ultrasonic noise produced by a bat flying within range of the microphone (zone of reception up to approximately 30 m).
Echolocation call data were downloaded to a computer using CFCread version 4.3 s then analysed using AnalookW version 3.8 s (Corben 2011, www.hoarybat.com). A call sequence was defined as a file containing at least three echolocation pulses (frequency sweeps) identified as bat echolocation calls (Hourigan et al. 2008), and each call sequence separated by >5 s was designated a unique file (Turbill 2008).
Temporal patterns of activity: February 2011 to May 2012
Sonograms (frequency versus time graphs) of recorded bat call sequences were manually sorted in order to define activity, pooling call sequences for all species to investigate activity patterns of the entire assemblage of bats recorded in the airspace above the two water bodies. All call sequences were then screened manually for buzz calls associated with pursuit and capture of prey and drinking on the wing. Terminal buzz calls are characterised by a rapid increase in pulse repetition rate, slope, frequency and speed (Griffin 1958), and are clearly audible in a frequency-divided audio file of a call sequence. The presence of audible buzz calls within call sequences was assessed to provide validation of buzz calls detected by visual inspection of sonograms. Sonograms were converting into audio (.WAV) files, at a frequency division ratio of 8, in AnalookW, then played in Windows Media Player version 12 (Microsoft, Redmond, Washington).
Mean monthly activity patterns were calculated for nights of matched recordings at the tailings dam and farm dam (i.e. nights when detectors worked at both water bodies) from the nightly number of call sequences recorded each month. Hourly activity patterns were defined as the mean number of call sequences recorded per hour after civil twilight, from Geoscience Australia (http://www.ga.gov.au/geodesy/astro/sunrise.jsp), for all species over all recording nights from February 2011 to May 2012 at both water bodies. Hourly patterns of foraging/drinking were also investigated by calculating the number of buzz calls recorded each hour after civil twilight. Minimum nightly ambient temperature, mean wind speed, mean barometric pressure and total rainfall were derived for each night of bat sampling from hourly recordings taken between dusk and dawn by a weather station (GRWS100, Campbell Scientific; 17 m above ground level) located at the CGM main administration building.
Activity recorded by multiple detectors within the tailings storage facility
In February 2012, two detectors were deployed in addition to the permanent detector station within the tailings dam (Fig. 2) to assess whether detector location within the central supernatant pond influenced the number of call sequences recorded. The three detectors were calibrated and enclosed in weatherproof housing as described above. The detectors were placed ≥50 m apart and recorded from sunset to sunrise for 13 consecutive nights from 27 January to 8 February 2012.
Aerial photograph showing approximate position of three bat detectors within the Cowal Gold Mine tailings dam central supernatant pond. The three detectors were operational on 13 consecutive nights from 27 January to 8 February 2012. Site 1 was the location of the permanent bat detector station used to collect monthly data (April 2006 to May 2012) for species composition analysis and continuous data from February 2011 to May 2012
Species identification: 2006–2012 monthly dataset
To supplement the nightly recordings from February 2011 to May 2012, a longer-term dataset was incorporated to investigate broad patterns of species diversity. Echolocation calls were recorded over four consecutive nights per month from April 2006 to December 2011 at the tailings dam and farm dam. A subset (four consecutive nights per month) of the February 2011 to May 2012 dataset was also included in the analysis. One observer (Donato) identified call sequences recorded in the first 4 h after civil twilight to taxa known to occur in the study area. Voucher calls presented in Pennay et al. (2004) were used as a reference library. Where individual species could not be clearly distinguished, call sequences were identified to genus, with three genera recognised: long-eared bats (Nyctophilus), broad-nosed bats (Scotorepens), forest bats (Vespadelus) and free-tailed bats (Mormopterus) (Supplementary material Table S1). A conservative approach to species identification was taken and any ambiguous calls were not identified.
Data analysis
Echolocation call data recorded on 212 matched nights at the tailings dam and farm dam were significantly non-normal (Shapiro–Wilk W test: P < 0.001) (Dytham 2011). Consequently, a non-parametric Wilcoxon signed-ranks test was used to assess differences in activity on matched nights at the tailings dam and farm dam (Quinn and Keough 2002). The 212 matched nights were separated into seasons (spring, summer, autumn and winter), and differences in activity among seasons were assessed using Wilcoxon signed-ranks tests. The Kolmogorov–Smirnov two-sample test was used to determine whether activity at each water body was evenly distributed throughout the night (Dytham 2011).
Using the same 212 matched nights, a non-parametric Wilcoxon signed-ranks test was used to test for differences in the number of terminal buzz calls recorded in the airspace above the two water bodies. The Kolmogorov–Smirnov two-sample test was used to assess if buzz calls were evenly distributed throughout the night. A one-way between-groups analysis of variance (ANOVA) was used to assess the influence of detector location within the tailings dam supernatant pond on bat activity (over 13 consecutive nights from 27 January to 8 February 2012) (Quinn and Keough 2002). To meet assumptions of normality and equal variances, bat activity data (call sequences per night) were square-root transformed.
For the April 2006 to May 2012 monthly species diversity dataset, a Wilcoxon signed-ranks test was used to assess differences in activity of four taxa (Gould’s wattled bat, Chalinolobus gouldii; white-striped free-tailed bat, Tadarida australis; free-tailed bat, Mormopterus spp.; forest bat, Vespadelus spp.) at the two water bodies. These taxa were selected because they collectively comprised 96.4 and 95.8 % of identified call sequences at the tailings dam and farm dam, respectively. All statistical analyses described above were carried out the using SPSS version 20.0 (IBM, Chicago, Illinois), and bat activity data are reported in the text as mean ± s.e. (except where stated otherwise).
A linear mixed-effects model was used to assess the influence of weather variables on bat activity using Genstat version 14 (VSN International Ltd., UK) (Bolker et al. 2009). As weather data were not available for every night, a subset (136 nights) of bat activity data recorded on 212 matched nights at the two water bodies was analysed. The maximal model contained four explanatory variables for the weather (minimum nightly temperature, mean nightly wind velocity, maximum nightly barometric pressure and nightly rainfall), with site (two levels: tailings dam and farm dam) and date (136 levels) and their interaction included as random factors; date was modelled using an AR1 correlated error structure. To help satisfy modelling assumptions, Ln(bat activity + 1) was used as the outcome variable. The most parsimonious model was derived by removal of non-significant (P > 0.05) terms.
Results
Comparison of activity at the tailings dam and farm dam: 2011–2012 dataset
Over 16 months from February 2011 to May 2012, bat activity was recorded in the airspace above both water bodies on almost all recording nights. Zero call sequences were recorded on only 23 nights (7.6 %) at the tailings dam and 19 nights (8.6 %) at the farm dam. A total of 50,620 bat call sequences were recorded on 521 detector nights, comprising 32,669 calls from 301 nights at the tailings dam (mean ± standard deviation = 108.8 ± 161.9) and 17,951 calls from 220 nights at the farm dam (mean ± standard deviation = 81.6 ± 102.3); reduced from a possible 450 nights at each because of mechanical failure or limitations in the availability of equipment.
Activity on matched nights
Matched nights of data were recorded on 212 nights from February 2011 to May 2012, yielding 25,022 and 17,829 echolocation call sequences at the tailings dam and farm dam respectively. Overall bat activity did not differ significantly between the two water bodies (Wilcoxon signed-ranks test: Z = −1.657, P = 0.098). Monthly bat activity at the tailings dam and farm dam varied considerably (Fig. 3). The highest mean activity at the tailings dam was in February 2011, with another peak in October 2011. Mean activity at the farm dam also peaked in February 2011 and October 2011. A higher mean activity was recorded at the tailings dam in the first 9 months when data were recorded; this pattern then reversed and mean activity was higher at the farm dam for the last 3 months (Fig. 3).
Monthly mean (±s.e.) echolocation call sequences per night on paired recording nights at the tailings dam and farm dam from February 2011 to May 2012. Numbers in brackets are the total number of recording nights per month when detectors functioned at both sites on the same night; no echolocation data were recorded (due to equipment malfunction) in the autumn months April and May 2011 and March and April 2012
At a finer resolution, there was considerable variation in nightly activity, including the occurrence of spikes in activity, documented at both water bodies. There was marked night-to-night variation during periods of both (a) low (June 2011) and (b) high (November 2011) activity (Fig. 4). Mean activity at the tailings dam in June 2011 was 23.2 ± 8.7 (range 0 to 249 calls). In November 2011, the mean was 136.9 ± 18.7 (range 11 to 395 calls). At the farm dam, monthly mean activity for June and November were 14.1 ± 3.6 (range 0 to 78) and 110.8 ± 14.4 (range 8 to 300), respectively. Similar spikes of activity occurred across the entire survey period; however, activity spikes did not occur at both water bodies on the same nights (Fig. 4). The maximum activity was 1,243 calls at the tailings dam in August 2011 and 642 calls at the farm dam in February 2012.
There were differences in relative bat activity among seasons at the two water bodies. Mean activity was greater at the tailings dam than the farm dam in winter (Wilcoxon signed-ranks test: Z = −3.499, P < 0.001; tailings dam = 117.0 ± 28.1, farm dam = 28.8 ± 4.1) and spring (Z = −2.122, P = 0.034; tailings dam = 146.9 ± 18.3, farm dam = 102.0 ± 14.8), while activity was greater at the farm dam than tailings dam in summer (Z = −1.974, P = 0.048; tailings dam = 148.3 ± 23.1, farm dam = 189.3 ± 17.5), with similar activity at both water bodies in autumn (Z = −0.292, P = 0.770; tailings dam = 153.0 ± 44.1, farm dam = 100.9 ± 16.0).
Although considerable variation occurred in night-to-night bat activity recorded at three detector sites within the tailings dam over 13 consecutive nights in January–February 2012, mean activity at the three sites was similar (F 2, 36 = 0.119, P = 0.888; site 1 = 91.1 ± 27.3, site 2 = 108.0 ± 24.3, site 3 = 104.1 ± 35.1).
Hourly activity
For all calls combined, highest activity occurred in the first hour after civil twilight (tailings dam 22.3 % and farm dam 23.2 % of call sequences), whereas more than 60 % of all calls occurred in the first 4 h after civil twilight at both water bodies. For calls on 212 matched nights at the two water bodies separated into seasons, the hourly distribution of activity was significantly non-uniform (Kolmogorov–Smirnov two-sample tests, P > 0.05) (Supplementary material Fig. S1). Nightly activity patterns were similar at both water bodies across all seasons, with highest activity occurring within 4–5 h of sunset, then steadily decreasing towards dawn. However, bats were consistently present in the airspace above both water bodies throughout the night. There was a slight pre-dawn increase in activity at the farm dam in autumn.
Effect of weather
Bat activity (Ln(echolocation call sequences + 1)) was positively related to minimum nightly temperature (linear mixed-effects model: estimate = 0.1611, s.e. = 0.0197; P < 0.001),and negatively related to mean nightly wind velocity (linear mixed-effects model: estimate = −0.3404, s.e. = 0.0764; P < 0.001). Bat activity was not significantly related to maximum nightly barometric pressure or nightly rainfall (P > 0.05 for both).
Terminal phase buzz calls
Of the 32,669 echolocation call sequences recorded at the tailings dam, 3.5 % (1,155 calls) contained a terminal buzz call, while 2.3 % (415 calls) of the 17,951 call sequences recorded at farm dam contained a buzz call. The maximum number of buzz calls in one night at the tailings dam was 103 in August 2011, while at the farm dam the nightly maximum was 34 in 14 October 2011. On matched recording nights, there were significantly more buzz calls (Wilcoxon signed-ranks test, Z = −3.733; P < 0.001) at the tailings dam (mean = 4.5 ± 0.7) than the farm dam (mean = 1.9 ± 0.3). The hourly distribution of buzz calls was significantly non-uniform (Kolmogorov–Smirnov two-sample tests, P > 0.05) at both water bodies. Although buzz calls were recorded throughout the night at both water bodies, the majority occurred in the first 4 h after civil twilight (69.8 % at the tailings dam, 78.6 % at the farm dam) (Fig. 5).
Percentage of foraging/drinking buzz calls detected each hour after civil twilight at the tailings dam and farm dam. Data presented are from 301 nights of echolocation recording at the tailings dam and 220 nights at the farm dam from February 2011 to May 2012. During spring and summer, dawn occurs in the 9th and 10th hours after civil twilight; whereas in autumn and winter, it occurs in the 11th and 12th hours
Species identification from long-term dataset (2006–2012)
A total of 13,233 and 8,424 echolocation call sequences were recorded in the first 4 h after civil twilight over 150 matched nights from April 2006 to May 2012 at the tailings dam and farm dam respectively. Of these, 75.3 % of call sequences from the tailings dam were identified to the level of species or genus, as were 68.4 % from the farm dam (Table 1). All bat genera present in the West Wyalong region of NSW were recorded at least once in the airspace above the tailings dam and/or the farm dam (Supplementary material Table S1). Pairwise comparisons (between water bodies) of identified calls indicated Gould’s wattled bat, free-tailed bat spp. and the white-striped free-tailed bat were significantly more active at the tailings dam than farm dam (Wilcoxon signed-ranks test: Gould’s wattled bat, Z = −3.624 (P = 0.001); free-tailed bat spp., Z = −4.7 (P < 0.001); white-striped free-tailed bat, Z = −6.250 (P < 0.001)), whereas activity of forest bats was similar at both water bodies (Z = −0.532; P = 0.595). The sample size for the other species/genera was too small for a statistical comparison.
Discussion
Activity patterns and cyanide toxicosis risks to bats
To our knowledge, this study represents the longest running survey of bat activity in the airspace above water bodies at a gold mine. Although activity was highly variable across nights and months, bats were present at both the tailings dam and farm dam on the majority of nights throughout the survey period, and all known genera from the West Wyalong region were recorded during the six years of sampling. This suggests that a wide range of species and large numbers of individuals have the potential to interact with cyanide in gold mining wastewater impoundments.
Our findings highlight the need to consider the variable nature of bat activity when monitoring wildlife at cyanide-bearing water bodies. Bat activity can be highly variable, both within and between nights (Fenton et al. 1977; Kuenzi and Morrison 2003). Nightly bat activity has been correlated with a range of abiotic factors, including nocturnal light intensity (Ciechanowski et al. 2007), temperature (Bullen and McKenzie 2005; Milne et al. 2005a), air pressure (Turbill 2008), rainfall and subsequent availability of drinking water (Geluso and Geluso 2012). However, the patterns documented are not universally consistent. Variation in nightly activity recorded during this study was significantly correlated to temperature and wind velocity, but not rainfall or air pressure. During this study, consecutive years of above average rainfall occurred, resulting in multiple significant region-wide flooding events in 2011 and 2012 (Australian Bureau of Meteorology 2012), and thus an abundance of water away from the two sampled water bodies. Consequently, correlations between nightly bat activity and weather variables during this study may differ under other environmental conditions. More generally, the factors influencing temporal activity in the airspace above water bodies at gold mines are likely to vary with the geographic location of the mine and the season in which monitoring is conducted.
Bats often display consistent temporal patterns of activity throughout the night (Law et al. 1998; Milne et al. 2005b; Adams and Thibault 2006). Concentrated activity at water bodies immediately after dusk has been reported previously (Cockrum and Cross 1964; Lumsden and Bennett 1995; Korine and Pinshow 2004; Geluso 2007; Geluso and Geluso 2012). A peak in activity at water bodies immediately after nightfall has been related to two primary factors: (1) concentrated foraging effort to exploit crepuscular activity patterns of invertebrate communities (Taylor 1963; Racey and Swift 1985; Rautenbach et al. 1988; Kalcounis-Rüeppell et al. 2007) and (2) the need to maintain a positive water balance by drinking from surface water upon emerging from diurnal roosts (Webb 1995; Kurta et al. 1989a, b; Adams and Hayes 2008). During this study, activity at both water bodies was greatest in the hour immediately after civil twilight, and more than 60 % of all activity occurred in the first 4 h after civil twilight. Furthermore, the majority of echolocation sequences containing terminal buzz calls were recorded in the first 4 h after civil twilight. Although terminal buzz calls are often used to infer a rate of bat foraging activity (Vaughan et al. 1996; Polak et al. 2011), buzz calls recorded in the airspace directly above water are also produced by echolocating bats drinking on the wing (Russo et al. 2012; Griffiths 2013). However, it is currently not possible to separate drinking and foraging buzz calls solely on characteristics of echolocation calls (Griffiths 2013). When dealing specifically with echolocation call data recorded at water bodies, it may therefore be appropriate to use the term ‘terminal buzz calls’ to incorporate both foraging and drinking behaviour (Griffiths et al. 2014). Monitoring techniques such as the use of infrared video recording could be employed to investigate this further, particularly if cyanide concentrations in open impoundments are above the 50 mg/L WAD industry protective limit.
When cyanide concentrations in gold mining wastewater impoundments exceed a critical toxicity threshold significant mortality events can occur in bats (Clark and Hothem 1991; Henny et al. 1994). Although there is little information relating specifically to cyanide tolerance in bats, one laboratory-based study has investigated lethal dose at 50 % (LD50) of little brown bats Myotis lucifugus. When dosed orally with sodium cyanide, the little brown bat, white-footed mouse Peromyscus leucopus, house mouse Mus musculus and mallard Anas platyrhinchos, showed 24 h LD50 values of 8.4, 28, 8.7 and 2.9 mg kg−1 body weight, respectively (Clark and Hothem 1991). By this measure, the sensitivity of the little brown bat was similar to the house mouse, but the threefold variation between the two mice species indicates that other bat species would need to be tested before any generalities can be drawn between groups of species (Clark et al. 1991). However, in the absence of any further toxicological studies, it is reasonable to predict that critical toxic thresholds for free-ranging bats interacting with gold mining wastewater impoundments would be similar to that reported in other small insectivorous mammals (see Eisler 2000). A maximum concentration of 50 mg/L WAD cyanide in solution is typically viewed as being protective of most terrestrial wildlife and livestock (Ma and Pritsos 1997; ICMI 2005; Hagelstein and Mudder 2006). The 50 mg/L WAD cyanide concentration limit has largely been determined by field observation at tailings systems with salinity concentrations potable to wildlife (Henny et al. 1994; Donato 1999; Griffiths et al. 2014). The cyanide concentration within the CGM tailings dam (spigot discharge and pooling supernatant) was consistently well below the 50 mg/L WAD industry protective limit during this study. Therefore, supernatant stored within the CGM tailings dam posed a minimal risk of cyanide toxicosis to wildlife, including bats.
Management implications
The highly variable nature of bat activity has significant implications for monitoring with echolocation recording techniques (Milne et al. 2005b). Accurate and precise measures of bat activity can only be obtained by using intensive sampling efforts that account for temporal variation (Hayes 1997, 2000; Skalak et al. 2012). To achieve certification under the Cyanide Code, specific protocols describing temporal frequency and duration of bat detector surveys must be developed on a site-specific basis (Donato et al. 2008). Gold mining operations that discharge tailings to open impoundments at cyanide concentrations consistently below 50 mg/L WAD are likely to pose minimal risk of cyanide toxicosis to bats (Donato et al. 2007). For routine wildlife monitoring under such operating conditions, bat detector surveys should be conducted for a similar time frame as those recommended in general fauna survey guidelines: a minimum of four consecutive nights per survey (Australasian Bat Society 2006; Eyre et al. 2012). However, the frequency at which these surveys occur (i.e. monthly or quarterly) will need to be determined via negotiation between the mining operation, the independent Cyanide Code certification auditor and the ICMI. As part of this process, the gold mining operation is required to demonstration a ‘good faith effort’ to investigate the risk cyanide-bearing water bodies pose to wildlife (ICMI 2005, 2007). For sites that have no previous records of bat activity, baseline bat detector surveys should be conducted to investigate the variation in bat activity above water bodies at that particular site (e.g. monthly surveys for 6–12 months).
Any gold mining operation discharging tailings to open impoundments at cyanide concentrations exceeding 50 mg/L WAD is required to satisfy an independent auditor that no impacts to vertebrate wildlife, including bats, are occurring (ICMI 2007). Currently, the ICMI has prescribed daily wildlife surveys (including a thorough search for the presence of carcases) as a standard operating procedure for gold mining operations storing wastewater in open impoundments at concentrations above 50 mg/L WAD cyanide (Adams et al. 2008; Donato 2009; Donato et al. 2010; ICMI 2010).
At two Australian gold mines currently conducting nocturnal surveys of bat activity as part of routine wildlife monitoring procedures, a single bat detector is deployed per water body (ICMI 2010, 2012a). Data from the CGM tailings dam suggest that when surveying a centrally located supernatant pool within a conventional paddock-style tailings dam, the specific location of the bat detector does not significantly influence the number of echolocation calls recorded. However, this finding would need re-testing at more irregularly shaped tailings dams, like those constructed by damming a natural valley. As the spigot discharge point within this type of impoundment changes, ephemeral water bodies can form around the periphery (Donato, personal observation). This can also be the case at dry-stacking central-discharge tailings dams, where ephemeral supernatant ponds can form around the periphery of the dam (Griffiths et al. 2014). The location of a bat detector may significantly influence the number of echolocation calls recorded within such complex tailings dams. In these cases, several detectors may need to be deployed to ensure an accurate measure of bat activity is recorded.
Although cyanide concentrations at the CGM tailings dam during this study were consistently low, sampling at other gold mines has shown that cyanide concentration within tailings dams, and other wastewater impoundments, is not stable (Donato 1999; Adams 2001; Donato and Smith 2007; Griffiths et al. 2009). The concentration of cyanide in tailings slurry discharged to tailings dams can vary temporally in relation to changes in ore characteristics and mill processing procedures (Botz et al. 1995), resulting in spikes in concentration of bioavailable cyanide within open impoundments (Environment Australia 2003; Adams et al. 2008). The combination of fluctuating cyanide concentration within open impoundments and sporadic high rates of bat visitation greatly increases the risk of cyanide toxicosis, and has been implicated in cyanide-related mortality at gold mines (Henny et al. 1994; Sinclair et al. 1997; Eisler et al. 1999; National Industrial Chemicals Notification and Assessment Scheme 2010). This highlights the need for paired monitoring of wildlife visitation and cyanide chemistry at gold mining wastewater bodies (Donato et al. 2007).
Cyanide monitoring at gold mining operations often relies on sampling data that bears little relevance to the cyanide concentrations to which wildlife may be exposed within a wastewater impoundment (Donato 1999; Environment Australia 2003). For example, sampling supernatant only after it is pumped from the tailings dam back to the mill can result in significant underestimation of the concentration of bioavailable cyanide within the tailings dam (Eisler 2000; Adams 2001). Systematic cyanide monitoring protocols developed by CGM address this deficiency by collecting water chemistry data that accurately document the hazard: at the tailings discharge point (spigot) into the tailings dam and in the supernatant decant pond in the centre of the tailings dam (ICMI 2012a). This monitoring method moves away from reactive procedures, which only document the cause of impacts (Sinclair et al. 1997), towards the development of preventative cyanide concentration trigger values, designed to detect an increase in the likelihood of wildlife cyanide toxicosis prior to the occurrence of wildlife deaths (Donato et al. 2007, 2008). To achieve compliance with the Cyanide Code, systematic sampling of cyanide concentrations within open impoundments should be conducted to document the hazard to wildlife prior to the occurrence of mortality incidents (ICMI 2005). The frequency of sampling required for compliance is typically determined on a site-by-site basis, however if cyanide concentrations at discharge into the tailings dam approach or exceed 50 mg/L WAD, then daily sampling is necessary (ICMI 2007).
Conclusions
Passively recorded echolocation data presented here provide empirical evidence of the presence and activity patterns of bats above gold mining water bodies. These observations are consistent with data presented on wildlife cyanide toxicosis risks at gold mining operations for a wide range of diurnal vertebrate taxa (Donato and Smith 2007; Smith et al. 2008; Adams et al. 2008; Hudson and Bouwman 2008; Griffiths et al. 2009; Donato et al. 2010). It is therefore clear that, despite the artificial nature of wastewater impoundments at gold mines, these structures present attractive habitats to vertebrate wildlife, including bats (Griffiths et al. 2014).
Both echolocation monitoring and systematic sampling of cyanide concentration in open wastewater impoundments can be incorporated into a gold mine risk-assessment model in order to evaluate the potential of bat exposure to cyanide. Documenting empirical evidence of the extent of bats drinking on the wing is logistically difficult when sampling remotely with bat detectors (Griffiths et al. 2009). However, echolocation buzz calls recorded in the airspace directly above water provide indirect evidence of foraging and/or drinking behaviour (Griffiths 2013). A high rate of buzz calls recorded above a cyanide-bearing water body may be used as a risk assessment trigger point to activate control measures to reduce cyanide concentrations below 50 mg/L WAD. Fewer options are available for operations consistently discharging tailings to open impoundments at cyanide concentrations exceeding 50 mg/L WAD, as the efficacy of control measures designed to physically exclude wildlife from accessing wastewater impoundments (e.g. bird balls; Ramirez 2010) are untested for nocturnally active taxa such as bats.
The most effective mechanism for preventing cyanide toxicosis to wildlife, including bats, is reducing the concentration of cyanide in tailings discharged to open impoundments to below 50 mg/L WAD (Donato et al. 2008; Griffiths et al. 2009). Limiting spigot discharge concentration to this level will result in habitats downstream of the spigot (i.e. fast-flowing tailings streams and ponding supernatant) being consistently well below 50 mg/L WAD (Donato et al. 2007; Adams et al. 2008).
Abbreviations
- CGM:
-
Cowal Gold Mine
- ICMI:
-
International Cyanide Management Institute
- LD50 :
-
Lethal dose at 50 %
- NSW:
-
New South Wales
- WAD:
-
Weak acid dissociable
References
Adams MD (2001) A methodology for determining the deportment of cyanide losses in gold plants. Miner Eng 14:383–90
Adams RA, Hayes MA (2008) Water availability and successful lactation by bats as related to climate change in arid regions of western North America. J Anim Ecol 77:1115–21
Adams RA, Simmons JA (2002) Directionality of drinking passes by bats at water holes: is there cooperation? Acta Chiropt 4:195–99
Adams RA, Thibault KM (2006) Temporal resource partitioning by bats at water holes. J Zool 270:466–72
Adams MD, Donato DB, Schulz RS, Smith GB (2008) Influences of hypersaline tailings on wildlife cyanide toxicosis; MERIWA Project M398 (II) 'cyanide ecotoxicity at hypersaline gold operations' final report volume 2—definitive investigation. Minerals and Energy Research Institute of Western Australia, Perth
Australasian Bat Society (2006) Recommendations of the Australasian Bat Society Inc for reporting standards for insectivorous bat surveys using bat detectors. Aust Bat Soc Newsl 27:6–9
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Botz MM, Devuyst E, Mudder TI, Norcross R, Ou B, Richins R, Robbins R, Smith A, Steiner N, Stevenson PE, Waterland R, Wilder A, Zaidi A (1995) Cyanide: an overview of cyanide treatment and recovery methods. Min Environ Manag 3:4–16
Bullen RD, McKenzie NL (2005) Seasonal range variation of Tadarida australis (Chiroptera: Molossidae) in Western Australia: the impact of enthalpy. Aust J Zool 53:145–56
Churchill SK (2008) Australian bats. Allen and Unwin, Sydney
Ciechanowski M (2002) Community structure and activity of bats (Chiroptera) over different water bodies. Mamm Biol 67:276–85
Ciechanowski M, Za T, Bieas A, Dunajski R (2007) Spatiotemporal variation in activity of bat species differing in hunting tactics: effects of weather, moonlight, food abundance, and structural clutter. Can J Zool 85:1249–63
Clark DR Jr (1991) Bats, cyanide and gold mining. Bats 9:17–8
Clark DR Jr, Hothem RL (1991) Mammal mortality at Arizona, California, and Nevada gold mines using cyanide extraction. Calif Fish Game 77:61–9
Clark DR Jr, Hill EF, Henry PFP (1991) Comparative sensitivity of little brown bats (Myotis lucifugus) to acute dosages of sodium cyanide. Bat Res News 32:68
Cockrum EL, Cross SP (1964) Time of bat activity over water holes. J Mammal 45:635–6
Creekmore LH (1999) Cyanide. In: Friend M, Franson JC (eds) Field manual of wildlife diseases: general field procedures and diseases of birds. U.S. Department of the Interior and U.S. Geological Survey, Washington, pp 341–46
Donato DB (1999) Bird usage patterns on Northern Territory mining water tailings and their management to reduce mortalities. Department of Mines and Energy, Northern Territory, Darwin
Donato DB (2009) Re-calibration of Sunrise Dam Gold Mine central-discharge thickened tailings system cyanide and salinity operating parameters: re-certification with the International Cyanide Management Code. Donato Environmental Services, Darwin
Donato DB, Smith GB (2007) Australian Centre for Mine and Environmental Research project 58, Anglogold Ashanti Australia Sunrise Dam Gold Mine sponsor’s report: summary of findings. Australian Centre for Mine and Environmental Research, Perth
Donato DB, Nichols O, Possingham H, Moore M, Ricci PF, Noller B (2007) A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife. Environ Int 33:974–84
Donato DB, Ricci PF, Noller B, Moore M, Possingham H, Nichols O (2008) The protection of wildlife from mortality: hypothesis and results for risk assessment. Environ Int 3:727–36
Donato DB, Smith GB, Madden-Hallett D (2010) Influences of hypersaline tailings on wildlife cyanide toxicosis. Granny Smith Gold Mine, Donato Environmental Services, Darwin
Dytham C (2011) Choosing and using statistics: a biologist’s guide, 3rd edn. Blackwell Science, United Kingdom
Eisler R (2000) Cyanide. In: Eisler R (ed) Handbook of chemical risk assessment volume 2: health hazards to humans, plants, and animals. Lewis Publishers, Washington, pp 903–52
Eisler R, Wiemeyer SN (2004) Cyanide hazards to plants and animals from gold mining and related water issues. Rev Environ Contam Toxicol 183:21–54
Eisler R, Clark DR Jr, Wiemeyer SN, Henny CJ (1999) Sodium cyanide hazards to fish and other wildlife from gold mining operations. In: Azcue JM (ed) Environmental impacts of mining activities: emphasis on mitigation and remedial measures. Springer-Verlag, Berlin, pp 55–67
Environment Australia (2003) Cyanide management: a booklet in the series on best practice environmental management in mining. Environment Australia, Canberra
Eyre TJ, Ferguson DJ, Hourigan CL, Smith GC, Mathieson MT, Kelly AL, Venz MF, Hogan LD (2012) Terrestrial vertebrate fauna survey assessment guidelines for Queensland. Department of Science, Information Technology, Innovation and the Arts, Brisbane.
Fenton MB, Boyle NGH, Harrison TM, Oxley DJ (1977) Activity patterns, habitat use, and prey selection by some African insectivorous bats. Biotropica 9:73–85
Geluso K (2007) Winter activity of bats over water and along flyways in New Mexico. Southwest Nat 52:482–92
Geluso KN, Geluso K (2012) Effects of environmental factors on capture rates of insectivorous bats, 1971–2005. J Mammal 93:161–9
Griffin DR (1958) Listening in the dark. Yale University Press, New Haven
Griffiths SR (2013) Insectivorous bats produce echolocation buzz calls while drinking on the wing. Behav Process 98:58–60
Griffiths SR, Smith GB, Donato DB, Gillespie CG (2009) Factors influencing the risk of wildlife cyanide poisoning on a tailings storage facility in the Eastern Goldfields of Western Australia. Ecotox Environ Safe 72:1579–86
Griffiths SR, Donato DB, Lumsden LF, Coulson G (2014) Hypersalinity reduces the risk of cyanide toxicosis to insectivorous bats interacting with wastewater impoundments at gold mines. Ecotox Environ Safe 99:28–34
Hagelstein K, Mudder TI (2006) Strategies and standards for control of bird mortality at mining operations. In: Mudder TI, Botz MM (eds) The cyanide monograph, 2nd edn. Mining Journal Books Ltd., London, pp 180–203
Hayes JP (1997) Temporal variation in activity of bats and the design of echolocation-monitoring studies. J Mammal 78:514–24
Hayes JP (2000) Assumptions and practical considerations in the design and interpretation of echolocation-monitoring studies. Acta Chiropt 2:225–36
Henny CJ, Hallock RJ, Hill EF (1994) Cyanide and migratory birds at gold-mines in Nevada, USA. Ecotoxicology 3:45–58
Higgins PJ, Peter JM, Cowling SJ (2006) Handbook of Australian, New Zealand and Antarctic birds, volume 7: boatbill to starlings. Oxford University Press, Melbourne
Hourigan CL, Catterall CP, Jones D, Rhodes M (2008) A comparison of the effectiveness of bat detectors and harp traps for surveying bats in an urban landscape. Wildl Res 35:768–74
Hudson A, Bouwman H (2008) Birds associated with a tailings storage facility and surrounding areas from a South African gold mine. Afr J Ecol 46:276–281
ICMI (2005) Implementation guidance for the International Cyanide Management Code. International Cyanide Management Institute, Washington
ICMI (2007) Auditor guidance for use of the Gold Mining Operations Verification Protocol. International Cyanide Management Institute, Washington
ICMI (2010) International Cyanide Management Code gold mining operations summary audit report: Sunrise Dam Gold Mine. International Cyanide Management Institute, Washington
ICMI (2012a) International Cyanide Management Code Recertification Audit: Barrick Gold of Australia Ltd - Cowal Gold Mine Summary Audit Report. International Cyanide Management Institute, Washington
ICMI (2012b) The International Cyanide Management Code. International Cyanide Management Institute, Washington
Kalcounis-Rüeppell MC, Payne VH, Huff SR, Boyko AL (2007) Effects of wastewater treatment plant effluent on bat foraging in an urban stream system. Biol Cons 138:120–30
Korine C, Pinshow B (2004) Guild structure, foraging space use, and distribution in a community of insectivorous bats in the Negev Desert. J Zool 262:187–96
Krausman PR, Rosenstock SS, Cain JW (2006) Developed waters for wildlife: science, perception, values, and controversy. Wildl Soc Bull 34:563–69
Kuenzi AJ, Morrison ML (2003) Temporal patterns of bat activity in southern Arizona. J Wildl Manag 67:52–64
Kurta A, Bell GP, Nagy KA, Kunz TH (1989a) Energetics of pregnancy and lactation in free-ranging little brown bats (Myotis lucifugus). Physiol Zool 62:804–18
Kurta A, Bell GP, Nagy KA, Kunz TH (1989b) Water balance of free-ranging little brown bats (Myotis lucifugus) during pregnancy and lactation. Can J Zool 67:2468–72
Kurta A, Kunz TH, Nagy KA (1990) Energetics and water flux of free-ranging big brown bats (Eptesicus fuscus) during pregnancy and lactation. J Mammal 71:59–65
Law B, Anderson J, Chidel M (1998) A bat survey in state forests on the south-west slopes region of New South Wales with suggestions of improvements for future surveys. Aust Zool 30:467–79
Lumsden LF, Bennett AF (1995) Bats of a semi-arid environment in south-eastern Australia: biogeography, ecology and conservation. Wildl Res 22:217–39
Ma J, Pritsos CA (1997) Tissue-specific bioenergetic effects and increased enzymatic activities following acute sublethal peroral exposure to cyanide in the mallard duck. Toxicol Appl Pharmacol 142:297–302
Milne DJ, Armstrong M, Fisher A, Flores T, Pavey CR (2005a) Structure and environmental relationships of insectivorous bat assemblages in tropical Australian savannas. Aust Ecol 30:906–19
Milne DJ, Fisher A, Rainey I, Pavey CR (2005b) Temporal patterns of bats in the Top End of the Northern Territory, Australia. J Mammal 86:909–20
National Industrial Chemicals Notification and Assessment Scheme (2010) Sodium cyanide: priority existing chemical assessment report number 31. Commonwealth of Australia Department of Health and Ageing, Sydney
O'Shea TJ, Clark DR Jr, Boyle TP (2001) Impacts of mine-related contaminants on bats. In: Vories KCT, Throgmorton D (eds) Bat conservation and mining. U.S. Department of the Interior, Office of Surface Mining, Alton, pp 205–16
Pennay M, Law BS, Reinhold K (2004) Bat calls of New South Wales: region based guide to the echolocation calls of microchiropteran bats. New South Wales Department of Environment and Conservation, Hurstville
Polak T, Korine C, Yair S, Holderied MW (2011) Differential effects of artificial lighting on flight and foraging behaviour of two sympatric bat species in a desert. J Zool 285:21–7
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Racey PA, Swift SM (1985) Feeding ecology of Pipistrellus pipistrellus (Chiroptera: Vespertilionidae) during pregnancy and lactation. J Anim Ecol 54:205–15
Ramirez P (2010) Bird mortality in oil field wastewater disposal facilities. Environ Manag 46:820–26
Rautenbach I, Kemp AC, Scholtz CH (1988) Fluctuations in availability of arthropods correlated with microchiropteran and avian predator activities. Koedoei 31:77–90
Razgour O, Korine C, Saltz D (2010) Pond characteristics as determinants of species diversity and community composition in desert bats. Anim Cons 13:505–13
Russo D, Cistrone L, Jones G (2012) Sensory ecology of water detection by bats: a field experiment. PLoS ONE 7:e48144
Sinclair G, McMullen A, Peters R (1997) A case study of bird mortality and cyanide management at Northparkes: short course notes on management of cyanide in mining. Australian Centre for Minesite Rehabilitation Research, Perth
Skalak SL, Sherwin RE, Brigham RM (2012) Sampling period, size and duration influence measures of bat species richness from acoustic surveys. Methods Ecol Evol 3:490–502
Smith GB, Donato DB (2007) Wildlife cyanide toxicosis: monitoring of cyanide-bearing tailing and heap leach facilities - compliance with the international cyanide management code. In: Avraamides G, Deschenes J, Tucker D (eds) World Gold 2007: By and co-products and the environment. Australasian Institute of Mining & Metallurgy, Parkville, pp 149–55
Smith GB, Donato DB, Gillespie CG, Griffiths SR, Rowntree J (2008) Ecology of a goldmining tailings facility in the Eastern Goldfields of Western Australia: a case study. Int J Min Reclam Environ 22:154–73
Taylor LR (1963) Analysis of the effect of temperature on insects in flight. J Anim Ecol 32:99–117
Turbill C (2008) Winter activity of Australian tree-roosting bats: influence of temperature and climatic patterns. J Zool 276:285–90
Vaughan N, Jones G, Harris S (1996) Effects of sewage effluent on the activity of bats (Chiroptera: Vespertilionidae) foraging along rivers. Biol Cons 78:337–43
Webb PI (1995) The comparative ecophysiology of water balance in microchiropteran bats. In: Racey PA, Swift SM (eds) Ecology, evolution, and behaviour of bats. Clarendon Press, Oxford, pp 203–16
Wiemeyer ST, Hill FH, Carpenter JW, Krynitsky J (1986) Acute oral toxicity of sodium cyanide in birds. J Wildl Dis 22:538–46
Acknowledgements
The authors are grateful to the CGM Environment Department personnel for logistical assistance during fieldwork. Financial support for this study was provided by Barrick Gold Corporation’s CGM and a Holsworth Wildlife Research Endowment to SRG. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
• Wastewater impoundments at gold mines present attractive habitats to bats.
• Echolocation calls provide empirical evidence of bat activity above water bodies.
• Buzz calls recorded above water provide indirect evidence of bats foraging and/or drinking.
• This study highlights the importance of developing site-specific bat monitoring protocols for compliance with the Cyanide Code.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Bat species found in the West Wyalong region, New South Wales: echolocation characteristics, presence recorded at the Cowal Gold Mine tailings dam and freshwater farm dam during monthly surveys from April 2006 to May 2012. Species distributions from Churchill (2008) and on advice from Pennay (Australasian Bat Society Inc.). Echolocation call characteristics and frequency range (AnalookW characteristic frequency) from Pennay et al. (2004). (DOCX 22 kb)
Fig. S1
Mean (±s.e.) number of echolocation call sequences per night detected each hour after civil twilight at the tailings dam and farm dam: a spring, b summer, c autumn and d winter. Data are from 212 matched nights recording from February 2011 to May 2012, comprising a total of 25,022 and 17,829 call sequences at the tailings dam and farm dam, respectively. During spring and summer, dawn occurs in the 9th and 10th hours after civil twilight; whereas in autumn and winter, it occurs in the 11th and 12th hours. (XLSX 23 kb)
Rights and permissions
About this article
Cite this article
Griffiths, S.R., Donato, D.B., Coulson, G. et al. High levels of activity of bats at gold mining water bodies: implications for compliance with the International Cyanide Management Code. Environ Sci Pollut Res 21, 7263–7275 (2014). https://doi.org/10.1007/s11356-014-2651-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2651-z