Abstract
Fluorene belongs to the polycyclic aromatic hydrocarbons (PAHs) which are potentially carcinogenic or mutagenic. However, very few studies on biodegradation of three-ring fluorene were investigated as compared to other three-ring PAHs such as phenanthrene and anthracene. The aim of this work is to evaluate fluorene degradation by fungal strain isolated from the decayed wood in tropical rain forest, Malaysia, and examine the effectiveness of the strain for degrading fluorene in liquid culture supplemented with the nonionic surfactants. Detailed taxonomic studies identified the organisms as Pestalotiopsis species and designated as strain Pestalotiopsis sp. W15. In this study, fluorene was totally degraded by Pestalotiopsis sp. W15 after incubation for 23 days. Various analytical studies confirmed the biotransformation of fluorene by detection of two metabolites in the treated medium: indanone (R f 0.45; λ max 240 and 290 nm; t R 7.1 min and m/z 132) and salicylic acid (λ max 205, 235, 290 nm; t R 9.4 min and m/z 382). Based on these products, a probable pathway has been proposed for the degradation of fluorene by Pestalotiopsis sp. W15. None of the intermediates were identified as dead-end metabolites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) are aromatic compounds consisting three or more fused benzene rings. The member of these compounds has been known as highly toxic to human and ecosystems as the results of their carcinogenic and mutagenic properties. Furthermore, their high affinity and low aqueous solubility to soils give rise to their persistence in the environment. Fluorene is a compound that consists three fused aromatic rings in its molecular structure which is found abundantly in the ecosystem. Therefore, fluorene is not genotoxic but the carcinogen aspect of its molecular structure has been used as an indicator to evaluate PAH-containing pollutants (Antizar-Ladislao et al. 2006; Hadibarata and Kristanti 2014; Lu and Zhu 2007; Teh and Hadibarata 2014).
Microbial degradation is an expected approach for the degradation of PAHs, and its application has been widely explored. In general, PAHs which contain two or three aromatic rings are readily degradable by indigenous fungus. Several fungi have been confirmed to utilize low molecular weight PAHs as a source of carbon and energy (Chakroun et al. 2010; Hadibarata et al. 2012a; Hadibarata et al. 2013; Mollea et al. 2005; Wu et al. 2008). Fungal oxidation of PAHs results in the production of metabolites with higher aqueous solubility and generally less biological reactivity than the parent compounds. The indigenous microorganisms isolated from contaminated sites have been exposed to be a highly effective bioremediation approach. Different groups of ascomycete fungi have shown the ability in removing organic pollutants in environments (Aghaie-Khouzani et al. 2012; da Silva et al. 2003; Forootanfar et al. 2011; Hadibarata et al. 2007; Liers et al. 2006).
Recently, a newly isolated laccase-producing ascomycete, identified as Pestalotiopsis species, was isolated from tropical rain forest. The aim of the present study was to investigate the ability of the mentioned fungus for elimination of fluorene in liquid cultures with addition of two synthetic surfactants. Removal studies in the relationship between enzyme activity and biomass production in the existence of Tween 80 and Brij35 were also determined.
2 Materials and Methods
2.1 Chemicals
Fluorene including 4-chlorobiphenyl and some standards for metabolites were obtained from Sigma-Aldrich (Milwaukee, WI). Malt extract and all other chemicals for liquid culture were purchased from Difco (Detroit, USA). Nonionic surfactant such as Brij35 and Tween 80 and extraction solvents were obtained from Acros Chemicals. All other chemicals were of analytical grade.
2.2 Fungi Identification Using 18S RNA Method
Identification of W15 strain was performed molecularly on the basis of 18S ribosomal RNA (rRNA) variation. The universal primers, NS1 and NS8, were used to amplify the extracted DNA. PCR was done according to previous condition (Hadibarata et al. 2012b). Amplification products were then sent to 1st BASE Laboratory Sdn. Bhd., Malaysia, for sequencing. BioEdit was performed to read and edit the DNA sequence. Finally, the sequence data was compared with other 18S rRNA gene sequences obtained from the National Center for Biotechnology Information GenBank database and construct the phylogenic tree of the fungus (Hadibarata et al. 2012b).
2.3 Microorganisms
Pestalotiopsis sp. W15 was isolated from the decayed wood in tropical rain forest, Malaysia, and examined for the effectiveness of the strain for degrading fluorene in liquid culture supplemented with the nonionic surfactants. The fungus was maintained on potato dextrose agar plates and on 2 % malt agar slants and stored at 4 °C.
2.4 Culture Conditions, Enzyme, and Biomass Determination
Spores and mycelia were aseptically inoculated into 100-mL Erlenmeyer flasks containing 20 mL of mineral salt medium (MSM). The flasks were incubated for specific time intervals at 25 °C. Control experiments were performed by incubating fluorene in autoclaved cultures and by incubating MSM with a fluorene without inoculum. All experiments were conducted in triplicate. Before incubation, a flask of each treatment was selected for immediate extraction. The culture broth was blended with ethyl acetate to extract the fluorene and metabolites from the mycelia. The ethyl acetate extraction procedure and enzyme analysis by fungi were described in our previous study (Hadibarata and Kristanti 2014). The enzyme production was expressed in units per liter. To determine fungal biomass during PAH degradation, 20 mL of culture broth was filtered on a Whatman no. 1 filter paper, washed with 40 mL distilled water, then dried at 105 °C for 24 h, and kept in desiccators until constant weight was achieved. The biomass of the fungi was expressed in grams per liter.
2.5 Detection of Fluorene and Metabolites
PAHs were quantified by an Agilent 5975E FID GC-MS (DB–1 capillary column; 0.25 μm ID; 0.25 mm diameter; 30 m length). The GC temperature was maintained as follows: initial 70 °C (1 min), raised at 18 °C/min to 150 °C, and increased 28 °C/min to 330 °C and hold 10 min in this temperature. The injector and interface temperatures were set at 260 °C with a splitless time of 2 min. The injection concentration was 1 μL in the splitless condition, and the flow rate of the helium was adjusted to 1 mL/min. The sylilation procedure for detecting sample containing hydroxyl and carboxyl group was performed with trimethylchlorosilane as described in our previous study (Hadibarata and Kristanti 2014). Identification of fluorene and metabolites was carried out by comparing their relative retention times and mass spectra with those of standards and the database library (Wiley 275L) (Hadibarata and Kristanti 2014).
3 Results and Discussion
3.1 Isolation and Identification of Ascomycete Fungus
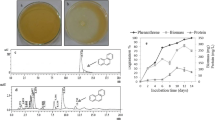
Of the 20 fungal isolates obtained, 11 were from decayed wood and 9 from fungal fruiting bodies isolated from nature. Using the remazol brilliant blue R dye to screen for ligninolytic enzyme-producing fungi, one isolate was found to have higher positive color zones, ranging from 5 to 7 cm with all chromogenic substances compared to 0 cm for the negative control. This fungus was subsequently selected for further studies. Microscopic characteristics were observed for the more complex conidiophores developing from the characteristic tufted or postulate areas of condition, usually 3–5 days after inoculation. Based on macroscopic morphological characteristics and 18S rRNA identification, phylogenetic tree was constructed, and W15 was classified as belonging to the Pestalotiopsis sp. W15 (Fig. 1).
3.2 Effect of Surfactant on Degradation of Fluorene, Biomass Production, and Laccase Production
Figure 2 shows biodegradation of fluorene in the presence of single or mixed surfactants. Fluorene dissolved in micelle phase and aqueous phase was rapidly biodegraded within 7 days that 0.61 g/day of fluorene in Tween 80 solution was removed followed by Tween 80-Brij35 (0.56 g/day) and Brij35 (0.33 g/day). A distinct inhibitory effect of fluorene biodegradation was observed in the presence of Brij35. A previous study reported that surfactants improve the mass transfer of solid hydrocarbons and increase the availability to microorganisms. However, with high CMC and low solubilizing capacity, Brij35 would not improve availability of contaminants significantly. Mixing Tween 80 with Brij35 inhibits the formation of mixed micelle and reduces CMC, which can be expected to enhance bioavailability of fluorene. These results are similar with previous studies that showed the effect of nonionic surfactant on the degradation of PAHs by increasing the oxygen consumption (Garon et al. 2002; Prak et al. 2002).
The effect of surfactant on biomass production is shown in Fig. 3. Pestalotiopsis sp. W15 culture supplemented with Tween 80 showed the highest biomass (4.8 g/L), indicating the presence of recent surfactant supported the cell growth, whereas for Brij35 and combination of Tween 80-Brij35 at the same level of concentration, the biomass production was less than cultures supplemented with Tween 80. Rapid biomass production (0.62 g/day) was achieved in the beginning of incubation in the cultures supplemented with Tween 80, followed by combination of Tween 80-Brij35 (0.46 g/day) and Brij 30 (0.24 g/day).
Among surfactants, Tween 80 showed significant laccase production (125 U/L) was produced in the Pestalotiopsis sp. W15 culture, respectively. The lowest laccase activity was obtained from Brij35. At the end of the experiment, about 4.2 U/L/day laccase was produced in Tween 80 cultures, followed by Tween 80-Brij35 (2.98 U/L/day) and Brij35 (1.88 U/L/d) (Fig. 4). These results indicate the increased laccase activity was resulted by the increased fluorene degradation as results of the addition of suitable surfactants. It is obvious that the preferential uptake of surfactants by the fungus at low surfactant concentration was taken account in the increased laccase activity. Hence, due to this reason, Tween 80 might be beneficial to the laccase production in aqueous culture. During the PAH degradation, many enzymes have been produced by fungi; however, laccase is playing an important role that has been acknowledged to be significantly produced in the degradation of some PAHs (Levin et al. 2003; Kamei et al. 2006; Zhang et al. 2008; Ting et al. 2011; Hadibarata and Kristanti 2014). In this study, laccase as the key enzyme responsible for the degradation process was attained.
3.3 Fluorene Metabolites
Fluorene is a three-ring PAH that is a major constituent of fossil fuels and coal derivatives. Several fungi able to use fluorene as a source of carbon and energy have been isolated and are in the genera of Pleurotus, Agrocybe, and Armillaria (Akdogan and Pazarlioglu 2011; Chupungars et al. 2009; Hadibarata and Kristanti 2014). The initial 3,4-dioxygenation of fluorene forms fluorene-3,4-diol that is further transformed to 2-formyl-1-indanone, 1-indanone-2-carboxylic acid, 1-indanone, 2-chromanone, and salicylic acid. Unfortunately, only 1-indanone and salicylic acid have been identified in our culture extract. In this study, the degradation products of fluorene were analyzed by GC-MS and UV-vis spectrophotometer. The results are shown in Table 1 and Fig. 5. Metabolite I (R f 0.45) showed λ max in 240 and 290 nm, similar to the standard 1-indanone. The compound I (t R of 7.1 min) had main spectrum at m/z 132 (M+) and the significant fragment ions at m/z 108 (M+ −28), sequential loss of C–O. Metabolite II (t R of 9.4 min) showed R f value of 0.28 and a main ion at m/z 382 (M+) and fragment ion at m/z 367 (M+ −15), representing methyl, as well as the expected fragment ion at m/z 193 (M+ −89) sequential lose of –OSi(CH3)3, as well as expected fragment ion at 73 [(CH3)3Si], 147, and 209. Based on the data of mass spectral obtained above, we suggested that compound II was salicylic acid. This study demonstrated that Pestalotiopsis sp. W15 could degrade fluorene with the use of their ligninolytic enzymatic systems.
4 Conclusions
In view of the biodegradation process, fluorene in Tween 80 solutions was readily degradable during the experiment. No inhibitory effects on the microorganism duo to the addition of Tween 80, Brij35, and combination of both were found. These results recommend the single surfactant (Tween 80) solution may increase the sorption rate of fluorene on the biomass and thus improve the removal process and reduce the level of remediation expanses. From the data obtained, it was observed that laccase was the key enzyme responsible for the degradation process. The fluorene-degrading fungus Pestalotiopsis sp. W15 appears to possess only the pathways initiated by dioxygenation at C-3,4, which may explain their lower versatility in PAH utilization.
References
Aghaie-Khouzani, M., Forootanfar, H., Moshfegh, M., Khoshayand, M. R., & Faramarzi, M. A. (2012). Decolorization of some synthetic dyes using optimized culture broth of laccase producing ascomycete Paraconiothyrium variabile. Biochemical Engineering Journal, 60, 9–15.
Akdogan, H. A., & Pazarlioglu, N. K. (2011). Fluorene biodegradation by P. ostreatus—part I: biodegradation by free cells. Process Biochemistry, 46, 834–839.
Antizar-Ladislao, B., Lopez-Real, J. M., & Beck, A. J. (2006). Bioremediation of polycyclic aromatic hydrocarbons (PAH) in an aged coal–tar-contaminated soil using different in-vessel composting approaches. Journal of Hazardous Material, 137, 1583–1588.
Chakroun, H., Mechichi, T., Martinez, M. J., Dhouib, A., & Sayadi, S. (2010). Purification and characterization of a novel laccase from the ascomycete Trichoderma atroviride: application on bioremediation of phenolic compounds. Process Biochemistry, 45, 507–513.
Chupungars, K., Rerngsamran, P., & Thaniyavarn, S. (2009). Polycyclic aromatic hydrocarbons degradation by Agrocybe sp. CU-43 and its fluorene transformation. International Biodeterioration & Biodegradation, 63, 93–99.
da Silva, M., Umbuzeiro, G. A., Pfenning, L. H., Canhos, V. P., & Esposito, E. (2003). Filamentous fungi isolated from estuarine sediments contaminated with industrial discharges. Soil and Sediment Contamination, 12, 345–356.
Forootanfar, H., Faramarzi, M. A., Shahverdi, A. R., & Tabatabaei Yazdi, M. (2011). Purification and biochemical characterization of extracellular laccase from the ascomycete Paraconiothyrium variabile. Bioresource Technology, 102, 1808–1814.
Garon, D., Krivobok, S., Wouessidjewe, D., & Seigle-Murandi, F. (2002). Influence of surfactants on the solubilization and fungal degradation of fluorene. Chemosphere, 47, 303–309.
Hadibarata, T., & Kristanti, R. A. (2014). Fluorene biodegradation and identification of transformation products by white-rot fungus Armillaria sp. F022. Biodegradation, 25, 373–382.
Hadibarata, T., Tachibana, S., & Itoh, K. (2007). Biodegradation of n-eicosane by fungi screened from nature. Pakistan Journal of Biological Sciences, 10, 1804–1810.
Hadibarata, T., Khudhair, A. B., & Salim, M. R. (2012a). Breakdown products in the metabolic pathway of anthracene degradation by a ligninolytic fungus Polyporus sp. S133. Water Air Soil Pollution, 223, 2201–2208.
Hadibarata, T., Yusoff, A. R. M., Aris, A., Salmiati, Hidayat, T., & Kristanti, R. A. (2012b). Decolorization of azo, triphenylmethane and anthraquinone dyes by laccase of a newly isolated Armillaria sp. F022. Water Air Soil Pollution, 223, 1045–1054.
Hadibarata, T., Yusoff, A. R. M., Adnan, L. A., Yuniarto, A., Rubiyatno, Zubir, M. M. F. A., Khudhair, A. B., Teh, Z. C., & Naser, M. A. (2013). Microbial decolorization of an azo dye reactive black 5 using white-rot fungus Pleurotus eryngii F032. Water Air Soil Pollution, 224, 1595–1604.
Kamei, I., Kogura, R., & Kondo, R. (2006). Metabolism of 4,40-dichlorobiphenyl by white-rot fungi Phanerochaete chrysosporium and Phanerochaete sp. MZ142. Applied Microbiology and Biotechnology, 72, 566–575.
Levin, L., Viale, A., & Forchiassin, A. (2003). Degradation of organic pollutants by the white rot basidiomycete Trametes trogii. International Biodeterioration & Biodegradation, 52, 1–5.
Liers, C., Ullrich, R., Steffen, K. T., Hatakka, A., & Hofrichter, M. (2006). Mineralization of C-14-labelled synthetic lignin and extracellular enzyme activities of the wood-colonizing ascomycetes Xylaria hypoxylon and Xylaria polymorpha. Applied Microbiology and Biotechnology, 69, 573–579.
Lu, H., & Zhu, L. (2007). Pollution patterns of polycyclic aromatic hydrocarbons in tobacco smoke. Journal of Hazardous Material, 139, 193–198.
Mollea, C., Bosco, F., & Ruggeri, R. (2005). Fungal biodegradation of naphthalene: microcosms studies. Chemosphere, 60, 636–643.
Prak, D. J. L., & Pritchard, P. H. (2002). Solubilization of polycyclic aromatic hydrocarbon mixtures in micellar nonionic surfactant solutions. Water Research, 36, 3463–3472.
Teh, Z. C., & Hadibarata, T. (2014). Enhanced degradation of pyrene and metabolites identification by Pleurotus eryngii F032. Water Air Soil Pollution, 225(1909), 1–8.
Ting, W. T. E., Yuan, S. Y., Wu, S. D., & Chang, B. V. (2011). Biodegradation of phenanthrene and pyrene by Ganoderma lucidum. International. Biodeterioration. Biodegradation, 65, 238–242.
Wu, Y., Luo, Y., Zou, D., Ni, J., Liu, W., Teng, Y., & Li, Z. (2008). Bioremediation of polycyclic aromatic hydrocarbons contaminated soil with Monilinia sp.: degradation and microbial community analysis. Biodegradation, 19, 247–257.
Zhang, J., Liu, X., Xu, Z., Chen, H., & Yang, Y. (2008). Degradation of chlorophenols catalyzed by laccase. International Biodeterioration Biodegradation, 61, 351–356.
Acknowledgments
A part of this research was financially supported by a Fundamental Research Grant Scheme (FRGS) of Ministry of Education Malaysia (Vote No. R.J130000.7809.4F465) and a Science Fund of Ministry of Science, Technology and Innovation Malaysia (Vote No. R.J130000.7909.4S110).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kristanti, R.A., Hadibarata, T. Biodegradation and Identification of Transformation Products of Fluorene by Ascomycete Fungi. Water Air Soil Pollut 226, 406 (2015). https://doi.org/10.1007/s11270-015-2674-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2674-1