Abstract
Two wood-dwelling ascomycetes, Xylaria hypoxylon and Xylaria polymorpha, were isolated from rotting beech wood. Lignin degradation was studied following the mineralization of a synthetic \({}^{{14}}{\text{C}}_{{\text{ $ \beta $ }}} \)-labelled lignin in solid and liquid media. Approximately 9% of the synthetic lignin was mineralized by X. polymorpha during the growth on beech wood meal, and the major fraction (65.5%) was polymerized into water- and dioxan-insoluble material. Both fungi produced laccase (up to 1,200 U l−1) in an agitated complex medium based on tomato juice; peroxidase activity (<80 U l−1) was only detected for X. polymorpha in soybean meal suspension. The enzymatic attack of X. polymorpha on beech wood resulted in the formation of three fractions of water-soluble lignocellulose fragments with molecular masses of 200, 30 (major fraction) and 3 kDa, as demonstrated by high-performance size exclusion chromatography. This fragment pattern differs considerably from that of the white-rot fungus Bjerkandera adusta, which preferentially released smaller lignocellulose fragments (0.8 kDa). The finding that X. polymorpha produced large lignocellulose fragments, along with the fact that high levels of hydrolytic enzymes (esterase 630 U l−1, xylanase 120 U l−1) were detected, indicates the cleavage of bonds between the lignin and hemicellulose moieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial degradation of lignocellulose, occurring in the cell walls of vascular plants, is of general significance for the recirculation of bound organic carbon. In particular, besides cellulose and hemicelluloses, the persistent macromolecule lignin provides strength and rigidity to plant cell wall and protects the polysaccharides from microbial attack (Monties and Fukushima 2001). The most efficient and extensively studied microorganisms degrading lignin are the wood-decaying basidiomycetes (white-rot fungi), which are able to chemically modify and mineralize the complex lignin polymer. During the last two decades, this remarkable metabolic pathway has been found to depend on two types of extracellular oxidoreductases. Both ligninolytic peroxidases and laccases can attack the lignin molecule in wood unspecifically via one-electron oxidations resulting in the formation of unstable radicals, which in turn disintegrate (Hatakka 1994). The removal of lignin gives fungi access to the plant polysaccharides (hemicelluloses, cellulose) which serve as their primary source of carbon and energy (Kirk and Farrell 1987). This complex process of lignocellulose decomposition requires the synergistic action of ligninolytic enzymes and a number of hydrolytic biocatalysts such as cellulases and hemicellulases (Hatakka 2001). In this context, the involvement of esterases, which may cleave bonds originating from the coupling of the carboxylic groups of hemicelluloses and the phenolic or alcoholic groups of lignin, has been widely discussed (Li and Helm 1995; Williamson et al. 1998).
Unlike basidiomycetous fungi, the knowledge about lignocellulose degradation by the second important group of wood-colonizing fungi—the ascomycetes—is limited. Wood decay by certain ascomycetes, which was first described in detail by Savory (1954) and designated as “soft-rot”, causes notable degradation of cellulose as compared to lignin. Furthermore, some studies have demonstrated the ability of different asco- and deuteromycetes to mineralize and disintegrate lignin or lignin model compounds to some extent (Haider and Trojanowski 1975; Rodríguez et al. 1996; Kluczek-Turpeinen et al. 2003). Ascomycetes of the family Xylariaceae have been reported to cause a special type of extensive wood-rot referred to as soft-rot type II (Blanchette 1995). These fungi have the ability to degrade both polysaccharides and lignin, although in contrast to white-rot basidiomycetes, the middle lamella is not attacked (Sutherland and Crawford 1981). In spite of their extensive occurrence in forests, there are only a few reports on the ligninolytic potential of Xylariaceae. Information about the extent of delignification and the production of ligninolytic enzymes is notably lacking. Thus in this study we describe the conversion and mineralization of a synthetic 14C-labelled lignin by two species of the genus Xylaria as well as the pattern of their extracellular enzymes.
Material and methods
Microorganisms
Xylaria hypoxylon CZi-002 and the bark-dwelling ascomycete Diatrype sp. CZi-001 were isolated from fruiting bodies collected from rotting beech wood in the geographical area of the Zittau mountains (“Zittauer Gebirge”, Germany). They were deposited in the fungal culture collection of the International Graduate School Zittau. Xylaria polymorpha RJe-004 and the reference white-rot fungus Bjerkandera adusta TM Ud1 were isolated from beech logs in the Hainich National Park (Germany) and were deposited in the culture collection of the Institute of Microbiology (University of Jena, Germany).
Media and culture conditions
The fungal organisms were routinely pre-cultured on 2% malt-extract agar for 2 weeks, and a single agar plug (8 mm in diameter) from the overgrown plates was used to inoculate solid-state cultures (SSCs). These consisted of 5 g of sterilized beech wood and 17 ml of distilled water in 100-ml Erlenmeyer flasks. The flasks were incubated at 23°C for 8 weeks. After 7, 14, 42 and 56 days, three flasks each were harvested and extracted with 35 ml of distilled water by sonication (5 min) and shaking (45 min on a rotatory shaker at 150 rpm). Aliquots (1 ml) were centrifuged and used for the measurement of extracellular enzyme activities as well as for the determination of the molecular mass distribution of lignocellulose fragments formed. In addition to the xylariaceous fungi, the basidiomycete B. adusta, which is known to cause intensive white-rot, served as a reference species in some experiments.
Secretion of ligninolytic enzymes (laccase, peroxidase) in liquid cultures was studied by using two complex plant-based media. Flasks contained 200 ml tomato juice (TJM; Albi & Co, Germany) diluted with distilled water (50:50 v/v), or a soybean meal suspension (SBS; 30 g l−1, Schoenenberger GmbH, Germany) (Robene-Soustrade et al. 1992; Ullrich et al. 2004). Liquid media were autoclaved and inoculated with 9 ml of a homogenized suspension of pregrown fungal mycelium (one agar plate in 80 ml water). Incubation occurred at 23°C on a rotary shaker (100 rpm) for 3 weeks. Samples (1 ml of the culture liquid) were taken daily from each flask and used for the determination of enzyme activities.
Experiments with 14C-labelled lignin
Mineralization studies using synthetic 14C-side-chain-labelled guaiacyl-type lignin were carried out in solid-state and liquid culture under the conditions described above. The 14C-lignin (\({}^{{14}}{\text{C}}_{{\text{ $ \beta $ }}} \)-labelled dehydrogenation polymer) was synthesized according to the method described by Brunow et al. (1998). A stock suspension of 14C-DHP was made as described by Vares et al. (1994) and added to give a final radioactivity of 400,000 dpm per flask in the SSCs and 200,000 dpm per flask in the liquid cultures. After the addition of 14C-lignin, the SSCs, containing 15 g of milled beech wood (water content 80%) in 200-ml flasks, were inoculated with three agar plugs of fresh mycelium; the liquid TJM and SBS cultures (20 ml) with 1.5 ml of homogenized mycelium. In addition, one set of 4-month-old SSCs were supplemented with 14C-lignin to prove the ligninolytic activity of the aged fungal mycelium.
Inoculated flasks and uninoculated controls were sealed with rubber septa and aluminium caps. Incubation was carried out at 24°C in the dark. 14C-labelled volatile compounds and 14CO2 were flushed out once a week with pure oxygen for 15 min and trapped by bubbling any gas released through two sequential flasks containing Opti-Fluor and Carbosorb/Opti-Fluor (Packard Instruments, Groningen, The Netherlands; Scheibner et al. 1997). After 11 weeks of cultivation, 14C-labelled water-soluble lignin fragments were extracted with 25 ml of pure water and the residual 14C-lignin with 15 ml of dioxan. Sequential extraction was done by sonication (10 min) and vigorous shaking (15 min). The residual wood, including the fungal biomass, was burned in a combustion chamber (Junitek, Turku, Finland) and the evolved 14CO2 was quantified. Liquid cultures were harvested after 10 weeks and the 14C-labelled water-soluble lignin fragments were determined after centrifugation. Bound radioactivity was analyzed after filtration of the cultures through paper filters, which were afterwards combusted in a combustion chamber. A liquid scintillation counter (model 1411; Wallac, Turku; Finland; Steffen et al. 2000) was used for all radioactivity measurements. Mineralization of the 14C-lignin was expressed as the percentage of total radioactivity added. All experiments were carried out in triplicate and the data given represent the mean values with standard deviations.
High-performance size exclusion chromatography
High-performance size exclusion chromatography (HPSEC) was used for the determination of the molecular mass distribution of lignocellulose fragments formed (Hofrichter et al. 2001). The high-performance liquid chromatography (HPLC) system (HP 1090 Liquid Chromatography; Hewlett-Packard, Waldbronn, Germany), equipped with a diode array detector, was fitted with a HEMA-Bio linear column (8×300 mm, 10 μm) from Polymer Standard Service (Mainz, Germany). The mobile phase consisted of 20% acetonitrile and 80% of an aqueous solution of 0.34% NaCl and 0.2% K2HPO4 (pH 10). The following separation parameters were used: flow rate 1 ml min−1, detection wavelength 260 nm and injection volume 25 μl. Sodium polystyrene sulphonates (0.8–150 kDa; Polymer Standard Service) were used as molecular mass standards.
Enzyme assays
Laccase activity was measured photometrically following the oxidation of 2,2′-azinobis(3-ethylbenzthiazoline-6-sulphonate) (ABTS) at 420 nm (Eggert et al. 1996). The assay mixture contained 100 mM McIlvaine buffer pH 4.5 and 0.3 mM ABTS. The reaction was started by adding 50 μl culture liquid or extract. Peroxidase activity was determined by the oxidation of 2,6-dimethoxyphenol (DMP; 5 mM) according to Bollag et al. (1979) but after the addition of H2O2 (100 μM), and was always corrected by the laccase activity (Eggert et al. 1996).
Acetylesterase activity was measured as described by Purdy and Kolattukudy (1973) using p-nitrophenylacetate as substrate in 100 mM phosphate buffer (pH 6.5). Measurements were performed at 405 nm with a microplate reader (Dynex Technologies, Denkendorf).
Determination of cellulolytic enzymes was based on the measurement of reducing sugars released according to the method described by Bailey and Nevalainen (1981). Endoglucanase was determined using a reaction mixture that contained in a total of 1 ml 900 μl of a 1% hydroxyethylcellulose solution in 50 mM sodium citrate buffer (pH 4.8) and 100 μl enzyme solution; reducing sugars formed were measured at 540 nm. Glucose was used as the standard. Glucosidase activity was detected by using an assay mixture consisting of 900 μl 4-nitrophenyl-d-pyranoside (1 mM) in 50 mM sodium citrate buffer (pH 4.8) and 100 μl enzyme solution. The mixture was incubated at 50°C for 20 min and the reaction stopped by addition of 1 ml Na2CO3 (1 M). The amount of p-nitrophenol liberated was determined by measuring the absorbance at 400 nm. A glucuron–xylan solution (1%) suspended in 50 mM sodium citrate buffer (pH 5.3) was used for the determination of xylanase. Substrate (900 μl) and enzyme solution (100 μl) were incubated as described above. Product formation was monitored photometrically at 540 nm. The mean values of triple determinations were calculated. All activities were expressed in international units (IU), defined as the amount of enzyme that forms 1 μmol product per minute under the assay conditions used; activities in SSC are expressed in relation to the initial water content of the beech wood (3.4 ml g−1=340%).
Results
Conversion of 14C-labelled synthetic lignin in solid-state and liquid cultures
The ligninolytic activity of X. hypoxylon and X. polymorpha was studied by following the mineralization of 14C-DHP both in SSC and liquid culture. Freshly prepared cultures of X. hypoxylon and X. polymorpha converted 5.0 and 8.7%, respectively, of the 14C-lignin into 14CO2 within 11 weeks (Fig. 1a). 14CO2 evolution by X. polymorpha was almost linear and lasted until the end of the experiment (∼0.8% per week). In contrast, X. hypoxylon showed a comparable activity only in the second and third weeks of cultivation; then, the mineralization rate declined constantly. 14CO2 release by the 4-month-old cultures of both fungi showed a similar curve progression, and approximately 6% of the labelled lignin was mineralized. This finding shows that these fungi are capable of mineralizing lignin slowly but over long periods.
Time courses of 14CO2 evolution from synthetic 14C side chain labelled lignin (400,000 dpm) during the growth of two wood-decaying ascomycetes on beech wood using cultures of different ages (a). X. hypoxylon, fresh culture (closed circles); X. polymorpha, fresh culture (closed triangles); X. hypoxylon, 4-month-old culture (open circles), X. polymorpha, 4-month-old culture (open triangles); uninoculated control (closed diamonds); and in liquid culture (b). Soybean meal suspension (SBS): X. hypoxylon (closed circles), X. polymorpha (closed triangles) and uninoculated control (closed diamonds); tomato juice medium (TJM): X. hypoxylon (open circles), X. polymorpha (open triangles) and uninoculated control (open diamonds). Data points represent means of three replicates with standard deviation
The balance of the radioactivity (total 14C) in the end of the experiment revealed that X. polymorpha in particular incorporated a substantial portion of 14C (more than 30% in comparison to the control) into the water- and dioxan-insoluble lignin fraction. On the other hand, water-soluble lignin, i.e. polar lignin fragments, were lacking in aqueous extracts. The same phenomenon, but less pronounced, was observed for the X. hypoxylon cultures. The bark dweller Diatrype sp. grew only hardly on beech wood and did not show any lignin mineralizing activity (Table 1).
The course of lignin mineralization in the liquid cultures resembled that in beech wood (Fig. 1b). Again, most of the 14CO2 was released by X. polymorpha (7.8% in SBS). Around 5% of the 14C-lignin was mineralized by X. polymorpha in TJM and by X. hypoxylon in both media. Polymerization was not observed and a significant part of the 14C-lignin was converted into water-soluble fragments (between 8 and 24% compared to the controls) (Table 2).
Enzyme activities
Both ascomycetous fungi grew relatively fast on beech wood chips, and 14 days after inoculation, the whole woody material was covered with whitish mycelium. After 8 weeks, the whole wood was tightly grown through and a network of black zone lines had developed. The pH of the beech wood decreased slightly in SSCs of X. hypoxylon (from 5.7 to 5.1) and stronger (from 5.9 to 4.5) in those of X. polymorpha.
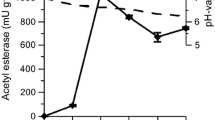
Laccase was the only ligninolytic enzyme that was found in low amounts in the aqueous extracts (max. activity in X. polymorpha SSCs: 50 U l−1, detected after 8 weeks). Peroxidase activity was completely lacking in the wood extracts. In contrast to the low oxidative enzyme activities, high levels of hydrolytic enzymes were detected. Thus, high titters of esterase were found in the extracts of X. polymorpha (up to 600 U l−1). The time course of esterase activity shows that the enzyme was present all throughout the cultivation (Fig. 2).
In addition to esterase, both fungi also secreted noticeable amounts of enzymes cleaving hemicelluloses and cellulose (Fig. 3a,b). The highest xylanase activity that permanently increased and reached 480 U l−1 at the last day of cultivation was observed in X. hypoxylon, along with moderate levels of endoglucanase (max. 90 U l−1) and a low but constant activity of glucosidase (∼30 U l−1). The cellulolytic activities of X. polymorpha were considerably lower but likewise present (max. activities of xylanase, 123 U l−1; endoglucanase 38 U l−1; glucosidase, 22 U l−1).
Because the oxidative activities in SSC were relatively low, they were studied in more detail with X. polymorpha in two complex liquid media based on tomato juice (TJM) or soybean meal (SBS). Both media promoted the fungal growth and facilitated the development of a high biomass density (exact biomasses could not be measured due to the presence of solid tomato and soybean particles). The pH increased during the course of cultivation from 6.2 to 8.6 and from 4.8 to 8.8. in SBS and TJM, respectively. X. polymorpha secreted laccase both in SBS and in TJM (Fig. 4). Activity in SBS increased almost permanently until the end of the experiment (max. 230 U l−1), while in TJM, a temporary maximum of 120 U l−1 was reached on day 15. Peroxidase activity, which was lower than that of laccase (max. 80 U l−1) and independent of Mn2+, was only detected in SBS; the enzyme oxidized both ABTS and DMP but not veratryl alcohol.
X. hypoxylon showed only low laccase activity in SBS (max. 16 U l−1). In TJM, the fungus secreted high amounts of laccase but only for a very short time on day 11 (1,200 U l−1) and day 12 (680 U l−1); peroxidase activity was lacking.
HPSEC analyses
Figure 6 shows the HPSEC elution profile of water-soluble lignocellulose fragments formed as the result of X. polymorpha activities during 8 weeks of growth on beech wood. For comparison, the corresponding chromatogram from B. adusta is given. The ascomycete released a small amount of extremely high molecular mass fragments (∼200 kDa); the major fraction had a molecular size around 30 kDa, whereas the amount of small- and medium-sized fragments was relatively low. In contrast, B. adusta formed exclusively small lignocellulose fragments with a predominant molecular mass around 0.8 kDa (Fig. 5).
Time-dependent changes in the molecular mass distribution of lignocellulose fragments released by X. polymorpha are given in Fig. 6. Starting on day 7, high molecular mass fractions (>30 kDa) increased permanently. However, the amount of smaller and medium-sized molecules was—in the end (after 8 weeks)—only moderately enhanced. In the meantime, changes were observed in the medium-sized fraction (around 3 kDa), which accumulated until day 42 and disappeared again in the further course of fungal growth.
Discussion
The wood-dwelling ascomycetes X. hypoxylon and X. polymorpha mineralized synthetic 14C-labelled lignin to a moderate extent both in solid-state and liquid cultures. They produced laccase and, in one case, low amounts of peroxidase as potential ligninolytic enzymes along with different polysaccharide-cleaving hydrolases as well as esterase. X. polymorpha released primarily water-soluble lignocellulose fragments of larger size (∼30 kDa) during their growth on beech wood, and the corresponding fragment pattern of the HPSEC elution profile differed considerably from that of the basidiomycetous white-rot fungus B. adusta.
X. polymorpha proved to be the most active species, converting about 0.8% of the labelled lignin per week into 14CO2 and 9% within 12 weeks. This was achieved by freshly inoculated SSC but even 4-month-old cultures still released 0.4–0.5% 14CO2 per week. Their mineralizing activities proceeded almost constantly over a prolonged period of 12 weeks, whereas wood-colonizing white-rot basidiomycetes (e.g. Phlebia radiata) typically mineralized most of the lignin in the first 4–6 weeks, reaching mineralization rates between 5 and 10% per week (Hatakka 2001). In this context, Xylaria spp. rather resemble certain litter-decomposing basidiomycetes (e.g. Agrocybe praecox) that were also found to mineralize lignin constantly with rates of about 2% 14CO2 per week (Steffen et al. 2000). On the other hand, the total extent of lignin mineralization of the two Xylaria spp. is comparable to those reported for the soil-inhabiting deuteromycetes Paecilomyces inflatus (Kluczek-Turpeinen et al. 2003), Fusarium oxysporum, Penicillium hirayamae and Penicillium chrysogenum (Carnicero et al. 1992). Unlike Xylaria spp., mineralization by all these microfungi did not proceed constantly and the highest rates were observed in the first weeks of cultivation (up to 1.8% per week) (Kluczek-Turpeinen et al. 2003).
Solubilization of 14C-lignin differed considerably, depending on culture conditions. Thus in SSC, part of the radioactivity was found in the dioxan-insoluble fraction, which means that a substantial portion (10–30%) of the 14C-labelled lignin was polymerized. Since the typical black zone lines of the Xylariaceae appeared in the wood cultures, we assume that part of the lignin was incorporated into fungal melanin (Schwarze et al. 2000). In contrast to SSC, 10–25% of the 14C was found in the water-soluble fraction in liquid cultures of Xylaria spp., i.e. part of the lignin was oxidized into more polar fragments. The extent of lignin solubilization was in the same range as that reported for wood- and litter-decomposing basidiomycetes in liquid culture (Steffen et al. 2000).
A few of the microfungi mineralizing lignin to some extent have been reported to produce laccase, but not peroxidases (Rodríguez et al. 1996; Kluczek-Turpeinen et al. 2003). In contrast, most white-rot basidiomycetes studied secreted high titers of laccase (e.g. Pycnoporus cinnabarinus; Eggert et al. 1996) along with at least one peroxidase (mostly Mn peroxidase; e.g. Ceriporiopsis subvermispora; Rüttimann-Johnson et al. 1993).
In complex liquid media (SBS, TJM), both X. polymorpha and X. hypoxylon reached appreciable laccase titers. Phenolic compounds, e.g. flavonoids or tannic acids, which are always present in such plant extracts, probably induced the production of ligninolytic enzymes (Carbajo et al. 2002). On the other hand, aromatic compounds (e.g. veratryl alcohol, guaiacol, xylidine) or metals (Cu2+, Mn2+), which have been found to stimulate the production of oxidative enzymes in other fungi (including certain ascomycetes; Botryosphaeria sp.; Dekker and Barbosa 2001), did not have any stimulating effect on the enzyme levels of both Xylaria species in complex liquid media (TJM); the use of synthetic media (e.g. Czapek-Dox or Kirk medium) did not lead to detectable activities of oxidative enzyme at all, neither in the absence nor in the presence of potential elicitors (unpublished results). Besides laccase, X. polymorpha showed a low but clearly detectable peroxidase activity in SBS. This enzyme seems to belong to the horseradish (HRP) and Coprinus cinereus (CiP)-type of peroxidase, since it did not oxidize veratryl alcohol or Mn2+ but ABTS and DMP (Dunford 1999).
Considering the moderate activities of ligninolytic enzymes, it is difficult to assume that they are exclusively responsible for the mineralization and solubilization of lignin. Based on recent findings, which principally demonstrated laccase-driven lipid peroxidation in vitro and in planta (Deighton et al. 1999), it seems reasonable that similar processes may be involved in the oxidation of lignin by ascomycetes. This assumption is supported by the similarity of lignin fragment patterns obtained after Mn peroxidase-driven lipid peroxidation of milled pine wood and those reported here (Figs. 5, 6; Hofrichter et al. 2001). In addition, hydrolytic enzymes, which were detected at high levels in our study, may cleave bonds within the hemicelluloses (xylan), facilitating the subsequent solubilization and diffusion of lignin and lignin–xylan complexes (Beg et al. 2001). The latter could be directly cleaved by esterases hydrolyzing the linkages between xylan uronic acid moieties and the lignin polymer (Li and Helm 1995). The preferred formation of high molecular mass lignin fragments during the growth of both Xylaria spp. on beech wood as well as the detection of high amounts of esterase in respective aqueous wood extracts of X. polymorpha further support this assumption.
Other extracellular hydrolases (endoglucanase, β-glucosidase) secreted by both Xylaria spp. may be directly involved in the degradation of beech wood cellulose. Wei et al. (1992) showed that there is a noticeable variation in cellulolytic enzymes within the genus Xylaria and they postulated the existence of a complex membrane-bound system of hydrolytic enzymes which can be switched under certain conditions.
All in all, our results show that ascomycetous wood dwellers have the ability to oxidize and modify recalcitrant synthetic lignin over a prolonged period, although at a lower extent than wood-rotting basidiomycetes do. Xylanases, cellulases and laccases seem to be the degradative key enzymes, probably along with ester cleaving hydrolases. Current studies focus on the purification of extracellular Xylaria enzymes and on the establishment of cell-free reaction mixtures based on esterase, laccase and lipids to disintegrate lignocelluloses in vitro.
References
Bailey MJ, Nevalainen KMH (1981) Induction, isolation and testing of stable Trichoderma reesei mutants with improved production of solubilizing cellulase. Enzyme Microb Technol 3:153–157
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications; a review. Appl Microbiol Biotechnol 56:326–338
Blanchette RA (1995) Degradation of the lignocellulose complex in wood. Can J Bot 73:999–1010
Bollag J-M, Sjoblad RD, Liu S-Y (1979) Characterization of an enzyme from Rhizoctonia praticola which polymerizes phenolic compounds. Can J Microbiol 25:229–233
Brunow G, Raiskila S, Sipliä J (1998) The incorporation of 3,4-dichloroaniline, a pesticide metabolite, into dehydrogenation polymers of coniferyl alcohol (DHPs). Acta Chem Scand 52:1338–1342
Carbajo JM, Junca H, Terrón MC, González T, Yagüe S, Zapico E, González AE (2002) Tannic acid induces transcription of laccase gene cglccl in the white-rot fungus Coriolopsis gallica. Can J Microbiol 48:1041–1047
Carnicero A, Trojanowski J, Falcón MA, De la Fuente G, Kharazipour A, Hüttermann A (1992) Lignin degrading capacities of several fungi imperfecti isolated from soils tested by the radiorespirometric method. Microbios 72:17–25
Deighton N, Muckenschnabel I, Goodman BA, Williamson B (1999) Lipid peroxidation and the oxidative burst associated with infection of Capsicum annuum by Botrytis cinerea. Plant J 20:485–492
Dekker FH, Barbosa AM (2001) The effect of aeration and veratryl alcohol on the production of two laccases by the ascomycete Botryosphaeria sp. Enzyme Microb Technol 28:81–88
Dunford HB (1999) Heme peroxidases. Wiley-VCH, New York
Eggert C, Temp U, Eriksson K-EL (1996) The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Haider K, Trojanowski J (1975) Decomposition of specifically 14C-labelled phenols and dehydropolymers as models for lignin degradation by soft and white rot fungi. Arch Microbiol 105:33–41
Hatakka A (1994) Lignin-modifying enzymes from selected white rot fungi—production and role in lignin degradation. FEMS Microbiol Rev 13:125–135
Hatakka A (2001) Biodegradation of lignin. In: Hofrichter M, Steinbüchel A (eds) Biopolymers. Vol 1, Lignin, humic substances and coal. Wiley-VCH, Weinheim, pp 129–180
Hofrichter M, Lundell T, Hatakka A (2001) Conversion of milled pine wood by manganese peroxidase from Phlebia radiata. Appl Environ Microbiol 67:4588–4593
Kirk TK, Farrell RL (1987) Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol 41:465–505
Kluczek-Turpeinen B, Tuomela M, Hatakka A, Hofrichter M (2003) Lignin degradation in a compost environment by the deuteromycete Paecilomyces inflatus. App Microbiol Biotechnol 61:374–379
Li K, Helm RF (1995) Synthesis and rearrangement reactions of ester-linked lignin-carbohydrate model compounds. J Agric Food Chem 43:2098–2103
Monties B, Fukushima K (2001) Occurrence, function and biosynthesis of lignin. In: Hofrichter M, Steinbüchel A (eds) Biopolymers. Vol 1—lignin, humic substances and coal. Wiley-VCH, Weinheim, pp 1–64
Purdy RE, Kolattukudy RE (1973) Depolymerization of a hydroxyl fatty acid biopolymer, cutin, by an extracellular enzyme from Fusarium solani pisi. Isolation and some properties of the enzyme. Arch Biochem Biophys 159:61–69
Robene-Soustrade I, Lung-Escarmant B, Bono JJ, Taris B (1992) Identification and partial characterization of an extracellular manganese-dependent peroxidase in Armillaria ostoyae and Armillaria mellea. Eur J For Pathol 22:227–236
Rodríguez A, Falcón MA, Carnicero A, Perestelo F, De la Fuente G, Trojanowski J (1996) Laccase activities of Penicillium chrysogenum in relation to lignin degradation. Appl Microbiol Biotechnol 45:399–403
Rüttimann-Johnson CL, Salas L, Vicuña R, Kirk TK (1993) Extracellular enzyme production and synthetic lignin mineralization by Ceriporiopsis subvermispora. Appl Environ Microbiol 59:1792–1797
Savory JG (1954) Breakdown of timber by ascomycetes and fungi imperfecti. Ann Appl Biol 41:336–357
Scheibner K, Hofrichter M, Herre A, Michels J, Fritsche W (1997) Screening for fungi intensively mineralising 2,4,6-trinitrotoluene. Appl Microbiol Biotechnol 47:452–457
Schwarze EWMR, Engels J, Mattheck C (2000) Fungal strategies of wood decay in trees. Springer Berlin Heidelberg New York
Steffen KT, Hofrichter M, Hatakka A (2000) Mineralization of 14C-labeled synthetic lignin and ligninolytic enzyme activities of litter-decomposing basidiomycetous fungi. Appl Microbiol Biotechnol 54:819–825
Sutherland JB, Crawford DL (1981) Lignin and glucan degradation by species of the Xylariaceae. Trans Br Mycol Soc 76:335–337
Ullrich R, Nüske J, Scheibner K, Spantzel J, Hofrichter M (2004) Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes. Appl Environ Microbiol 70:4575–4581
Vares T, Niemenmaa O, Hatakka A (1994) Secretion of ligninolytic enzymes and mineralization of 14C-ring-labelled synthetic lignin by three Phlebia tremellosa strains. Appl Environ Microbiol 60:569–575
Wei DL, Chang SC, Wei YH, Lin YW, Chuang CL, Jong SC (1992) Production of cellulolytic enzymes from the Xylaria and Hypoxylon species of Xylariaceae. World J Microbiol Biotechnol 8:141–146
Williamson G, Kroon PA, Faulds CB (1998) Hairy plant polysaccharides: a close shave with microbial esterases. Microbiology 144:2011–2023
Acknowledgements
The work was supported by the Federal State of Saxony (HWP program to C.L.), the German Federation of Industrial Cooperative Research Associations Otto von Guericke (project KF 0094004KMD3 to R.U. and M.H.), the Academy of Finland (project 52063 to M.H. and 106213 to K.T.S.) and the administration of the International Graduate School Zittau (Dr. R. Konschak). We thank U. Schneider and A. Elsner for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liers, C., Ullrich, R., Steffen, K.T. et al. Mineralization of 14C-labelled synthetic lignin and extracellular enzyme activities of the wood-colonizing ascomycetes Xylaria hypoxylon and Xylaria polymorpha . Appl Microbiol Biotechnol 69, 573–579 (2006). https://doi.org/10.1007/s00253-005-0010-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0010-1