Abstract
In this work, we compared the performance of Phragmites australis and Typha latifolia for depurating primary-treated urban wastewater and evaluated their suitability for irrigation reuse. Macrophytes were planted in two pilot-scale constructed wetland systems (CWs) and monitored during a 2-year experiment (2002–2003). CW efficiency was evaluated in terms of both mass removal and water quality considering boron (B) and the following heavy metals: aluminium (Al), arsenic (As), beryllium (Be) manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), cadmium (Cd), lead (Pb), selenium (Se) and vanadium (V). The accumulation of the elements, both in plant tissues and sandy substrate layer, and their offtake with the macrophyte harvest were also measured. In quantitative terms, the established CW systems showed high removal efficiency for Al (96 %), Cu (91 %), Pb (88 %) and Zn (85 %), while lower efficiencies were observed for Fe (44 %), Co (31 %) and B (40 %). The sediment played a strategic role in the adsorption and accumulation of wastewater pollutants, while plants acted as phytostabilizers since element root concentrations were generally from one to two orders of magnitude higher than those observed in the other parts. The results were less favourable in terms of water quality because the high evapotranspiration counteracted the depuration process by concentrating the elements in the outflow water. Outflow water contained more B (68 %), Mn (196 %) and, in the case of CW managed with Phragmites, also Fe (73 %) than inflow water, breaking the Italian guidelines for irrigation reuse. Integrating solutions to reduce the high evapotranspiration of CWs with more efficient pre-cleaning systems are necessary to obtain better removal efficiencies that reduce the effect of ET on water quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The interest in the reuse of urban wastewater to avoid future problems of water scarcity is now growing. The use of raw wastewater is nevertheless risky because it contains biodegradable organic matter, inorganic and organic chemicals, toxic substances and disease-causing agents (Salgot et al. 2006). Accumulation of heavy metals in soil (Mireles et al. 2004; Mapanda et al. 2005) and plants (Yadav et al. 2002; Solis et al. 2005) has been observed in fields long-term irrigated with wastewater. Conversely, municipal wastewater treated with wetland technologies has been found suitable for crop irrigation, meeting specific national guidelines (e.g. Belmont et al. 2004; Wang et al. 2005). Indeed constructed wetlands have proved to be efficient in removing the main chemicals (organic substances, heavy metals, etc.) and biological organisms (bacteria, viruses, parasites, etc.) from municipal and domestic wastewaters (Kivaisi 2001; Gross et al. 2007).
Adsorption to sediments (e.g. humic or clay colloids), precipitation and co-precipitation with secondary minerals and complexation are well-known mechanisms of heavy metals removal in wetlands (Gambrell 1994). Macrophytes play also an important role in depuration process, assimilating metals into the tissues, acting as catalysts for purification reactions, increasing environmental diversity in the rhizosphere and promoting a variety of chemical and biological reactions (Hadad et al. 2006). For instance, biological volatilisation of selenium, which involves element take up in the form of selenate or selenite and plant metabolization into volatile forms, takes place mainly in the roots region (Hansen et al. 1998). Volatilisation from the roots was observed also for mercury (Moreno et al. 2008).
Evaluating the mechanisms of depuration is crucial to understand the extent of the pollutants uptake and how they are distributed within the plants. Indeed for persistent elements as heavy metals, wetlands may be a source as well as a sinks for contaminants. Schaller et al. (2013) reported that in most of wetlands, the main sink for storing elements is the sediment, while the elemental blockage in macrophytes is generally low and mainly concentrated in the below-ground tissues (Sekomo et al. 2011; Bonanno 2013), although in some cases (e.g. Galletti et al. 2010) found that leaves, flowers and stems of Phragmites australis removed more Ni and Zn than roots and rhizomes.
Restriction of shoot translocation is believed to be the strategy of metal tolerance for non-hyperaccumulator plants (Bragato et al. 2006). However, metals can be also taken up by roots, transported upward to above-ground tissues, from which they can be excreted or released with the decaying litter (Weis and Weis 2004). Conversely, senescent plant tissues can be a sink for elements through litter adsorption or microbial immobilization. Du Laing et al. (2006), for instance, proved as Cd, Cu, Cr, Ni, Pb and Zn content of leaf blades and stems of P. australis increased considerably during the decomposition for effect of physico-chemical sorption, infiltration of mud particles and incorporation by microbial litter.
In Europe, there is no unified legislation regarding the reclamation of treated wastewater for irrigation purposes and the plurality of aspects makes difficult to evaluate the suitability of reclamation systems (Norton-Brandão et al. 2013). For example, the Italian guidelines concerning wastewater reuse and irrigation are more specific than former existing regulations in other parts of the world (Salgot et al. 2006), including a list of 60 physical (e.g. suspended solids), chemical (e.g. pH, COD, BOD, heavy metals) and biological (e.g. Escherichia coli, Salmonella) parameters. Recently, in a pilot-scale constructed wetland, we evaluated the mechanisms of purification of municipal wastewaters treated by P. australis and Typha latifolia (Morari and Giardini 2009) considering a list of representative chemical parameters (e.g. COD, BOD, Ntot). Here, we aimed to complete our previous analysis evaluating the depuration efficiency of such system and the suitability of treated wastewater for irrigation purposes with respect to boron and heavy metals.
2 Material and Methods
2.1 The Experimental Design
The experiment was conducted in 2002–2003 at the Experimental Farm of the University of Padova, in northeastern Italy. In a battery of 20 lysimeters, set up to evaluate the effects of irrigation with urban wastewater on crops (maize and tomato), four units were modified to simulate at pilot scale the functionality of two type of constructed wetlands (CWs), one with T. latifolia (CW-T) and the other with P. australis (CW-P), that were characterized by vertical flow.
Although the systems operated in a discontinuous way to fulfil the crop water requirements, the water retention time was estimated of ca. 2–3 weeks, with lower values corresponding to the period of higher evapotranspirative demand. The experimental layout was a randomized block with two replicates. The macrophyte lysimeters (Fig. 1) were surrounded by the crop lysimeters and had a surface area of 1 m2 (1 m × 1 m) and were 1.5 m deep. They were filled with sand (effective size of 0.16 mm and uniformity coefficient of 2.2) in the first 16 cm, followed by two 22-cm layers of gravel with a diameter of 4–8 and 8–12 mm and a 90 cm layer of gravel 30–50 mm in diameter (IWA 2000). A mobile roof automatically covered the experimental area when rain fell, thus avoiding rainfall input in the CWs.
The macrophytes were planted in May 2002 at a density of 12 rhizomes m−2. After 3 weeks, half of the T. latifolia rhizomes had rotted, so were replaced. Plants were watered until the beginning of June 2002, then fed with urban wastewater. Wastewater was collected from a nearby conventional municipal purification plant that treats primarily domestic wastewater of medium strength (e.g. 273 mg L−1 of total suspended solids; 202 mg L−1 of biochemical oxygen demand). Wastewater used in the experiment had received only the primary sedimentation treatment.
The CWs operated in a discontinuous way, on the basis of the water requirements of the crops cultivated in the nearby lysimeters: on average, charge/discharge operations were carried out weekly during the irrigation season (May–September) and every 2 weeks in the other months. Operations were suspended during the winter (December 2002-February 2003). Inflow and outflow volumes were measured manually, and during each charge/discharge operation a representative 2-L water sample was collected for chemical analysis. During the inflow operations, CWs were completely flooded up to surface level, controlling the level by means of the piezometer. Macrophytes were harvested at the end of October 2002 and 2003.
The fresh and dry biomass of the leaves, infructescences (spikes in T. latifolia and panicles in P. australis) and stems were weighed separately. Samples of the rhizomes and roots were also collected from an area of 0.09 m2. To measure the dry weight, the biomass was first dried at 65 °C in a forced draft oven for 36 h, the dried tissues were then ground and three 1-g powdered samples were dried at 130 °C to measure the residual moisture content.
2.2 Chemical Analyses
Organs of the macrophytes (leaves, infructescences, stems, rhizomes and roots), inflow/outflow water and sandy layer were analysed for boron (B), and the following heavy metals: aluminium (Al), arsenic (As), beryllium (Be) manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), cadmium (Cd), lead (Pb), selenium (Se) and vanadium (V).
Macrophytes organs were analysed after mineralisation of 400 mg dry material in a microwave oven (Milestone Ethos 900, Shelton, CT) with 5 mL of nitric acid (69 % v/v) and 1 mL H2O2 (30 % v/v). The digest was made up to 25 mL final volume with deionised water, filtered (0.45 μm, Millipore) and then analysed using ICP/OES (Spectro Ciros CCD, Kleve, Germany). Water samples were analysed with ICP/OES (Spectro Ciros CCD), with preliminary acid treatment of unfiltered samples (CNR-IRSA, 1994). Water samples were analysed every one out of three charge/discharge operations (21 days on average) the first year and every charge/discharge operation the second year, when the CWs were fully functioning. A general overview of inflow main physico-chemical water parameters was reported in Table 1 (Morari and Giardini 2009).
Samples of the sandy layer (0–16 cm) were collected at the start of the experiment and at the end of October 2002 and October 2003 (after the plant harvest). Sand samples were analysed by ICP/OES (Spectro Ciros CCD), after digesting the sample (1 g) with 3 mL HNO3 (69 % v/v) and 9 mL HCl (37 % v/v) in a microwave oven (Milestone Ethos 900).
2.3 Statistical Analyses
In some water samples, As, Cd, Co, Cr, Ni, Pb and V were found below the detection limit (DL) (censored data) (Gilbert 1987). To estimate the summary statistics of these elements, two different approaches were followed: (a) if the censored data were less than the 50 % of the total data, the Helsel’s robust method was applied (Helsel 1990). Helsel’s approach fits a regression line to the log-transformed observation values above the DL and their corresponding z scores. Next, the regression is used to predict “fill in” values for the below DL observations. All values including the predicted “fill in” values are then back-transformed to arithmetic units. The median and percentiles are estimated using the dataset that now includes “fill in” values for the censored observations (Newman 1995); (b) if the censored data were more than the 50 %, we calculated the median, including DL values and reported the result preceded by a “<” sign (Gilbert 1987). The first approach (Helsel’s) was applied to Cr and Pb data in 2003, whereas the second one was applied to As, Be, Co, Cr, Pb, V and Se in 2002, and As, Co, Ni and Se in 2003.
Non-parametric Kruskal-Wallis method was used to compare the four treatments (inflow and outflow concentrations in CW-T and CW-P) in the 2 years. Kruskal-Wallis was applied also to datasets including censored data (Gilbert 1987; Helsel 1990). Post hoc comparisons of mean ranks for all groups were then performed according to the study of Siegel and Castellan (1988). Correlations between water quality variables were determined using non-parametric Spearman’s coefficient. To clarify the structure of these interdependences, a joint principal components analysis (PCA) of concentration data on in the inflow and outflow water was performed. PCA was restricted only to data collected in 2003, since the high number of “<DL” values observed in 2002. Moreover, according to Farnham et al. (2002) only variables with less the 30 % of DL values were considered, substituting “<DL” values with DL/2. The standardized variables were submitted to the PCA; rotated orthogonal components (varimax method of rotation) were extracted and the relative scores were determined. Only PCs with eigenvalue >1 were considered for the discussion.
CW performances were evaluated in terms of both relative concentration reduction and mass removal (Kadlec and Knight 1996). Plant and soil data were analysed with ANOVA and significantly different means were differentiated with the Student-Newman Keuls test. It was not possible to test the year effect in all the plant organs as the macrophytes experienced incomplete growth and morphogenesis in the first year, without the formation of inflorescences and rhizomes in P. australis and inflorescences and stems in T. latifolia. The year effect was therefore tested only on roots and leaves, which were present on both plant species in 2002 and 2003 (three-way ANOVA year × plant structure × macrophyte species). A two-way ANOVA (structure × macrophyte species) was then applied to the 2003 plant dataset. ANOVA was not applied to As, Cd, Co, Cr, Ni, Pb and V datasets, since these elements were found in some of the macrophyte organs below the detection limit. Differences were therefore tested applying Kruskal-Wallis (Gilbert 1987; Helsel 1990).
3 Results
3.1 Water Balance
Details of water balance in the 2 years have been already reported by Morari and Giardini (2009). CWs’ evapotraspiration (ET) varied according to the year and species. In 2002, ET was higher in CW-T (1,249 ± 103 L m−2) than CW-P (871 ± 38 L m−2) whereas the opposite was observed in 2003 (3,575 ± 136 L m−2 in CW-T vs. 4,362 ± 234 L m−2 in CW-P). On average, inflow volumes were 2,560 ± 118 L m−2 in 2002 and 5,748 ± 253 L m−2 in 2003. Outflow was discharged in equal volumes for both CWs: 1,500 L m−2 in 2002 and 1,780 L m−2 in 2003. Water lost by ET (expressed as % of inflow volumes) was 45 % (2002) and 67 % (2003) in CW-T and 37 % (2002) and 71 % (2003) in CW-P. Consequently, to guarantee crop water needs, a mean hydraulic loading rate of 18 mm day−1 in 2002 and 28 mm day−1 in 2003 was applied to the CWs, with peaks of 40 mm day−1 in July and August 2003.
3.2 Plants Analysis
In 2002, T. latifolia was more productive than P. australis (dry biomass of 1.2 ± 0.20 vs 0.52 ± 0.02 kg m−2), with a different repartition of the aerial biomass: 100 % leaves in T. latifolia and 64 % leaves and 36 % stems in P. australis. On the contrary, in 2003, biomass was higher in P. australis (dry biomass 7.3 ± 0.08 kg m−2) than T. latifolia (dry biomass 6.8 ± 0.21 kg m−2) with a leaf-stem-infructescence repartition of 67, 30 and 3 % in P. australis and 58, 22 and 20 % in T. latifolia.
Element concentrations in the plant tissues varied according to species and their organs (Fig. 2 and Table 2). B was more concentrated in the infructe-scences (23.6 mg kg−1) and leaves (21.4 mg kg−1) without significant differences between the macrophytes. Moreover, no change in the concentrations of leaves and roots was observed between the 2 years. Higher Mn concentrations were observed in Phragmites (129 mg kg−1) than Typha (77 mg kg−1) (p < 0.05), with the highest values observed in the leaves and the lowest in the stem (Fig. 2). Leaf concentration decreased in the 2 years for both species, whereas only in Phragmites root concentration increased from 75 mg kg−1 in 2002 to 185 mg kg−1 in 2003 (p < 0.01).
The other elements (Fe, Al, Cu, Zn, As, Cd, Co, Cr, Pb, Ni and V) (Fig. 2 and Table 2) accumulated mainly in the root tissues with concentrations from one to two orders of magnitude higher than those observed in the other parts (p < 0.01), but Cu in Typha (n.s.). On average, element root concentrations in Phragmites ranked as follows, Fe > Al > Zn > Cu > Cr > Ni > Pb > V > As > Co > Cd, similar to Typha, Fe > Al > Zn > Cu > Ni > Cr > V > Pb > As > Co > Cd. Root concentrations of Fe, Al, Cu, Cr and Ni were significantly higher in Phragmites than in Typha (p < 0.05). In the second year, roots of Phragmites showed a significant increase in the concentration of metals, whose values were in general one order of magnitude higher than those observed in 2002. In the case of Cr, Pb, Ni and V, the increase in root concentration was accompanied by a contemporary decrease in the concentration of the leaves (Table 2). Differences among the above-ground organs and rhizomes were not statistically significant, even if the seconds showed an apparent higher concentration of heavy metals. Besides, above-ground concentrations of As, Cd and Co were frequently below the DLs in both the years. In the case of Se, values were always, even in the roots and rhizomes, below the DL and therefore not reported.
Element offtake (i.e. the quantity of element removed with the harvest) was influenced by the organ concentration and repartition in the harvested biomass (Fig. 3 and Table 3). It was higher in the second year than the first, with a significant interaction with the macrophyte factor. Higher Cu, Zn, Mn and Cr offtake was observed in Phragmites than in Typha in 2003. No significant differences were observed for Al and Fe, even if apparent higher values were observed the second year in Phragmites (+34 and +28 %, respectively). In spite of the fact that other elements (As, Cd, Co, Pb, V) were frequently below the DLs, uptake followed the same trend with higher removal capacity observed in 2003 with Phragmites.
In the fully established macrophytes, the leaves and stem gave the major contribution to the element offset. In Phragmites, elements were removed prevalently with the leaves, from 65 to more than 90 % (Fe, Al and Mn) of the total offset. In Typha, for the majority of the element the contribution of the fruits in the removal process was relevant (>15 %), particularly in the case of Cu (45 %). Only for Al and Mn it was lesser than 6 %. However, as well as in Phragmites, elements were removed prevalently with the leaves.
3.3 CWs Performances
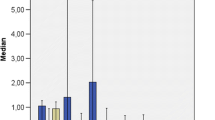
CWs had only a partial effect on water quality in the first year (Fig. 4). A significant abatement was observed only for Al (73 %) and Cu (44 %) in CW-P. By contrast, in the same CW, Fe concentration in outflow increased to 220 %. As, Cd, Co, Cr, Pb and V resulted often below the detection limits without any significant difference between averaged inflow and outflow data (Table 4). In 2003, quality of inflow water worsened for Al, Fe, As and Cd (p < 0.01). Contemporarily, CW functionality increased showing a significant (p < 0.01) abatement for Al (86 %), Cu (69 %), Cr (81 %), Pb and V (15.4 %) in both CWs and Zn (63 %) only in CW-T. By contrast, higher concentration levels (p < 0.05) in outflow water were measured for B (+68 %) and Mn (+196 %), and in the case of CW managed with Phragmites, also Fe (+73 %) and Co.
Concentrations of the chemical parameters in the inflow and outflow water. The dashed lines for B, Zn, Fe, Al and Mn represent the Italian guideline limits for irrigation reuse. Cu Italian guideline limit is 1 mg L−1. phr = TP. australis, ty = T. latifolia. Medians with the same letters in the same year are not statistically different at p = 0.05
The functionality of the CW changed according to ET demand. In fact, positive correlations were found between ET and abatement of Cr (r = 0.41), Al (r = 0.48), Pb (r = 0.30) and V (r = 0.43), whereas negative correlation was found between ET and abatement of B (r = −0.51) and Co (r = −0.48) and Mn (r = −0.29).
Application of PCA to inflow quality data allowed to extract two factors explaining more than 78 % of the variability (Table 5). The first factor, which accounted for the 66 % of the variance, was highly correlated (factor loadings ≥ 0.8) with Cu, Zn, Cr, Cd, Al, Fe and V, while the second factor explained only 12 % of the variance and was correlated with Co. The process of phytodepuration altered the equilibrium among the parameters as was evidenced by PCA on outflow quality data. Four factors with eingenvalue >1 were extracted on Phragmites outflow data. The first two factors explained the 56 % of variance and were correlated with B, V and Co (principal component 1) and Cu and Al (principal component 2). The remaining two factors explained the 23 % of the variability were correlated with Cr and Zn (principal component 3) and Fe and Mn (principal component 4). Same results were obtained with application of PCA on Typha’s outflow data. They differed mainly because Fe and Mn had a higher weight in explaining variability, while a lower weight was that one of B. Moreover, no factor was correlated (factor loadings > 0.6) with Al. Indeed four principal component with eingenvalue >1 were extracted. The first factor was highly correlated (factor loadings > 0.9) with Fe and Mn and explained the 31 % of the variance, while the fourth one was correlated with B and explained only the 10 % of the variance. The remaining two factors were correlated (factor loadings > 0.6) with Cu, Zn and V (principal component 2) and Cr (principal component 3).
Expressed in quantitative terms, the abatement efficiency was remarkably better in 2003 than in 2002 (the average of each event), despite the higher input observed during the second year (Fig. 5 and Table 3). High abatement efficiencies (>96 %) were always observed in 2003 for Al in both Phragmites and Typha, and for Cr and Zn in Typha (90 vs. 76 %). No significant effect was observed for Mn and Fe in 2002 and 2003. The contribution of plant offtake in the removal processes was generally higher for Fe and Cu than for Cr and finally B, Al and Zn. Phragmites tended to adsorb Cr more efficiently than Typha. This was also found for Mn (uptake = 1.1 and 0.76 g m−2 in Phragmites and Typha, respectively), although the element was not reduced by the CW since outflow water had more Mn than inflow water (+7 %).
3.4 Sandy Layer Analysis
After the first year, a sharp accumulation of the majority of elements was measured, but B and As (Table 6). At the end of the second year, only As contents remained stable while other elements significantly decreased compared to October 2002 (Table 6). The type of macrophyte affected the sand composition with higher average contents of Fe, Cu and Pb in CW-T than CW-P (p < 0.05). Conversely, Cr was more concentrated (p < 0.05) under Phragmites (109.3 mg kg−1 on average) than Typha phytodepuration system (92.8 mg kg−1).
4 Discussion
CWs reached the full functionality in the second year (2003) when macrophytes were fully established and their growth cycle was completed. During the first year, CW systems performed partly as unplanted beds (Morari and Giardini 2009) with a significant accumulation of heavy metals in the sediment at the end of the first year of experiment (October 2002). As already reported in previous studies (Liu et al. 2007; Schaller et al. 2013), the upper sediment layer, generally combined with a low diffusion rate of the element, plays a strategic role in the adsorption and accumulation of heavy metals, especially when plant biomass is strongly lower than sediment compartment (Hadad et al. 2006). The concentration levels, observed in the final date with respect to the initial one (Table 6), emphasized that sedimentation was the main process in the removal of heavy metals (Arroyo et al. 2013). Nevertheless in 2003 plant biomass was significantly higher than 2002, increasing its capacity to adsorb heavy metals and lowering the concentration of almost all the elements in the substrate. Accordingly, Vymazal et al. (2010) argued that the concentration of heavy metals remained steady during a 16-year study conducted on a constructed wetland due to increase in the sediment biomass.
Macrophytes accumulated significantly greater concentrations of heavy metals in roots than shoots. This is consistent with previous results reported in the literature (Carranza-Alvarez et al. 2008; Grisey et al. 2012; Bonanno 2013). For example Carranza-Alvarez et al. (2008), studying the accumulation of Pb, Cd, Co and Fe in macrophytes from an artificial lagoon treated by municipal and industrial wastewater, which found that Scirpus americanus and T. latifolia may be considered phytostabilizers for treating polluted water systems. The capacity of below-ground tissues to adsorb more heavy metals than above-ground tissues was also reported by Sasmaz et al. (2008). The former, calculating the plant’s potential to translocate metals from below-ground to above-ground tissues (i.e. translocation factor), showed that Typha did not effectively transfer heavy metals from root to body (translocation factor <1). However, the authors suggested the use of Typha as a bio-accumulator for sediments and water polluted by metals since the bio-accumulator factor (roots/sediments) was generally >1. Similarly, in our experiment, both Phragmites and Typha had a translocation factor <1 for almost all the elements (except for Mn and B), while the roots were suitable for accumulating heavy metals, especially Cu, Pb and Zn (bio-accumulation factor >1). Therefore, they can play an important role in constructed wetlands acting mainly as phytostabilizers, particularly in the long term when root biomass greatly increases.
Results indicated that CWs were efficient systems for removing Zn, Al, Cu, Cr, Pb and V. The influent concentration of Al and Zn (25th percentile) during 2003 was greater than the Italian legal limits for discharge into a surface water body (1 mg L−1), while the effluent concentration (25th percentile) was significantly reduced below the Italian standards, suggesting that wetland systems can be highly effective in removing pollutants. Conversely, previous studies documented the inability of CWs to remove some pollutants from wastewater. For example, Vymazal (2005) showed an increase of Mn concentration from 109 to 402 μg L−1, suggesting that immobilization in wetland sediments was delayed by anoxic conditions that favoured Mn2+, which is quite soluble, while Kröpfelová et al. (2009) found very low removal of B and Co in horizontal sub-surface flow constructed wetlands. The low B removal was also reported by Türker et al. (2013), arguing that optimal B uptake ranged from 6.3 to 7.5 pH values and that absorption decreased with increasing pH. Our study showed even more unsatisfactory results since B, Co (only in CW-P), Fe and Mn during 2003 were higher in the effluents than in the influents and in some cases the pollutants (Fe and Mn) did not meet the legal limits of Italian guidelines. Most likely the accumulation in the sediments during the first year (2002), coupled with the low plant removal/stabilization, involved the worsening of water quality. Fe and Mn, as well as B and Co, were joined in the same factors by PCA in the outflow water (both in Phragmites and Typha), suggesting the prevalence of a common concentrating mechanism. Most likely, in our experiment, the combined effect of unfavourable physico-chemical conditions (e.g. pH = 8.4, on average) and high evapotranspiration (Morari and Giardini 2009) strongly affected CWs efficiency, concentrating the elements in the water and lowering its quality both with Phragmites than Typha. Substantial water losses due to high evapotranspiration were considered a major component in the water balance of constructed wetlands, particularly where treated wastewater are destined for reuse (Borin et al. 2011; Shelef et al. 2013). If on the one hand the water lost through plant transpiration can increase hydraulic retention time enhancing nutrients removal (Gagnon et al. 2012), non-degradable contaminants increase their concentration in the effluents on the other hand (Katsenovich et al. 2009).
High removal efficiencies were required to improve water quality. In fact, a depuration effect was observed when quantitative removal efficiency (during 2003) was higher than 87.7 % in Phragmites on average, whereas in Typha this threshold rose to 91.5 %. By contrast, water quality worsened when quantitative removal efficiencies were lower than 43 and 67 % in Phragmites and Typha, respectively.
5 Conclusions
The established CW systems showed contrasting results in terms of pollutants removal. Most of the elements accumulated in the sediment, which played a strategic role in the depuration process. The translocation of pollutants to above-ground tissues was not always significant; consequently harvesting the vegetation would have a partial effect as a strategy to reduce pollutants from the system. However, macrophytes can act as phytostabilizers and enhance the pollutants immobilization, particularly in the long-term when root biomass greatly increases. Results in terms of water quality showed that CWs can be inefficient systems for depurating primary-treated urban wastewater. The high ET losses counteracted the depuration process by concentrating the elements in the outflow water. As a consequence, only parameters with high removal efficiency can be effectively removed, respecting the Italian guidelines for irrigation reuse. This is particularly important since CWs have a significant role in the ET process and the period of higher ET for hygrophytes coincides with the periods of higher water demand for crops.
Nevertheless, adjunctive studies are necessary to evaluate the long-term performances of continuous 12-month operation CWs. Indeed, this experiment represents a specific application of CWs, which operated in a discontinuous way to fulfil the crop water requirements. Results obtained under these conditions could be different from those of continuous 12-month operation and therefore cannot be used to evaluate CWs for wastewater treatment, in general. Anyway, these results indicate that integrated solutions to reduce the water loss through evapotranspiration, as well as improving wastewater pre-treatments, are key strategies to progress in CW systems.
References
Arroyo, P., Blanco, I., Cortijo, R., de Luis Calabuig, E., & Ansola, G. (2013). Twelve-year performance of a constructed wetland for municipal wastewater treatment: water quality improvement, metal distribution in wastewater, sediments, and vegetation. Water, Air, and Soil Pollution, 224, 1–14.
Belmont, M. A., Cantellano, E., Thompson, S., Williamson, M., Sanchez, A., & Metcalfe, C. D. (2004). Treatment of domestic wastewater in a pilot-scale natural treatment system in central Mexico. Ecological Engineering, 23, 299–311.
Bonanno, G. (2013). Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicology and Environmental Safety, 97, 124–130.
Borin, M., Milani, M., Salvato, M., & Toscano, A. (2011). Evaluation of Phragmites australis (Cav.) Trin. evapotranspiration in Northern and Southern Italy. Ecological Engineering, 37, 721–728.
Bragato, C., Brix, H., & Malagoli, M. (2006). Accumulation of nutrients and heavy metals in Phragmites australis (Cav.) Trin. ex Steudel and Bolboschoenus maritimus (L.) Palla in a constructed wetland of the Venice lagoon watershed. Environmental Pollution, 144, 967–975.
Carranza-Alvarez, C., Alonso-Castro, A. J., Alfaro-De La Torre, M. C., & Garcia-De La Cruz, R. F. (2008). Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial lagoon in San Luis Potosi, Mexico. Water, Air, and Soil Pollution, 188, 297–309.
CNR-IRSA. (1994). Quaderni IRSA N° 100. Metodi analitici per le acque. Istituto Poligrafico e Zecca dello Stato, Rome, Italy
Du Laing, G., Van Ryckegem, G., Tack, F. M. G., & Verloo, M. G. (2006). Metal accumulation in intertidal litter through decomposing leaf blades, sheaths and stems of Phragmites australis. Chemosphere, 63, 1815–1823.
Farnham, I. M., Singh, A. K., Stetzenbach, K. J., & Johannesson, K. H. (2002). Treatment of nondetects in multivariate analysis of groundwater geochemistry data. Chemometrics and Intelligent Laboratory Systems, 60, 265–281.
Gagnon, V., Chazarenc, F., Kõiv, M., & Brisson, J. (2012). Effect of plant species on water quality at the outlet of a sludge treatment wetland. Water Resources, 46, 5305–5315.
Galletti, A., Verlicchi, P., & Ranieri, E. (2010). Removal and accumulation of Cu, Ni and Zn in horizontal subsurface flow constructed wetlands: contribution of vegetation and filling medium. Science of the Total Environment, 408, 5097–5105.
Gambrell, R. P. (1994). Trace and toxic metals in wetlands—a review. Journal of Environmental Quality, 23, 883–891.
Gilbert, R. (1987). Statistical methods for environmental pollution monitoring. New York: van Nostrand Reinhold.
Grisey, E., Laffray, X., Contoz, O., Cavalli, E., Mudry, J., & Aleya, L. (2012). The bioaccumulation performance of reeds and cattails in a constructed treatment wetland for removal of heavy metals in landfill leachate treatment (Etueffont, France). Water, Air, and Soil Pollution, 223, 1723–1741.
Gross, A., Shmueli, O., Ronen, Z., & Raveh, E. (2007). Recycled vertical flow constructed wetland (RVFCW)—a novel method of recycling greywater for irrigation in small communities and households. Chemosphere, 66, 916–923.
Hadad, H. R., Maine, M. A., & Bonetto, C. A. (2006). Macrophyte growth in a pilot-scale constructed wetland for industrial wastewater treatment. Chemosphere, 63, 1744–1753.
Hansen, D., Duda, P. J., Zayed, A., & Terry, N. (1998). Selenium removal by constructed wetlands: role of biological volatilization. Environmental Science and Technology, 32, 591–597.
Helsel, D. R. (1990). Less than obvious-statistical treatment of data below the detection limit. Environmental Science and Technology, 24, 1766–1774.
IWA. (2000). Constructed wetlands for water pollution control, processes, performance, design and operation. IWA scientific and Technical report No 8. London: IWA Publishing.
Kadlec, R. H., & Knight, R. L. (1996). Treatment wetlands. Boca Raton: Lewis Publishers.
Katsenovich, Y. P., Hummel-Batista, A., Ravinet, A. J., & Miller, J. F. (2009). Performance evaluation of constructed wetlands in a tropical region. Ecological Engineering, 35, 1529–1537.
Kivaisi, A. (2001). The potential for constructed wetlands for wastewater treatment and reuse in developing countries: a review. Ecological Engineering, 16, 545–560.
Kröpfelová, L., Vymazal, J., Švehla, J., & Štíchová, J. (2009). Removal of trace elements in three horizontal sub-surface flow constructed wetlands in the Czech Republic. Environmental Pollution, 157, 1186–1194.
Liu, J., Dong, Y., Xu, H., Wang, D., & Xu, J. (2007). Accumulation of Cd, Pb and Zn by 19 wetland plant species in constructed wetland. Journal of Hazardous Materials, 147, 947–953.
Mapanda, F., Mangwayana, E. N., Nyamangara, J., & Giller, K. E. (2005). The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agriculture, Ecosystems & Environment, 107, 151–165.
Mireles, A., Solis, C., Andrade, E., Lagunas-Solar, M., Pina, C., & Flocchini, R. G. (2004). Heavy metal accumulation in plants and soil irrigated with wastewater from Mexico City. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 219, 187–190.
Morari, F., & Giardini, L. (2009). Municipal wastewater treatment with vertical flow constructed wetlands for irrigation reuse. Ecological Engineering, 35, 643–653.
Moreno, F. N., Anderson, C. W. N., Stewart, R. B., & Robinson, B. H. (2008). Phytofiltration of mercury-contaminated water: volatilisation and plant-accumulation aspects. Environmental and Experimental Botany, 62, 78–85.
Newman, M. C. (1995). Quantitative methods in aquatic ecotoxicology. Boca Raton: CRC Press.
Norton-Brandão, D., Scherrenberg, S. M., & van Lier, J. B. (2013). Reclamation of used urban waters for irrigation purposes—a review of treatment technologies. Journal of Environmental Management, 122, 85–98.
Salgot, M., Huertas, E., Weber, S., Dott, W., & Hollender, J. (2006). Wastewater reuse and risk: definition of key objectives. Desalination, 187, 29–40.
Sasmaz, A., Obek, E., & Hasar, H. (2008). The accumulation of heavy metals in Typha latifolia L. grown in a stream carrying secondary effluent. Ecological Engineering, 33, 278–284.
Schaller, J., Vymazal, J., & Brackhage, C. (2013). Retention of resources (metals, metalloids and rare earth elements) by autochthonously/allochthonously dominated wetlands: a review. Ecological Engineering, 53, 106–114.
Sekomo, C. B., Nkuranga, E., Rousseau, D. P. L., & Lens, P. N. L. (2011). Fate of heavy metals in an urban natural wetland: the Nyabugogo Swamp (Rwanda). Water, Air, and Soil Pollution, 214, 321–333.
Shelef, O., Gross, A., & Rachmilevitch, S. (2013). Role of plants in a constructed wetland: current and new perspectives. Water, 5, 405–419.
Siegel, S., & Castellan, N. J. (1988). Nonparametric statistics for the behavioral sciences. New York: McGraw-Hill.
Solis, C., Andrade, E., Mireles, A., Reyes-Solis, I. E., Garcia-Calderon, N., Lagunas-Solar, M. C., Pina, C. U., & Flocchini, R. G. (2005). Distribution of heavy metals in plants cultivated with wastewater irrigated soils during different periods of time. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 241, 351–355.
Türker, O. C., Böcük, H., & Yakar, A. (2013). The phytoremediation ability of a polyculture constructed wetland to treat boron from mine effluent. Journal of Hazardous, 252–253, 132–141.
Vymazal, J. (2005). Removal of heavy metals in a horizontal sub-surface flow constructed wetland. Journal of Environmental Science and Health, Part A Toxic/Hazardous Substances and Environmental Engineering, 40, 1369–1379.
Vymazal, J., Švehla, J., Kröpfelová, L., Němcová, J., & Suchý, V. (2010). Heavy metals in sediments from constructed wetlands treating municipal wastewater. Biogeochemistry, 101, 335–356.
Wang, X., Bai, X., Qiu, J., & Wang, B. (2005). Municipal wastewater treatment with pond-constructed wetland system: a case study. Water Science and Technology, 51, 325–329.
Weis, J. S., & Weis, P. (2004). Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environment International, 30, 685–700.
Yadav, R. K., Goyal, B., Sharma, R. K., Dubey, S. K., & Minhas, P. S. (2002). Post-irrigation impact of domestic sewage effluent on composition of soils, crops and ground water—a case study. Environment International, 28, 481–486.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morari, F., Dal Ferro, N. & Cocco, E. Municipal Wastewater Treatment with Phragmites australis L. and Typha latifolia L. for Irrigation Reuse. Boron and Heavy Metals. Water Air Soil Pollut 226, 56 (2015). https://doi.org/10.1007/s11270-015-2336-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2336-3