Abstract
Purpose
This study was undertaken to determine the feasibility of using three aquatic macrophytes, Phragmites australis, Juncus effusus and Iris pseudacorus, to phytoextract potentially toxic elements (PTEs) from a contaminated area by mining activities.
Materials and methods
An artificial pond was constructed with two topsoils (yellow and black samples) collected from Portman Bay. In order to simulate the mixing with carbonate materials, which naturally occurs in this area, a stabilisation approach was applied by mixing with 30 % of limestone filler. Three replicates of each type of soil have been prepared in pots for the selected species. The total PTEs content (arsenic, cadmium, copper, iron, lead and zinc) was determined and the bioconcentration factor (BCF) and transfer factor (TF) calculated.
Results and discussion
Soil samples showed high PTEs content as a result of mining activities. As regards the root contents, the PTEs is higher in yellow samples (YS) than in black ones, because in these samples the PTEs content that could be mobilised is higher. The BCF results were higher than unity for arsenic, copper, lead and cadmium for I. pseudacorus and P. australis growing on YS soil. Overall, copper and manganese showed a larger number of plants with BCF higher than unity. The PTEs content in leaves is low, and the TF results are lower than unity in almost all samples.
Conclusions
The results indicate that it is possible to use the selected species for phytostabilisation of soils contaminated with PTEs. J. effusus, P. australis and I. pseudacorus could be considered as tolerant, and natural or artificial wetlands containing these species could be used for remediation purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mining activity is considered as one of the most hazardous anthropogenic activities (Forghani et al. 2015), and its disruptive effects on the environment have been known for decades. Abandoned mine activities have produced huge quantities of waste-rock dumps containing low-grade ore and tailings in several regions of the world. Effluents of mine workings containing residual sulphides typically leads to acid mine drainage (AMD) (Silva et al. 2014). The release and dispersion of potentially toxic elements (PTEs) to the environment occurs through AMD, and the erosion of waste-rock dumps and tailings. Acid mine drainage can severely contaminate soils, affect water quality and pollute ecological environments because of the low pH and high concentrations of heavy metals and other toxic elements.
An important issue to be considered in soil recuperation studies is the environmental risk assessment for human health, which is a basic element for selecting reclamation techniques. However, it is less common to apply the Ecological Risk Assessment (ERA), which emerged at the beginning of the 1990s with dawning awareness of the risks liable to impact ecosystems when they are exposed to substances of anthropic origin (Alhashemi et al. 2011; Perrodin et al. 2011; Topuz et al. 2011).
The application of ERA methodology involves further study of PTEs mobility through the food chain by an in situ calculation of indicators which allows assessing the present risk (Ciszewski et al. 2013; Guo and Cutright 2014).
The importance of the role played by wetlands in the biosphere is fully recognised at the social, economic, scientific and environmental level by the Convention on Wetlands of International Importance (commonly known as the RAMSAR Convention 1971).
In wetlands, there is a close relationship between the vegetation and the soil and water conditions, being complex systems in which the exchange between soil and biota is performed in dilute media and sometimes in reducing environment, providing environments that differ from most soil surface horizons, which are usually under aerobic conditions. These environmental conditions determine the transport and absorption of nutrients by the root system of plants, causing significant physiological and morphological adaptations (Almeida et al. 2005; Caçador et al. 2013).
In addition, wetlands have an important role in the preservation of environmental quality due to their high capacity for retention and/or inactivation of harmful substances (González-Alcaraz et al. 2014).
Aquatic macrophytes are widely distributed in various wet environments, from fresh to salt water (Bonanno and Lo Giudice 2010). They have a large capacity for metal accumulation from soil and sediments through their root/rhizome system which is a good indicator of their potential use for phytoremediation purposes. Wetland plants that can survive under low pH and high concentrations of metals, such as Phragmites australis and Typha latifolia, are commonly used to remediate AMD-contaminated soil (Caldelas et al. 2012a; Guo and Cutright 2014). Phytoremediation is an effective, low cost, preferred clean-up option for moderately contaminated areas. Wetland plants generally are not “hyperaccumulator”; they store more metals in the below ground organ than in the above ground organ (Park et al. 2008; Soda et al. 2012). Phytoremediation has been successfully tested in degraded areas, acting as a green filter and providing a simple and efficient solution for contamination problems (González-Alcaraz et al. 2011; Teuchies et al. 2012; Sun et al. 2013). When phytoremediation techniques are applied in abandoned mining areas, the soil-plant transfer of PTEs has to be studied in order to thoroughly assess the environmental risk of PTEs with carcinogen effects entering the human food chain (Madejón and Lepp 2007; Anawar 2013).
The aim of this study was to study the feasibility of using three plant species frequently used in artificial wetlands, J. effusus, I. pseudacorus and P. australis, for remediation purposes. The selected species grew on soils affected by mining activities amended with limestone filler, and data on their vegetative growth, nutrient uptake and PTEs transfer were determined to select the most suitable species in terms of remediation.
2 Materials and methods
2.1 Study area
Sierra Minera is a lineation of about 25 km of low rising hills (maximum height, 431 m.a.s.l) in south-east of the province of Murcia (Spain), in the Cartagena-La Unión area. The mining district of La Unión used to be one of the most important mining sites in Spain and a perfect example of the close relationship between the Miocene magmatism, tectonics and metallogenic processes in SE Spain. Mining activities originally (from the seventh century B.C.) focused on silver and lead. Following long centuries of intermittent activity, mining resumed in the nineteenth century, with a renewed interest in zinc and iron. From the mid-twentieth century, large open pits predominated, until 1990, when mining ceased altogether (Peña et al. 2013; Martínez-Sánchez et al. 2014).
From 1957 to 1991, the materials mined were transported to “Lavadero Roberto”, the benefiting plant located in Portman Bay, which used seawater for flotation processes. Sterile materials were dumped directly into the bay, which was flooded by sediments, resulting in the coastline retracting several hundred metres (Martínez-Sánchez and Pérez-Sirvent 2008). In this treatment plant, the mineral was crushed and passed through flotation circuits for the differential separation of sulphides (galena, sphalerite and pyrite). Once the ores had been separated, the waste materials were discharged directly into the sea, originally in the inner part of the bay; later on, wastes were discharged a little further offshore. Up to 1967, magnetite was also recovered, but this practice ceased, in order to prioritise the recovery of sulphides, reducing magnetite to a residue. In addition, the wastes showed a high content of trace elements and an important proportion of iron oxides including magnetite, goethite and hematite. As a result of dumping, the whole bay has filled up with wastes, which also extended into the Mediterranean Sea (García-Lorenzo et al. 2014; Pérez-Sirvent et al. 2016).
In the sea, coastal dynamics transported fine particles offshore while the sand fraction remained to fill up the bay. Finally, wastes were also discharged directly over the bay surface. These wastes showed a high content of fine particulates and are affected by weathering processes. However, materials in the deepest experienced reducing conditions. In summary, the bay contains largely unaltered mine wastes, while a small proportion of the infilling materials have experienced weathering.
In 2007, a recuperation pilot project (Martínez-Sánchez et al. 2013; González-Ciudad 2014; Pérez-Sirvent et al. 2014) was developed and financed by the Spanish Government. In the first step of this project, the complete physical, chemical and mineralogical characterisation of topsoils, both in surface and at depth, was carried out. In this project, the construction of wetlands to mitigate the effect of acid mine drainage in restored areas as a result of torrential rains is planned (Navarro et al. 2008; Navarro-Hervás et al. 2012).
2.2 Experimental design
The experiments were conducted using two topsoils collected from the most recent exploitation stage in Portman Bay: a black sandy sample (BS) and a yellow fine texture sample (YS). Three samples of each soil were collected.
Two areas are present in Portman Bay: a wetland with vegetation, where the two types of soils (black sand and yellow sand) are intercalated. This area receives carbonate materials from surrounding areas and is mainly colonised by P. australis and J. effusus. The other area is also flooded at different times of the year but does not present vegetation. This area receives AMD and constitutes a contamination focus with high environmental risk.
Topsoils selected for the experience correspond to the most extreme situation that can be found in the bay. The YS sample is the result of the direct discharge of waste material in the beach. These materials have been affected by weathering processes for 25 years, being the pyrite oxidised and secondary minerals, such as jarosite, akaganeite and gypsum, were formed. The YS is also characterised by acid pH and high PTEs contents (Table 1 and Table 2). This material showed a fine texture because minerals were finely grounded in order to float the ore, which was separated from the rest (such as silicates, carbonates or oxides). The pyrite was also rejected and discharged with the steriles, reaching sometimes concentrations ranging from 3 to 15 %. These materials occupy a large space in the bay and could appear covered with other materials, black sands, also considered in this work.
The BS sample has a sandy texture because of grain sorting by sea currents, concentrating the denser and larger minerals and forming a mixture of unreactive minerals, such as magnetite, hematite, goethite, siderite, mica or pyrite, with a coarse texture. These materials constitute the beach sand, are stable and are not affected by weathering processes. Due to the action of waves and wind, they appear in the bay with different thicknesses.
Previous studies (Pérez-Sirvent et al. 1998, 2016; García-Lorenzo et al. 2014) showed that no phases of lead, arsenic or cadmium are present, being these elements included in the crystal lattice of identified minerals. Then, the mobility of these metals depends on the solubility of the host minerals.
A control soil was also collected and corresponds to a cultivated area close to the studied zone. Is a Luvic Calcisol with PTEs content slightly higher than the geogenic values established in the area (Martínez-Sánchez and Pérez-Sirvent 2008; Pérez-Sirvent et al. 2009)? This soil is affected by tertiary contamination (Lottermoser 2007; García-Lorenzo et al. 2012, 2014), it is mixed with mining soils and carried by rain (Navarro et al. 2008). The mineralogical composition of this sample (Table 2) showed that clay minerals, quartz and calcite are the main minerals, together with hematite.
In order to simulate the mixing with carbonate materials, which naturally occurs in the study area (García-Lorenzo et al. 2012), a stabilisation approach was applied by mixing the topsoil YS with 30 % of limestone filler. The limestone filler is a residue of crushing of limestone from a stone quarry. The limestone filler was characterised by a basic pH and the PTEs content was below the detection limit, except for iron. The X-ray diffraction analysis showed that it is mainly composed of calcite (86 %) (Pérez-Sirvent et al. 2007, 2011a, 2011b; Martínez-Sánchez et al. 2014).
Three plant species, which usually grow in the study area, has been selected for this study: I. pseudacorus, J. effusus and P. australis.
Iris pseudacorus (Linneo) usually grows in wet conditions and is commonly found in marshes, tolerating immersion, low pH and anoxic soil conditions. This species spreads rapidly through rhizomes and seeds dispersed by water. Although it is an aquatic plant, rhizomes could survive extended dry periods. I. pseudacorus has been used in the remediation of contaminated waters, mainly in marshy areas (Barbolani et al. 1986; Lee and Kim 2011), because of its ability to absorb PTEs through their roots.
Juncus effusus (Linneo) (soft rush or common rush) is an ecologically important plant, common in fresh and brackish water habitats, and is widely used in water gardens, contamination mitigation and constructed wetlands. It serves several purposes in wetlands, including metal accumulation, wastewater treatment, food and cover, nitrification-denitrification, stimulation of microbial activity and biological processing (Almeida et al. 2004, 2011; Bhatia and Goyal 2014).
P. australis (Adanson) (common reed) is one of the most distributed macrophytes in aquatic ecosystems, and numerous studies showed its capacity of trace elements bioaccumulation (i.e. Bragato et al. 2009; Maddison et al. 2009; Bonanno 2014; Conesa et al. 2014). P. australis is a large perennial grass living in lakes and rivers or brackish wetlands, such as marshes, across temperate and tropical regions all over the world and has colonised many coastal salt marshes. It belongs to the Poaceae family and is the most common species of the Phragmites genus. This species prefers eutrophic and stagnating waters, and tolerates a moderate salinity (Cooper et al. 1996; Kumari and Tripathi 2015). It is a rhizomatous hemicryptophyte/geophyte and forms wide stands known as reed beds that provide microhabitats for many birds and mammals. In addition, it can accumulate several metals (Rocha et al. 2014).
An artificial pond was constructed and filled with layers of BS and YS intercalated as follows: 40 cm of BS, 30 cm of YS stabilised with 30 % of limestone filler and 10 cm of BS in the surface. After filling the pond with water, it was left in contact with the soil for a month and, after this time, water samples were collected (Table 1).
Three replicates of each type of soil were prepared in pots for the three selected species. In addition, three replicates with the control soil, without mining influence, were prepared for each species. The experiment was developed by immersing the plant pots in 20 cm of water in the pond. After 1 year, samples of the rhizosphere soil, roots and leaves were collected in each pot, obtaining a total of 27 samples.
2.3 Analytical determinations
Soil samples were air-dried and sieved through a 2-mm screen for general analytical determinations. The pH and EC were determined in a 1:5 (m/V) suspension of soil in pure, deionised water (Milli-Q; resistivity ≥18 MΩ cm).
To determine the total PTEs content, the soil samples were first ground to a fine powder using a zirconium ball mill. Aliquots (0.1 g) of soil samples were placed in Teflon vessels, and a mixture of 5 ml concentrated HF (37 %), 200 μl concentrated HNO3 (65 %) and 5 ml water was added. When digestion in the microwave system was complete, the samples were transferred to a volumetric flask and brought to 50 ml before measurement. Teflon or other suitable plastic ware was used for handling these liquids.
Fresh plant materials were separated into root and above-ground part, carefully washed with fresh water, cleaned using an ultrasonic bath to remove dust contamination and finally rinsed with deionised water, air-dried and then lyophilized. Then, 200 mg of lyophilized vegetal tissue was placed in Teflon vessels with 3 ml water, 2 ml concentrated H2O2 and 5 ml concentrated HNO3 acid solution, and subjected to digestion in the microwave oven, finally obtaining 50 ml solutions, which were analysed.
The soils and plants samples were digested using a Milestone ETHOS Plus microwave system operating with a standard programme (applied power in watts 150, 0, 150, 0, 150, 0, 350, 400, 0, 450 and 0 for 1, 1, 1, 1, 2, 1, 5, 5, 1, 1 and 20 min, respectively).
The reliability of the results was assessed through analysis of the NIST standard reference materials: SRM 2711 Montana Soil and SRM 1515 Apple leaves. Spikes, duplicates and reagent blanks were also used as a part of the quality control.
Zinc and Fe content in soils were determined by flame atomic absorption spectrometry (FAAS) using a Perkin-Elmer 1100B Atomic Absorption Spectrophotometer. Lead, cadmium and copper contents were determined by electrothermal atomization atomic absorption spectrometry (ETAAS) using a high-resolution continuum source ContrAA spectrometer from Analytik Jena AG. Arsenic was measured by atomic fluorescence spectrometry using an automated continuous flow hydride generation spectrometer (PSA Millenium Merlin 10055).
The limit of quantification for the selected elements was 0.3 μg kg−1 for arsenic, 10 μg kg−1 for lead, 0.5 μg kg−1 for copper, 0.6 μg kg−1 for cadmium, 50 μg kg−1 for zinc and manganese and 100 μg kg−1 for iron.
A semiquantitative estimation of the mineralogical composition of the solid samples was made by powder X-ray diffraction (XRD) analysis using Cu-Kα radiation with a PW3040 Philips Diffractometer. An X-powder software was used to analyse diffractograms. The powder diffraction file (PDF2) database was used for peak identification.
After the pond filling, the PTEs content and the pH and EC of the water were determined in the same way as in the soil samples.
With the soil, root and shoot tissue concentrations of PTEs, the bioconcentration factor (BCF) and the transfer factors were calculated. The BCF is defined as the ratio between the concentration of the elements in the root and that present in the soil while the transfer factor (TF) is defined as the ratio between the concentration of the element in leaves and in roots.
2.4 Data treatment
The statistical analysis has been applied in order to clarify the influence of soil type in the PTEs behaviour for the three species and the influence of each plant species in the PTEs behaviour. Two multivariate techniques have been carried out: a general linear model (GLM) and a multiple linear regression.
A factorial ANOVA was applied using the GLM univariate. GLM is an ANOVA procedure in which the calculations are performed using a least squares regression approach to describe the statistical relationship between one or more predictors and a continuous response variable.
To find which of the selected elements has more influence on the rest, a multiple linear regression was applied, where predictor variables were introduced to study its influence on the dependent variable. All statistical calculations were made using SPSS version 20 software.
3 Results and discussion
3.1 Rhizosphere soil and plant results
Table 3 summarises the average values obtained for the PTEs in the rhizosphere soils, roots and shoots of the studied plants. In relation to the rhizosphere soil samples, differences were observed for the different plants grown in the same medium. The differences, that were particularly noticeable for arsenic, lead and iron, could be attributed to the sampling stage, but several authors (Weiss et al. 2004; Yang et al. 2010) have reported that plants may modify the rhizosphere by concentrating elements that are retained by the roots, or facilitating the formation of iron oxide plaques that immobilise and concentrate PTEs. The effect increased in the order P. australis > J. effusus > I. pseudacorus. This fact could be related to the morphological and physiological characteristics of each species that, as reported elsewhere (Whalley et al. 2005; Hinsinger et al. 2009), result in the formation of soil aggregates with different physicochemical properties despite the plants are growing in the same soil. In the case here studied, the differences were more evident for the control soil and the fine texture soil (YS) and for iron and arsenic, which is attributed to the reduced mobility of iron at a pH above 5, and the affinity between Fe (III) and arsenic (Yang et al. 2016). The levels of PTEs found in roots also showed this behaviour, the highest content of arsenic being found for P. australis.
Zinc and cadmium contents in root samples were higher in plants growing in YS for J. effusus and P. australis. No differences were noticed among the selected species or among the soil types. On the contrary, the maximum lead content for the three media were found for I. pseudacorus and the level was higher in the roots of the plants grown in YS. I. pseudacorus developed in YS showed the highest content in roots (132 mg kg−1) while the other species showed similar values for both soil types.
Even if no significant differences were found in the copper concentrations of the plant roots developed in YS, they are slightly higher than in the plants growing in BS soil. For manganese, a similar behaviour was observed, YS being the soil in which more element was incorporated in the root. In addition, the highest concentration effect was found for the case of P. australis.
For shoot samples, the arsenic level was low in all cases, indicating that the species studied limit the mobility of this element from roots to shoots. Only J. effusus growing in YS soil showed an average content of 21 mg kg−1 while the other plants showed concentration lower than 2 mg kg−1 (Table 3). Iron, lead and copper showed a similar behaviour, the highest contents being found in samples growing in YS soil, with no differences among species. When cadmium concentration was evaluated in shoots, the highest values were found in P. australis, both growing in YS and BS soils, while the lowest concentration was determined in I. pseudacorus. No significant differences were noticed among species and soil type.
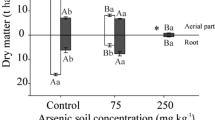
Phytostabilisation is a process which depends on roots ability to limit the contaminant mobility and bioavailability in the soils. Species tolerant to potentially toxic elements showing high BCF and low TF can be used for phytostabilisation of contaminated soils. Then, the elevated concentration of PTEs in roots and low translocation to the above-ground parts of the plants indicated their suitability for phytostabilisation. In order to study the possible accumulation of PTEs, the BCF and TF were calculated, and the results obtained for arsenic, manganese and iron, that are summarised in Fig. 1, showed that for arsenic, both I. pseudacorus and P. australis growing in YS had BCF > 1. On the other hand, the TF value was lower than unity in all species. No differences in BCF for manganese were observed among the plants studied.
The results for other elements are given in Fig. 2. The BCF and TF values for zinc were in the 0.1–0.9 range, so that the plant species could be classified as tolerant for this metal. The results obtained for lead indicated that this element was not translocated to the aerial part of the plants. The BCF values for copper and cadmium were above the unity for the three species growing in YS but conclusions as regards hyperaccumulation are not reliable due to the low content of these elements in the rhizosphere soil.
In general, the PTEs concentrations both for roots and leaves are slightly higher than those reported in other studies for the selected plant species (Almeida et al. 2006; Zhang et al. 2007; Caldelas et al. 2012b; Ahmad et al. 2014) but they do not reach the minimum values to be considered as phytoextractor plants. Therefore, the selected species could be considered as tolerant and used as phytostabilisers.
3.2 Statistical analysis
The obtained results of the GLM univariate (Table 4) for the target elements determined in roots using as fixed factor the soil sample showed that for all elements, the p value was lower than 0.05. Based on this analysis, our data support the conclusion that the selected soil affects the PTEs content determined in roots. As regards the mean values, copper and cadmium are higher in plants growing in YS and the lowest value was found in the control soil. For iron, zinc, arsenic and lead, the highest value was also found in the YS substrate, while the lowest content was found in plants growing in BS. However, for manganese, the highest value was found for the control soil and the lowest in the BS. The applied treatment explains the high values in the variance of arsenic, lead, iron and manganese (maximum of 92 %), the intermediate variance values for copper and cadmium and low values of zinc (Table 4).
Applying the same procedure but taking into account the PTEs content in leaves, the obtained p values were also lower than the significance level (0.05). Similarly to roots, the PTEs content in shoots depends on the soil type. A different situation was found in iron content. For this element, the p value was 0.166. Based on this analysis, our data do not support the conclusion that Fe content differ significantly by soil sample.
As regards the mean values, the highest values for copper, iron, arsenic and cadmium were found in leaves of plants growing on YS, while the lowest were determined in plants growing on the control soil. The percentage of variance explained is low in all cases.
The obtained results confirmed that the soil type (BS or YS) could affect in the PTEs content, both in roots and leaves. However, the transfer from root to leaves is not important.
When the influence of soil type in the PTEs content in the rhizosphere soil is evaluated, lead, arsenic, zinc and manganese contents depend on the soil sample, while cadmium, copper and iron showed no dependence.
An ANOVA procedure was also used to determine whether the average PTEs content in roots and leaves differed between the plants species. In the output, summarised in Table 4, the obtained p values were lower than 0.05, suggesting that the PTEs content in roots depends on the plant species. However, for arsenic and iron, the obtained p values are higher than the significance level and then, we can affirm that PTEs content in roots does not depend on the plant type. The mean values obtained in different plants vary widely, and the percentage of variance explained is moderate, ranging from 15 to 40 % in all cases.
The results obtained applying the same procedure but taking into account the PTEs content in leaves showed that copper, iron, lead and manganese are independent on the plant species, while arsenic, zinc and cadmium showed p values >0.05 and then their content depends on the selected plant species. Arsenic and cadmium, typically present in metalophytes, showed higher average values in J. effusus, while in I. pseudacorus the lowest were found.
The plant substrate influences the PTEs content in plants, conditioning the concentration from rhizosphere soil to roots and the shoot content. Only cadmium does not present significant variations in the rhizosphere soil. As regards the plant species, only copper and manganese showed significant variations in the rhizosphere soil.
In addition to the ANOVA procedure, a multiple linear regression was applied in order to establish the possible relationship between PTEs in the rhizosphere soil, root and leaf samples. In the rhizosphere soil, a low set of predictors was obtained regarding the analysed variables, with percentage of the variance explained higher than 65 % except for lead, with a 2 %. The p value was lower than the significance level and positive in all the variables and predictors, except for lead/manganese, where this p value was negative. The main predictor for copper and iron is arsenic, and zinc in the case of cadmium and manganese.
Similarly, in roots, the results showed a low set of predictors, except for copper. The percentages of variance explained are dissimilar. Positive and lower than 0.05 p values are found except for the pairs copper-zinc, arsenic-zinc/copper and cadmium-manganese. In leaves, the predictors are even lower than those calculated for roots and rhizosphere soil, except for lead, which present three predictors, explaining the 64 % of the variance. For iron and manganese, no predictive relationships with any PTEs were found, so we have to assume that under these experimental conditions these metals are independent from the rest in terms of its concentration in leaves. The relationship between the dependent variable and each predictor (p ≤ 0.000) was positive and statistically significant, except for lead/copper and cadmium/lead, which showed negative values.
4 Conclusions
The results indicate that it is feasible to use the selected species for phytostabilisation of soils contaminated with PTEs. J. effusus, P. australis and I. pseudacorus could be considered as tolerant, and natural or artificial wetlands containing these species could be used for remediation purposes. Taking into account that these species showed high BCF and low TF, they can be used for phytostabilisation of contaminated soils. Since the transfer to the aerial parts of the plants is so scant, the risk of incorporation to the trophic chain is very low.
References
Ahmad SS, Reshi ZA, Shah MA, Rashid I, Ara R, Andrabi SMA (2014) Phytoremediation potential of Phragmites australis in Hokersar wetland: a RAMSAR site of Kashmir Himalaya. Int J Phytoremediation 16:1183–1191
Alhashemi ASH, Karbassi AR, Kiabi BH, Monavari SM, Nabavi SMB, Sekhavatjou MS (2011) Bioaccumulation of trace elements in trophic levels of wetland plants and waterfowl birds. Biol Trace Elem Res 142:500–516
Almeida CMR, Mucha AP, Vasconcelos M (2004) Influence of the sea rush Juncus maritimus on metal concentration and speciation in estuarine sediment colonized by the plant. Environ Sci Technol 38:3112–3118
Almeida CMR, Mucha AP, Vasconcelos M (2005) The role of a salt marsh plant on trace metal bioavailability in sediments—estimation by different chemical approaches. Environ Sci Pollut R 12:271–277
Almeida CMR, Mucha AP, Vasconcelos M (2006) Variability of metal contents in the sea rush Juncus maritimus—estuarine sediment system through one year of plant’s life. Mar Environ Res 61:424–438
Almeida CMR, Mucha AP, Vasconcelos MT (2011) Role of different salt marsh plants on metal retention in an urban estuary (Lima estuary, NW Portugal). Estuar Coastal Shelf S 91:243–249
Anawar HM (2013) Impact of climate change on acid mine drainage generation and contaminant transport in water ecosystems of semi-arid and arid mining areas. Phys Chem Earth 58-60:13–21
Barbolani E, Clauser M, Pantani F, Gellini R (1986) Residual heavy metal (Cu and Cd) removal by Iris pseudacorus. Water Air Soil Pollut 28:277–282
Bhatia M, Goyal D (2014) Analyzing remediation potential of wastewater through wetland plants: a review. Environ Prog Sustain Energy 33:9–27
Bonanno G, Lo Giudice R (2010) Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol Indic 10:639–645
Bonanno G (2014) Trace element accumulation and distribution in the organs of Phragmites australis (common reed) and biomonitoring applications. Ecotox Environ Safe 74:1057–1064
Bragato C, Schiavon M, Polese R, Ertani A, Pittarello M, Malagoli M (2009) Seasonal variations of Cu, Zn, Ni and Cr concentration in Phragmites australis (Cav.) Trin. ex Steud. in a constructed wetland of North Italy. Desalination 246:35–44
Caçador I, Neto JM, Duarte B, Barroso DV, Pinto M, Marques JC (2013) Development of an angiosperm quality assessment index (AQuA-index) for ecological quality evaluation of Portuguese water bodies—a multimetric approach. Ecol Indic 25:141–148
Caldelas C, Araus JL, Febrero A, Bort J (2012a) Accumulation and toxic effects of chromium and zinc in Iris pseudacorus L. Acta Physiol Plant 34:1217–1228
Caldelas C, Bort J, Febrero A (2012b) Ultrastructure and subcellular distribution of Cr in Iris pseudacorus L. using TEM and X-ray microanalysis. Cell Biol Toxicol 28:57–68
Ciszewski D, Aleksander-Kwaterczak U, Pociecha A, Szarek-Gwiazda E, Waloszek A, Wilk-Wozniak E (2013) Small effects of a large sediment contamination with heavy metals on aquatic organisms in the vicinity of an abandoned lead and zinc mine. Environ Monit Assess 185:9825–9842
Conesa HM, Maria-Cervantes A, Álvarez-Rogel J, González-Alcaraz MN (2014) Role of rhizosphere and soil properties for the phytomanagement of a salt marsh polluted by mining wastes. Int J Environ Sci Tec 11:1353–1364
Cooper PF, Job GD, Green MB, Shutes RBE (1996) Reed beds and constructed wetlands for wastewater treatment. WRc Publications, Medmenham, Marlow
Forghani G, Mokhtari AR, Kazemi GA, Fard MD (2015) Total concentration, speciation and mobility of potentially toxic elements in soils around a mining area in central Iran. Chem Erde 75(3):323–334
García-Lorenzo ML, Martínez-Sánchez MJ, Pérez-Sirvent C (2014) Application of a plant bioassay for the evaluation of ecotoxicological risks of heavy metals in sediments affected by mining activities. J Soils Sediments 14:1753–1765
García-Lorenzo ML, Pérez-Sirvent C, Martínez-Sánchez MJ, Molina-Ruiz J (2012) Trace elements contamination in an abandoned mining site in a semiarid zone. J Geochem Explor 113:23–35
González-Alcaraz MN, Jiménez-Cárceles FJ, Álvarez Y, Álvarez-Rogel J (2014) Gradients of soil salinity and moisture, and plant distribution, in a Mediterranean semiarid saline watershed: a model of soil-plant relationships for contributing to the management. Catena 115:150–158
González-Alcaraz MN, Conesa HM, Tercero MC, Schulin R, Álvarez-Rogel J, Egea C (2011) The combined use of liming and Sarcocornia fruticosa development for phytomanagement of salt marsh soils polluted by mine wastes. J Hazard Mater 186:805–813
González-Ciudad E (2014) Evaluación en nave cerrada de los riesgos para la salud en tecnosoles procedentes de residuos de minería polimetálica. Doctoral thesis, University of Murcia
Guo L, Cutright TJ (2014) Remediation of acid mine drainage (AMD)-contaminated soil by Phragmites australis and rhizosphere bacteria. Environ Sci Pollut Res 21:7350–7360
Hinsinger P, Glyn Bengough A, Vetterlein D, Young IM (2009) Rhizosphere: biophysiscs, biogeochemistry and ecological relevance. Plant Soil 321:117–152
Kumari M, Tripathi BD (2015) Efficiency of Phragmites australis and Typha latifolia for heavy metal removal from wastewater. Ecotox Environ Safe 112:80–86
Lee SC, Kim WS (2011) Cadmium accumulation and tolerance of Iris pseudacorus and Acorus calamus as aquatic plants native to Korea. Korean J Hortic Sci 29:413–419
Lottermoser B (2007) Mine wastes-characterization, treatment and environmental impacts. Springer, Berlin
Madejón P, Lepp NW (2007) Arsenic in soils and plants of woodland regenerated on an arsenic-contaminated substrate: a sustainable natural remediation. Sci Total Environ 379(2):256–262
Maddison M, Soosaar K, Mauring T, Mander U (2009) The biomass and nutrient and heavy metal content of cattails and reeds in wastewater treatment wetlands for the production of construction material in Estonia. Desalination 246:120–128
Martínez-Sánchez MJ, García-Lorenzo ML, Pérez-Sirvent C, González E, Pérez V, Martínez S, Martínez L, Molina J (2014) Heavy metal immobilisation by limestone filler in soils contaminated by mining activities: effects on metal leaching and ecotoxicity. Int J Min Reclam Environ 28(6):414–425
Martínez-Sánchez MJ, Martínez-López S, Martínez-Martínez LB, Pérez-Sirvent C (2013) Importance of the oral arsenic bioaccessibility factor for characterising the risk associated with soil ingestion in a mining-influenced zone. J Environ Manag 116:10–17
Martínez-Sánchez MJ, Navarro MC, Pérez-Sirvent C, Marimon J, Vidal J, García-Lorenzo ML, Bech J (2008) Assessment of the mobility of metals in a mining-impacted coastal area (Spain, Western Mediterranean). J Geochem Explor 96:171–182
Martínez-Sánchez MJ, Pérez-Sirvent C (2008) Niveles defondo y niveles genéricos de referencia de metales pesadosen suelos de la Región de Murcia. Consejería de Desarrollo sostenible y Ordenación del territorio, Murcia
Navarro-Hervás C, Pérez-Sirvent C, Martínez-Sánchez ML, García-LorenzoML MJ (2012) Weathering processes in waste materials from a mining area in a semiarid zone. Appl Geochem 27:1991–2000
Navarro MC, Pérez-Sirvent C, Martínez-Sánchez MJ, Vidal J, Tovar PJ, Bech J (2008) Abandoned mine sites as a source of contamination by heavy metals: a case study in a semi-arid zone. J Geochem Explor 96:183–193
Park EJ, Kim DS, Park K (2008) Monitoring of ambient particles and heavy metals in a residential area of Seoul, Korea. Environ Monit Assess 137:441–449
Peña JA, Manteca JI, Martínez-Pagán P, Teixidó T (2013) Magnetic gradient map of the mine tailings in Portman Bay (Murcia, Spain) and its contribution to the understanding of the bay infilling process. J Appl Geophys 95:115–120
Pérez-Sirvent C, Hernández-Pérez C, Martínez-Sánchez MJ, García-Lorenzo ML, Bech J (2016) Geochemical characterisation of surface waters, topsoils and efflorescences in a historic metal-mining area in Spain. J Soils Sediments 16:1238–1252
Pérez-Sirvent C, Martínez-Sánchez MJ, García-Lorenzo ML, Hernández-Córdoba M, Molina J, Martínez S, González E, Pérez-Espinosa V (2014) A preliminary zonation to support the remediation and the risk assessment of an area contaminated by potentially toxic elements in Murcia Region (SE, Spain). Procedia Earth Plan Sci 10:388–391
Pérez-Sirvent C, García-Lorenzo ML, Martínez-Sánchez MJ, Molina-Ruiz J, Marimon J, Navarro MC (2011a) Use of marble cutting sludges for remediating soils and sediments contaminated by heavy metals. Environ Prog Sustainable Eng 30:533–539
Pérez-Sirvent C, Martínez-Sánchez MJ, Martínez-López S, Hernández-Córdoba M (2011b) Antimony distribution in soils and plants near an abandoned mining site. Microchem J 97:52–56
Pérez-Sirvent C, Martínez-Sánchez MJ, García-Lorenzo ML, Molina J, Tudela ML (2009) Geochemical background levels of zinc, cadmium and mercury in anthropically influenced soils located in a semi-arid zone (SE, Spain). Geoderma 148:307–317
Pérez-Sirvent C, García-Lorenzo ML, Martínez-Sánchez MJ, Navarro MC, Marimon J, Bech J (2007) Metal-contaminated soil remediation by using sludges of the marble industry: toxicological evaluation. Environ Int 33:502–504
Pérez-Sirvent C, Martínez-Sánchez J, García-Rizo C (1998) Lead mobilization in calcareous agricultural soils. In: Iskandar IK, Magdi Selim H (eds) Fate and transport of heavy metals in the vadose zone. CRC Press, Boca Raton, pp. 177–199
Perrodin Y, Boillot C, Angerville R, Donguy G, Evens E (2011) Review ecological risk assessment of urban and industrial systems: a review. Sci Total Environ 409:5162–5176
Rocha AC, Almedia CMR, Basto MCP, Vasconcelos MTSD (2014) Antioxidant response of Phragmites australis to Cu and Cd contamination. Ecotox Environ Safe 109:152–160
Silva MMVG, Lopes SP, Gomes EC (2014) Geochemistry and behavior of REE in stream sediments close to an old Sn-W mine, Ribeira, Northeast Portugal. Chem Erde 74:545–555
Soda S, Hamada T, Yamaoka Y, Ike M, Nakazato H, Saeki Y, Kasamatsu T, Sakurai Y (2012) Constructed wetlands for advanced treatment of wastewater with a complex matrix from a metal-processing plant: bioconcentration and translocation factors of various metals in Acorus gramineus and Cyperus alternifolius. Ecol Eng 39:63–70
Sun H, Wang Z, Gao P, Liu P (2013) Selection of aquatic plants for phytoremediation of heavy metal in electroplate wastewater. Acta Physiol Plant 35:355–364
Teuchies J, Beauchard O, Jacobs S, Meire P (2012) Evolution of sediment metal concentrations in a tidal marsh restoration project. Sci Total Environ 419:187–195
Topuz E, Talinli I, Aydin E (2011) Integration of environmental and human health risk assessment for industries using hazardous materials: a quantitative multi criteria approach for environmental decision makers. Environ Int 37:393–403
Whalley WR, Riseley B, Leeds-Harrison PB, Paul Adderley W (2005) Structural differences between bulk and rhizosphere soil. Eur J Soil Sci 56(3):353–360
Weiss JV, Emerson D, Megonigal JP (2004) Geochemical control of microbial Fe (III) reduction potential in wetlands: comparison of the rhizosphere to non-rhizosphere soil. FEMS Microbiol Ecol 48:89–100
Yang J, Ma Z, Ye Z, Guo X, Qiu R (2010) Heavy metal (Pb, Zn) uptake and chemical changes in rhizosphere soils of four wetland plants with different radial oxygen loss. J Environ Sci 22(5):696–702
Yang JX, Guo QJ, Yang J, Zhou XY, Ren HY, Zhang HZ, Xu RX, Wang XD, Peters M, Zhu GX, Wei RF, Tian LY, Han XK (2016) Red mud (RM)-induced enhancement of iron plaque formation reduces arsenic and metal accumulation in two wetland plant species. Int J Phytoremediation 18(3):269–277
Zhang XB, Liu P, Yang YS, Chen WR (2007) Phytoremediation of urban wastewater by model wetlands with ornamental hydrophytes. J Environ Sci (China) 19:902–909
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Maria Manuela Abreu
Rights and permissions
About this article
Cite this article
Pérez-Sirvent, C., Hernández-Pérez, C., Martínez-Sánchez, M.J. et al. Metal uptake by wetland plants: implications for phytoremediation and restoration. J Soils Sediments 17, 1384–1393 (2017). https://doi.org/10.1007/s11368-016-1520-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1520-4