Abstract
Constructed wetlands are commonly used for treatment of municipal sewage. The treatment is usually aimed at removal of organics, suspended solids, nutrients and microbial pollution. The information on removal and fate of heavy metals is very limited. The purpose of this study was to evaluate the amount of sediments and heavy metal concentration in the sediments in filtration beds of seven constructed wetlands with horizontal subsurface flow treating municipal sewage with various length of operation. The results revealed that concentrations of Cd, Ni, Pb, Cu, Cr and Zn in the sediment are mostly comparable with concentrations occurred in natural unpolluted or slightly polluted wetlands. The concentrations are much lower than those found in wetlands impacted with mine drainage waters or wastewater from industrial operations. Concentrations of studied heavy metals exceeded only occasionally limits set by the Czech legislation. However, when heavy metal concentrations are evaluated within the filtration material the concentrations are well below the limits set for soils in the Czech Republic. The results also revealed that concentrations of heavy metals in the sediment do not reflect the time of operation probably due to build-up of sediments from suspended solids contained in wastewaters. However, the sediment mass increases during the course of operation and consequently the metal mass increases as well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During last two decades the constructed wetlands with horizontal subsurface flow (HF CWs) have increasingly been used in the Czech Republic to treat municipal wastewater (Vymazal 1995, 1996, 2002, 2009; Vymazal and Kröpfelová 2005). The first constructed wetland was put in operation in 1989 and at present, there are about 300 systems in operation. Trace elements are usually not the target of the treatment of municipal wastewater but their concentrations in the sediments within the filtration bed may be the concern when the filtration bed would need to be excavated and disposed. So far only few investigations have been aimed at the heavy metals concentrations in the filtration beds of constructed wetlands with subsurface flow treating sewage (e.g. Obarska-Pempkowiak and Klimkowska 1999; Obarska-Pempkowiak 2001; Vymazal 2003; Vymazal and Krása 2003; Lesage et al. 2007a, b).
Redox potential and pH of the sediment–water system are the major factors known to influence the mobility of trace elements in wetlands (DeLaune et al. 1998; Koretsky et al. 2008). However, in most municipal sewage the pH is around neutral and, therefore, this parameter does not affect mobility and retention of heavy metals in constructed wetland too much. Particularly in wetlands, oxidation and reduction reactions are of prime importance (Du Laing et al. 2008). Under aerobic conditions, the most important process affecting accumulation of heavy metals is precipitation of Fe/Mn hydrous oxides (Singer and Stumm 1970). The most important processes affecting heavy metals accumulation/mobilization under anoxic and anaerobic conditions are creation of hydrogen sulfide via sulfate reduction and dissolution of Fe/Mn hydrous oxides (Khalid et al. 1978; Green et al. 2003; De Volder et al. 2003; Mansfeldt 2004).
Sedimentation has long been recognized as the principle process in removal of heavy metals from wastewater in constructed wetlands. However, it is not a simple straightforward physical reaction and other chemical processes such as precipitation and co-precipitation have to occur first (Yao and Gao 2007). Iron, manganese and also aluminum can form under aerobic conditions insoluble compounds through hydrolysis and/or oxidation. This leads to formation of variety of oxides, oxyhydroxides and hydroxides (Wieder 1989; Batty et al. 2002; Woulds and Ngwenya 2004; Sheoran and Sheoran 2006). Once associated with the particulate phase, these elements become subject to removal from the water via sedimentation. The stability of these inorganic compounds is controlled primarily by the system pH, the solubility of the product, and concentrations of the metals and relevant anions (Gambrell 1994; Sheoran and Sheoran 2006). At near-neutral to slightly alkaline pH levels, metals tend to be effectively immobilized (Gambrell 1994). Co-precipitation is an adsorptive phenomenon in wetland sediments. The concentration and distribution of many elements, such as Ni, Cu, Zn or Cd, in sediments and overlying waters are strongly influenced by adsorption and/or co-precipitation with Fe and Mn oxides (Krauskopf 1956; Jenne 1968; Feely et al. 1983; Ferris et al. 1989). Copper, nickel, zinc and manganese are co-precipitated in Fe oxides and cobalt, iron, nickel and zinc are co-precipitated in manganese oxides (Stumm and Morgan 1981). In addition, zinc is reported to be retained on iron plaques at the surface of plant roots (Otte et al. 1995). However, in filtration beds of HF CWs anoxic/anaerobic conditions prevail (e.g., Dušek et al. 2008) and therefore precipitation of Fe/Mn compounds is not the major retention mechanism as Mn and Fe precipitates dissolute under these conditions (Laanbroek 1990; Jacobson 1994; Lovley 1995; Green et al. 2003; Cooper et al. 2006).
Under reducing conditions, dissimilatory sulfate reduction transforms SO4 2− to H2S during respiration by several genera of strictly anaerobic bacteria by reaction with a variety of organic substrates (Gambrell and Patrick 1978; Laanbroek and Veldkamp 1982; Mandernack et al. 2000; Megonikal et al. 2004). Most of the heavy metals react with hydrogen sulfide to form highly insoluble metal sulfides (Krauskopf 1956; Stumm and Morgan 1981; Kosolapov et al. 2004):
where M2+ represents a divalent metal ion such as Fe2+ (pyrite, FeS2; pyrrhotite, FeS), Pb2+ (galena, PbS), Cd2+ (CdS), Cu2+ (covellite, CuS; chalcocite, CuS2; chalcopyrite, CuFeS2), Ni2+ (NiS) or Zn2+ (sphalerite, ZnS). These compounds are very stable and insoluble under anaerobic conditions. However, under oxidized conditions sulfides dissolute and release metals. This may occur, for example, as a consequence of oxygen release from plant roots in the rhizosphere (Engler and Patrick 1975; Gambrell et al. 1980; Jacob and Otte 2003).
Heavy metals may also form carbonates when the bicarbonate concentration in water is high. Although carbonates are less stable than sulfides, they can still perform a significant role in initial trapping of metals (Ramos et al. 1994; Sobolewski 1996; Sheoran and Sheoran 2006; Du Laing et al. 2008). Carbonate precipitation is especially effective for the accumulation of lead and nickel in wetlands (Lin 1995).
Metal complexes with large molecular weight organics tend to be effectively immobilized. There is some evidence that at least some metals are more tightly bound by organics under anoxic or reducing conditions compared with upland conditions because humic material may become structurally less complex under oxic conditions (Gambrell and Patrick 1978; Gambrell et al. 1980; Guo et al. 1997). However, complex formation with soluble and insoluble organic matter under all conditions of pH and oxidation intensity occurs (Verloo and Cottenie 1972).
The purpose of this study was to evaluate the amount of sediments and heavy metal concentration in the sediments in filtration beds of constructed wetlands with horizontal subsurface flow treating municipal sewage with various time of operation.
Materials and methods
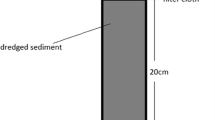
Seven constructed wetlands with horizontal subsurface flow (Fig. 1) with time of operation varying between 2 and 16 years (Table 1) were sampled in 2008. In each constructed wetland samples were taken in the inflow, middle and outflow zones (three samples in each zone). Gravel or crushed rock samples were taken using the reinforced stainless steel soil sampler (so called “Russian corer”) which was driven into the filtration substrate to a depth of 60 cm. Samples were divided into surface (0–20 cm) and bottom sections (20–60 cm) in order to evaluate vertical distribution of sediments. In the laboratory, samples were cleaned from roots and freeze-dried under low pressure and temperature. Dried sediment material was weighed, homogenized and passed through a 0.5 mesh sieve. After drying both sediment and filtration material volume were determined in order to calculate the volume ratio between sediment and filtration material. 500 mg of the dry sediment was digested in reverted (Löfelt) aqua-regia (4.5 ml HNO3 and 1.5 ml HCl) under high pressure and temperature in microwave apparatus (MARS-5, CEM, USA) according to the modified U.S. EPA method 3052 (U.S. EPA 1995). After digestion, the sample was filtered in order to obtain a clear sample. Heavy metals were analyzed by ICP-MS (PQ-ExCell, VG-Thermo Elemental, Winsford, Cheshire, UK) according to U.S. EPA method 200.8 (U.S. EPA 1994). For statistical analyses of heavy metal concentrations along the filtration beds paired-sample t-test (P < 0.05) was used. Differences between sediment concentrations in the filtration beds were statistically evaluated through the two-way ANOVA (P < 0.05) for vertical (top and bottom) and horizontal (inflow, middle and outflow) profiles.
Schematic representation of a constructed wetland with horizontal sub-surface flow. 1—distribution zone filled with large stones, 2—surface of the bed, 3—water level in the bed, 4—impermeable liner, 5—medium (e.g., gravel, crushed stones), 6—collection zone filled with large stones, 7—collection drainage pipe, 8—outlet structure for maintaining of water level in the bed. The arrows indicate only a general flow pattern (Vymazal 2001)
Results and discussion
Concentrations of heavy metals in the sediments
Cadmium
Under anoxic conditions, cadmium forms very insoluble compounds with sulfide (CdS) and under slightly reduced to oxidized conditions solid carbonate (CdCO3) is a major control mechanism for cadmium solubility (Khalid (1980). Precipitation of carbonate can be microbially mediated, for example, by Alcaligenes denitrificans (Remacle et al. 1992). Under aerobic conditions, cadmium could be adsorbed or co-precipitated with oxides, hydroxides, and hydrous oxides of Fe, Mn and possible Al (Khalid 1980). Cadmium complexed with the organic fraction may be divided into chelated and organic bound. Chelated Cd is the fraction that is loosely attached to immediately mobile and easily decomposable organic material while organic-bound Cd is the fraction incorporated into the insoluble organic material and can be solubilized only after intense oxidation of the organic matter (Khalid 1980).
The concentration of cadmium in sediments of monitored constructed wetlands varied between 0.095 and 1.35 mg/kg (Fig. 2). In all systems, the concentrations in the top layer did not significantly differed from those found in bottom layers with the exception of Příbraz and Spálené Poříčí where the Cd concentration was significantly higher at the outflow zone in the top layer. The results shown in Fig. 2 indicate that in Břehov, Libníč, Mořina and Spálené Poříčí the concentration of Cd near the inflow was significantly higher than in the middle of the bed and near the outflow. Similar observations were also reported by Lesage et al. (2007a, b) from HF CWs in Zemst and Zevergem, De Pinte, Flanders, Belgium and by Vymazal (2003) from HF CW Nučice in the Czech Republic. Also, the Cd concentrations found in our study were comparable with those reported by Lesage et al. (2007b). The values are slightly lower than those reported by Lesage et al. (2007a), Haberl and Perfler (1990), Samecka-Cymerman et al. (2004), Gschlössl and Stuible (2000) or Zuidervaart (1996) from HF CWs in Belgium, Austria, Poland, Germany and the Czech Republic, respectively. On the other hand, the concentrations were lower than concentrations reported from constructed wetlands for road runoff or mine wastewater treatment (Table 2). The data on sediment concentration in various wetlands (Table 2) indicate that concentrations found in our study are also comparable with those found in natural unpolluted wetlands. It is obvious that the highest Cd concentrations are found in wetlands receiving industrial wastewaters or mine drainage waters.
Nickel
Under oxic or suboxic conditions, Ni sorbs to Mn oxides and can substitute for Ni in the lattice of some Mn oxides (Green-Pedersen et al. 1997; Tonkin et al. 2004). Under anoxic/anaerobic conditions nickel forms insoluble sulfides (Sobolewski 1999) and is incorporated into pyrite (Morse and Luther 1999). Also carbonates could be an effective sink for nickel (Lin 1995).
The concentration of nickel in sediments of monitored constructed wetlands varied between 7.0 and 111 mg/kg (Fig. 2). In all systems, the concentrations in the top layer did not significantly differed from those found in bottom layers. The results shown in Fig. 2 indicate that in Břehov, and Spálené Poříčí the concentration of Ni near the inflow was significantly higher than in the middle of the bed and near the outflow. In Libníč, Příbraz, Slavošovice and Mořina, the Ni concentration in the sediments did not vary too much. In Čejkovice, the highest Ni concentration was measured at the outflow. The literature results on nickel distribution along the filtration bed also vary. Vymazal (2003) found significantly higher Ni concentration in the inflow zone while Lesage et al. (2007b) observed only a slight decrease along the bed and Lesage et al. (2007a) a slight increase in Ni concentration in the sediments along the bed. Nickel concentrations were comparable with Ni concentrations reported from constructed wetlands treating municipal sewage (Table 3). Also, the Ni concentrations are within the range of Ni concentrations reported from both unpolluted and polluted wetlands. Results presented in Table 3 revealed that by far the highest Ni concentrations are found in wetlands receiving industrial wastewater, mine drainage waters and also road runoff.
Lead
Koretsky et al. (2008) pointed out that lead, like Zn and Cu, is a chalcophile that forms discrete sulfide phases and may also bind strongly to organic matter. Also carbonates could be an effective sink for lead (Lin 1995). It has been shown that lead also strongly adsorbs to Fe/Mn oxides and it has been found in association with rhizosphere Fe(III) plaques (Dzombak and Morel 1990). However, it has been concluded that the Pb is not trapped by Fe oxides, but rather is complexed to organic matter either in the rhizosphere solution or on the root surface (Sundby et al. 2005).
The concentration of lead in sediments of monitored constructed wetlands varied between 9.3 and 125 mg/kg but most values were lower than 30 mg/kg (Fig. 2). In all systems, the concentrations in the top layer did not significantly differed from those found in bottom layers. The results shown in Fig. 2 indicate that in Břehov, Libníč, Mořina and Spálené Poříčí the concentration of Pb near the inflow was significantly higher than in the middle of the bed and near the outflow. In Příbraz and Slavošovice the Pb concentration in the sediments did not vary too much and decreased slightly along the bed. In Čejkovice, similarly to Ni, the concentrations gradually increase along the bed. Lesage et al. (2007a, b) reported that lead concentration in the sediment decreased along the bed. Vymazal (2003) found a significant decrease after 16 m of the bed but than the concentration increased again and after 48 m the Pb concentration was only slightly lower as compared to the concentration near the inflow. Lead concentrations found in our study were comparable with Pb concentrations reported from natural unpolluted and lightly polluted wetlands (Table 4) In comparison with the results reported from various constructed wetlands the measured concentrations are slightly lower. The data in Table 4 also clearly indicate that Pb concentrations in sediments of wetlands receiving mining drainage waters and waters affected by smelters are up to two orders of magnitude higher.
Copper
Copper forms under anoxic conditions very insoluble compounds with sulfur, including both cupric and cuprous sulfides (Sobolewski 1999; Morse and Luther 1999) and may also associate with pyrite (Huerta-Diaz et al. 1993). Copper also forms insoluble hydroxides and carbonates (Morel and Hering 1993) but those are important in presence of sulfides. Copper also forms strong complexes with organic matter and can be bound to Fe/Mn oxides under oxic conditions via formation of ternary complexes with organic matter (Achterberg et al. 1997).
The concentration of copper in sediments of monitored constructed wetlands varied between 6.3 and 139 mg/kg but most values were lower than 75 mg/kg (Fig. 3). The concentrations of Cu in the top layer did not differ from those found in the bottom layers in all seven systems. With the exception of Čejkovice and Příbraz, in all other system the Cu concentration was significantly higher in the inflow zone as compared to the middle and outflow zones. Lesage et al. (2007a, b) observed a steep decrease in Cu sediment concentration in two HF CWS in Belgium. Vymazal and Krása (2003) reported slight decrease in Cu concentration along the filtration bed of a HF CW in the Czech Republic. The copper concentrations found in our study were comparable with higher values found in unpolluted wetlands and with lower end of the range reported for polluted wetlands (Table 5). The copper concentrations shown in Fig. 3 were similar to the concentrations reported from Poland and Italy and also by Zuidervaart (1996) who studied heavy metals in the Czech constructed wetlands more than 10 years ago (Table 5). On the other hand, copper concentrations found in our study were lower than concentrations reported from Belgium (Table 5). The data in Table 5 also clearly indicate that Cu concentrations in sediments of wetlands receiving mining drainage waters and waters affected by smelters are up to two orders of magnitude higher.
Chromium
Contrary to most heavy metals such as Zn, Cd, Pb or Ni, chromium undergoes a change in oxidation state as a consequence of soil oxidation–reduction conditions (Gambrell 1994). These conditions play a major role in chromium speciation, solubility and mobility with reduction transformations being microbially mediated (Masscheleyn et al. 1992; Cervantes et al. 2001). DeLaune et al. (1998) reported that reduction of Cr(VI) occurs at approximately same redox levels as nitrate reduction. Under oxic and suboxic conditions chromium typically sorbs to Fe, and especially Mn, oxides (Davison 1993; Guo et al. 1997; Achterberg et al. 1997). Under anoxic sediments, reduced chromium is not readily incorporated into sulfides (Huerta-Diaz et al. 1998) but instead tends to associate with organic matter (Otero and Macias 2002). Also Guo et al. (1997) reported that under reducing conditions, the behavior of Cr is controlled primarily by insoluble large molecular humic materials.
The concentration of chromium in sediments of monitored constructed wetlands varied between 13 and 163 mg/kg but in Příbraz, Slavošovice, Mořina and Spálené Poříčí the average Cr concentrations in sediments did not exceed 45 mg/kg (Fig. 3). The concentrations of Cu in the top layer did not differ from those found in the bottom layers in all seven systems. In Břehov and Spálené Poříčí the highest Cr concentrations were recorded in the inflow zone while in Čejkovice and Příbraz the highest concentrations were recorded in the outflow zone. Lesage et al. (2007a, b) observed a slight decrease in Cr sediment concentration in Belgium. The chromium concentrations found in our study were higher as compared to values found in natural unpolluted wetlands and are similar to concentrations found in polluted wetlands (Table 6). The data in Table 6 indicate that Cr concentration in sediments in studied HF CWs was slightly higher than most data reported in the literature from constructed wetlands. The range of concentrations found in our study is comparable with concentrations found in a constructed wetland treating road runoff (Scholes et al. 1998). However, the highest values found in our study were lower than concentrations reported by Gschlössl and Stuible (2000) from Germany.
Zinc
Under aerobic conditions zinc is commonly associated with Fe and Mn oxides, hydroxides and oxyhydroxides (Krauskopf 1956; Jenne 1968; Ferris et al. 1989; Bostick et al. 2001). Zinc is also retained in iron plaques on plant root surface (Otte et al. 1995). Under anoxic conditions zinc forms very insoluble sulfides (Huerta-Diaz et al. 1993; Achterberg et al. 1997; Stumm and Morgan 1981; Kosolapov et al. 2004) and carbonates Hansel et al. 2001; Bostick et al. 2001).
The concentration of zinc in sediments of monitored constructed wetlands varied widely between 1.0 and 1,768 mg/kg (Fig. 3). In Příbraz, Slavošovice and Spálené Poříčí significantly more zinc was found in the top layer. In most surveyed constructed wetlands significantly more Zn was found in the inflow zone. The extremely high concentrations of Zn in sediments in Mořina are influenced by naturally high inflow Zn concentrations (Kröpfelová et al. 2009). Very high accumulation of zinc in the inflow zone of HF CWs was also reported by Lesage et al. (2007a, b) from systems in Zemst and Zevergem in Belgium and by Vymazal and Krása (2003) from the HF CW in the Czech Republic. Zinc concentrations found in our study were comparable with higher Zn concentrations reported from natural unpolluted wetlands and with lower range of concentrations reported from polluted wetlands (Table 7). Zinc concentrations found in our study has never reached concentrations reported from wetlands impacted by mining activity (Table 7). In comparison with the results reported from various constructed wetlands the measured Zn concentrations are within the same range with the exception of Zn concentrations reported from a constructed wetland treating mining waters from Pb/Zn mine in China (Table 7).
In Table 8, average concentrations of studied heavy metals in seven HF constructed wetlands are shown. The data could be compared with background values and legal limits (Table 9). The data indicate that concentrations of Cd exceeded the Czech limits for light soils in Mořina and Příbraz but in general the concentrations were only slightly elevated as compared to concentrations found in unpolluted soils and sediments (Bowen 1979). Also concentrations of nickel were only slightly elevated as compared to unpolluted soils and sediments and only in Čejkovice the average Ni concentration exceeded the Czech limit for other soils.
Concentrations of lead were very low and comparable with unpolluted soils and sediments (Bowen 1979). Also for Cu, concentrations in the sediments were quite low and only in Mořina the average value exceeded slightly the Czech limit for light soils. Concentrations of Cr exceeded slightly the Czech limit for light soils in Čejkovice and Libníč, otherwise the concentrations were low and comparable with unpolluted soils and sediments. Concentrations of zinc showed the greatest variation among studied constructed wetlands. In Mořina and Spálené Poříčí the average values exceeded the Czech limits for other soils.
The results did not show any relationship between the concentration of heavy metals and the time of operation. This is probably a consequence of the sediment build-up in the filtration beds where the sediments are also formed by suspended solids. Haberl and Perfler (1990) documented that concentration of Zn, Cu and Cd remained steady during the 7-year study. While the concentrations do not change substantially during the course of constructed wetland operation, due to increase in the sediment biomass the amount of heavy metals increases. This was also observed in our study.
Concentrations of sediment in the filtration beds
For constructed wetlands in the Czech Republic, washed gravel or crushed stones are used. In the beginning of operation, the amount of sediment is zero and its concentration increases during the time of operation. In Table 10, concentrations of sediment expressed in %DM of the filtration bed material are shown. The results indicate the increase of sediment concentration with increasing time of operation. The amount of sediment was usually greater in the inflow zone as compared to outflow zone but the difference was not always statistically significant (Table 10). In Slavošovice, significantly more sediment mass was found at the bottom layer while in Spálené Poříčí significantly more sediment mass was found in the top layer in the inflow and middle zones. Also, in Břehov and Mořina, more sediment was found in the top layer. This variation is probably affected by the placement of the distribution pipes. While in Slavošovice the distribution pipes are buried near the bottom of the bed, in Spálené Poříčí, Břehov and Mořina, the distribution systems is either laid down on the surface of the filtration bed or it is buried only shallowly bellow the bed surface. Taking into consideration the sediment/filtration material mass ratio it was possible to calculate average heavy metal concentrations in the filtration material including sediments (Table 11). As sediment mass varied between 0.42 and 10.55% of the filtration material, the final heavy metal concentrations are much lower than legal limits (Table 9).
Conclusions
Concentrations of Cd, Ni, Pb, Cu, Cr and Zn were evaluated in seven constructed wetlands with horizontal subsurface flow treating municipal wastewater in the Czech Republic. The time of operation varied between 2 and 16 years among systems. The results revealed that concentrations of heavy metals were low and comparable with concentrations found in unpolluted or lightly polluted natural wetlands. The concentrations were much lower than concentrations found in wetlands receiving mine drainage or industrial wastewaters. The concentrations of heavy metals did not reflect the length of operation but the amount of sediment mass increases with the length of operation. This will result in greater heavy metal mass in the system. The concentrations of heavy metals in the sediment exceeded occasionally the limits for agricultural soils but when filtration material was taken into consideration, the concentrations were well below the limits.
References
Accornero A, Gnerre R, Manfra L (2008) Sediment concentrations of trace metals in the Berre Lagoon (France): An assessment of contamination. Bull Environ Contam Toxicol 54:372–385
Achterberg EP, Van den Berg CGM, Boussemart M, Davison W (1997) Speciation and cycling of trace metals in Esthwaite Water: a productive English lake with seasonal deep-water anoxia. Geochim Cosmochim Acta 61:5233–5253
Aksoy A, Demirezen D, Duman F (2005) Bioaccumulation, detection and analyses of heavy metal pollution in Sultan Marsh and its environment. Water Air Soil Pollut 164:241–255
Babcock MF, Evans DW, Alberts JJ (1983) Comparative uptake and translocation of trace elements from coal ash by Typha latifolia. Sci Total Environ 28:203–214
Baldantoni D, Ligrone R, Alfani A (2009) Macro- and trace-element concentrations in leaves and roots of Phragmites australis in a volcanic lake in Southern Italy. J Geochem Explor 101:166–174
Batty LC, Baker AJ, Wheeler BD (2002) Aluminum and phosphate uptake by Phragmites australis: the role of Fe, Mn and Al root plaques. Ann Bot 89:443–449
Baudo R, Canzian E, Galanti G, Guilizzoni P, Rapetti G (1985) Relationship between heavy metals and aquatic organisms in Lake Mezzola hydrographic system (Northern Italy). 6. Metal concentrations in two species emergent of macrophytes. Mem Ist Ital Idrobiol 43:161–180
Bi X, Feng X, Yang Y, Li X, Sin GPY, Qiu G, Qian X, Li F, He T, Li P, Liu T, Fu Z (2007) Heavy metals in an impacted wetland system: a typical case from southwestern China. Sci Tot Environ 387:257–268
Bostick BC, Hansel CM, Force MJL, Fendorf S (2001) Seasonal fluctuations in zinc speciation within a contaminated wetland. Environ Sci Technol 35:3823–3829
Bowen HJM (1979) Environmental chemistry of the elements. Academic Press, London
Bragato C, Brix H, Malagoli M (2006) Accumulation of nutrients and heavy metals in Phragmites australis (Cav.) Trin ex Steudel and Bolboschoenus maritimus (L.) Palla in a constructed wetland of the Venice lagoon watershed. Environ Pollut 144:967–975
Carapeto C, Purchase D (2002) Artificial wetlands and their importance for water quality. In: Pries J (ed) Treatment Wetlands for Water Quality Improvement. CH2M HILL Canada, Waterloo, pp 45–52
Cardwell AJ, Hawker DW, Greenway M (2002) Metal accumulation in aquatic macrophytes from southeast Queensland, Australia. Chemosphere 48:653–663
Carranza-Álvarez C, Alonso-Castro AJ, Alfaro-De La Torre MC, Garcia-De La Cruz RF (2008) Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial lagoon in San Luis Potosí, México. Water Air Soil Pollut 188:297–309
Cervantes C, Campo-Garcia J, Devars S, Gutierrez-Corona F, Loza-Tavera H, Torres-Guzman JC, Moreno-Sanchez R (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347
Cooper DC, Picardal EF, Coby AJ (2006) Interaction between microbial iron reduction and metal geochemistry: effect of redox cycling on transition, metal speciation in iron bearing sediments. Environ Sci Technol 40:1884–1891
Davison W (1993) Iron and manganese in lakes. Earth Sci Rev 34:119–163
De Volder PS, Brown SL, Hesterberg D, Pandya K (2003) Metal bioavailability and speciation in a wetland tailings repository amended with biosolids compost, wood ash and sulfate. J Environ Qual 32:851–864
DeLaune RD, Gambrell RP, Knox RS (1989) Accumulation of heavy metals and PCB’s in an urban lake. Environ Technol Lett 10:753–762
DeLaune RD, Patrick WH Jr, Guo T (1998) The redox-pH chemistry of chromium in water and sediment. In: Allen HE, Garrison AW, Luther GW (eds) Metals in surface waters. Sleeping Bear Press Inc., Chelsea, pp 241–255
Deng H, Ye ZH, Wong MH (2004) Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ Pollut 132:29–40
Deng H, Ye ZH, Wong MH (2006) Lead and zinc accumulation and tolerance in populations of six wetland plants. Environ Pollut 141:69–80
Deng PY, Shu WS, Lan CY, Liu W (2008) Metal contamination in the sediment, pondweed, and snails of a stream receiving effluent from a lead/zinc mine in southern China. Bull Environ Contam Toxicol 81:69–74
Department of Soil Protection, Netherlands (1994) The Netherlands soil contamination guidelines. Netherlands Intervention Values for Soil Remediation, DBO/07494013
Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2008) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Tot Environ 407:3972–3985
Duman F, Cicek M, Sezen G (2007) Seasonal changes of metal accumulation and distribution in common club rush (Schoenoplectus lacustris) and common reed (Phragmites australis). Ecotoxicology 16:457–463
Dušek J, Picek T, Čížková H (2008) Redox potential dynamics in a horizontal subsurface flow constructed wetland for wastewater treatment: diel, seasonal and spatial fluctuations. Ecol Eng 34:223–232
Dzombak DA, Morel FMM (1990) Surface complexation modeling: hydrous ferric oxide. Wiley, New York
Eckhardt DAV, Surface JM, Peverly JH (1999) A constructed wetland system for treatment of landfill leachate, Monroe County, New York. In: Mulamoottil G, McBean EA, Rovers F (eds) Constructed wetlands for the treatment of landfill leachate. CRC Press/Lewis Publishers, Boca Raton, pp 205–222
Engler RP, Patrick WH Jr (1975) Stability of sulfides of manganese, iron, zinc, copper, and mercury in flooded and nonflooded soil. Soil Sci 119:217–221
Feely RD, Massoth GJ, Paulson AJ, Gendron JF (1983) Possible evidence for enrichment of trace elements in the hydrous manganese oxide phases of suspended matter from an urbanized embayment. Estuar Coast Shelf Sci 7:693–708
Fejitel TC, DeLaune RD, Patrick WH Jr (1988) Biogeochemical control of metal distribution and accumulation in Louisiana sediments. J Environ Qual 17:88–94
Ferris FG, Schultze S, Witten TC, Fyfe WS, Beveridge TJ (1989) Metal interactions with microbial biofilms in acidic and neutral pH environments. Appl Environ Microbiol 55:1249–1257
Folsom BL, Lee CR (1981) Zinc and cadmium uptake by the freshwater marsh plant Cyperus esculentus grown in contaminated sediments under reduced (flooded) and oxidized (upland) conditions. J Plant Nutr 3:233–244
Gambrell RP (1994) Trace and toxic metals in wetlands—a review. J Environ Qual 23:883–891
Gambrell RP, Patrick WH Jr (1978) Chemical and microbiological properties of anaerobic soils and sediment. In: Hook DD, Crawford RMM (eds) Plant life in anaerobic environments. Ann Arbor Science Publishers, Ann Arbor, pp 375–423
Gambrell RP, Khalid RA, Patrick WH Jr (1980) Chemical availability of mercury, lead, and zinc in mobile bay sediment suspensions as affected by pH and oxidation-reduction conditions. Environ Sci Technol 14:431–436
Green CH, Heil DM, Cardon GE, Butters GL, Kelly EF (2003) Solubilization of manganese and trace metals in soils affected by acid mine runoff. J Environ Qual 32:1323–1334
Green-Pedersen H, Jensen BT, Pind N (1997) Nickel adsorption on MnO2, Fe(OH)3, montmorillonite, humic acid and calcite: a comparative study. Environ Technol 18:807–815
Gschlössl T, Stuible H (2000) Reed bed systems: design, performance and maintainability. Water Sci Technol 41(1):73–76
Guo T, DeLaune RD, Patrick WH (1997) The influence of sediment redox chemistry on chemically active forms of arsenic, cadmium, chromium, and zinc in estuarine sediments. Environ Int 23:305–316
Haberl R, Perfler R (1990) Seven years of research work and experience with wastewater treatment by a reed bed system. In: Cooper PF, Findlater BC (eds) Constructed wetlands in water pollution control. Pergamon Press, Oxford, pp 205–214
Hansel CM, Fendorf S, Sutton S, Newville M (2001) Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants. Environ Sci Technol 35:3863–3868
Higgins J, Brown T (1999) The use of constructed wetland to treat landfarm leachate at the Sunoco Refinery in Sarnia, Ontario. In: Mulamoottil G, McBean EA, Rovers F (eds) Constructed wetlands for the treatment of landfill leachates. CRC Press/Lewis Publishers, Boca Raton, pp 235–250
Huerta-Diaz MA, Carigan R, Tessier A (1993) Measurement of trace metals associated with acid volatile sulfides and pyrite in organic freshwater sediments. Environ Sci Technol 27:2367–2372
Huerta-Diaz MA, Tessier A, Carignan R (1998) Geochemistry of trace metals associated with reduced sulphur in freshwater sediments. Appl Geochem 13:13–33
Jacob DL, Otte ML (2003) Conflicting processes in the wetland plant rhizosphere: metal retention or mobilization. Water Air Soil Pollut 3:91–104
Jacobson ME (1994) Chemical and biological mobilization of Fe(III) in marsh sediments. Biogeochemistry 25:41–60
Jenne EA (1968) Controls of Mn, Fe, Co, Ni, Cu, and Zn concentration in soils and water: significant role of hydrous Mn and Fe oxides. Adv Chem Ser 73:337–387
Jiménez-Cárceles FJ, Álvarez-Rogel J, Conesa Alcazar HM (2008) Trace elements concentrations in saltmarsh soils strongly affected by wastes from metal sulphide mining areas. Water Air Soil Pollut 188:283–295
Khalid RA (1980) Chemical mobility of cadmium in sediment-water systems. In: Nriagu JO (ed) Cadmium in the environment. Part I. Wiley, New York, pp 258–304
Khalid RA, Patrick WH Jr, Gambrell RP (1978) Effect of dissolved oxygen on chemical transformations of heavy metals, phosphorus, and nitrogen in an estuarine sediment. Estuar Coast Mar Sci 6:21–35
Koretsky CM, Cuellar A, Haveman M, Beuving L, Shattuck T, Wagner M (2008) Influence of Spartina and Juncus on saltmarsh sediments. II. Trace element geochemistry. Chem Geol 255:100–113
Kosolapov DB, Kuschk P, Vainshtein MB, Vatsourina AV, Wiessner A, Kästner M, Müller RA (2004) Microbial processes of heavy metal removal from carbon-deficient effluents in constructed wetlands. Eng Life Sci 4:403–411
Krauskopf KB (1956) Separation of manganese from iron in sedimentary processes. Geochim Cosmochim Acta 12:61–84
Kröpfelová L, Vymazal J, Švehla J, Štíchová J (2009) Removal of trace elements in three horizontal sub-surface flow constructed wetlands in the Czech Republic. Environ Pollut 157:1186–1194
Laanbroek HJ (1990) Bacterial cycling of minerals that affect plant growth in waterlogged soils: a review. Aquat Bot 38:109–125
Laanbroek HJ, Veldkamp H (1982) Microbial interactions in sediment communities. Philos Trans R Soc Lond B 297:533–550
Lan C, Chen G, Li L, Wong MH (1990) Purification of wastewater from a Pb/Zn mine using hydrophytes. In: Cooper PF, Findlater BC (eds) Constructed wetlands in water pollution control. Pergamon Press, Oxford, pp 419–427
Lesage E (2006) Behaviour of heavy metals in constructed treatment wetlands. Dissertation, Ghent University, Ghent, Belgium
Lesage E, Rousseau DPL, Meers E, Van de Moortel AMK, Du Laing G, Tack FMG, De Pauw N, Verloo MG (2007a) Accumulation of metals in the sediment and reed biomass of a combined constructed wetland treating domestic wastewater. Water Air Soil Pollut 183:253–264
Lesage E, Rousseau DPL, Meers E, Tack FMG, De Pauw N (2007b) Accumulation of metals in a horizontal subsurface flow constructed wetland treating domestic wastewater in Flanders, Belgium. Sci Tot Environ 380:102–115
Li QS, Wu ZF, Chu B, Zhang N, Cai SS, Fang JH (2007) Heavy metals in coastal wetland sediments of the Pearl River Estuary, China. Environ Pollut 149:158–164
Liang Y, Wong MH (2003) Spatial and temporal organic and heavy metal pollution at Mai Po Marshes Nature Reserve, Hong Kong. Chemosphere 52:1647–1658
Lin LY (1995) Wastewater treatment for inorganics. In: Encyclopedia of environmental biology, vol 3. Academic Press, San Diego, pp 479–484
Lindau CW, Hossner LR (1982) Sediment fractionation of Cu, NI, Zn, Cr, Mn, and Fe in one experimental and three natural marshes. J Environ Qual 11:540–545
Lovley DR (1995) Microbial reduction of iron, manganese, and other metals. Adv Agron 54:175–231
Lwanga MS, Kansiime F, Denny P, Scullion J (2003) Heavy metals in Lake George, Uganda, with relation to metal concentrations in tissues of common fish species. Hydrobiologia 499:83–93
Madejón P, Murillo JM, Maraňón T, Espinar JL, Cabrera F (2006) Accumulation of As, Cd and selected trace elements in tubers of Scirpus maritimus L. from Daňana marshes (South Spain). Chemosphere 64:742–748
Mandernack KW, Lynch L, Krouse HR, Morgan MD (2000) Sulfur cycling in wetland peat of the New Jersey Pinelands and its effect on stream water chemistry. Geochim Cosmochim Acta 64:3949–3964
Mansfeldt T (2004) Redox potential of bulk soil and soil solution concentration of nitrate, manganese, iron, and sulfate in two Gleysols. J Plant Nutr Soil Sci 167:7–16
Marcussen H, Joergensen K, Holm PE, Brocca D, Simmons RW, Dalsgaard A (2008) Element contents and food safety of water spinach (Ipomea aquatica Forssk.) cultivated with wastewater in Hanoi, Vietnam. Environ Monit Assess 139:77–91
Masscheleyn PH, Pardue JH, DeLaune RD, Patrick WH Jr (1992) Chromium redox chemistry in lower Mississippi Valley Bottomland Hardwood wetland. Environ Sci Technol 26:1217–1227
Mattiuzzo E, Favero L, Zennaro F, Franco D (2007) Heavy metal behavior in an experimental free water surface wetland in the Venice Lagoon watershed. Water Air Soil Pollut 183:143–151
Mazej Z, Germ M (2009) Trace element accumulation and distribution in four aquatic macrophytes. Chemosphere 74:642–647
McDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Tocixol 39:20–31
Megonikal JP, Hines ME, Visscher PT (2004) Anaerobic metabolism: linkage to trace gases and aerobic processes. In: Schlesinger WH (ed) Biogeochemistry. Elsevier-Pergamon, Oxford, pp 317–424
Mishra VK, Upadhyay AR, Pandey SK, Tripathi BD (2008) Concentrations of heavy metals and aquatic macrophytes of Govind Ballabh Pant Sagar an anthropogenic lake affected by coal mining effluent. Environ Monit Assess 141:49–58
Moore JW, Sutherland DJ (1981) Distribution of heavy metals and radionuclides in sediments, water, and fish in an area of Great Bear Lake contaminated with mine waters. Arch Environ Contam Toxicol 10:329–338
Morel FMM, Hering JG (1993) Principles and applications of aquatic chemistry. Wiley, New York
Morse JW, Luther GW (1999) Chemical influences on trace metal–sulfide interaction in anoxic sediments. Geochim Cosmochim Acta 63:3373–3378
Mungur AS, Shutes RBE, Revitt DM, House MA (1994) An assessment of highway runoff treatment by natural wetlands. In: Proceedings of 4th international conference on wetland systems for water pollution control. ICWS Secretariat, Guangzhou, P. R. China, pp 669–676
Murdoch A, Capobianco J (1978) Study of selected metals in marshes on Lake St. Clair, Ontario. Arch Hydrobiol 84:87–108
National Standard of PR China (1995) Soil Environmental Quality (GB 15618–1995). Standards Press of China, Beijing
Obarska-Pempkowiak H (2001) Retention of selected heavy metals: Cd, Cu, Pb in a hybrid wetland system. Watere Sci Technol 44(11–12):463–468
Obarska-Pempkowiak H, Klimkowska K (1999) Distribution of nutrients and heavy metals in a constructed wetland system. Chemosphere 39:303–312
Ojo OE, Mashauri DA (1996) Uptake of heavy metals in the root-zone of Msimbazi reeds. In: Proceedings of 5th international conference on wetland systems for water pollution control. Universität für Bodenkultur, Vienna, Austria
Oke BH, Juwarkar AS (1996) Removal of heavy metals from domestic wastewater using constructed wetland system. In: Proceedings of 5th international conference on wetland systems for water pollution control. Universität für Bodenkultur, Vienna, Austria
Orru H, Orru M (2006) Sources and distribution of trace elements in Estonian peat. Glob Planet Change 53:249–258
Otero XL, Macias F (2002) Variation with depth and season in metal sulfides in salt marsh soils. Biogeochemistry 61:247–268
Otte ML, Kearns CC, Doyle MO (1995) Accumulation of arsenic and zinc in the rhizosphere of wetland plants. Bull Environ Contam Toxicol 55:154–161
PAS (1994) Protection of Agricultural Soils. Czech Law 13/1994 (in Czech)
Peng K, Luo C, Lou L, Li X, Shen Z (2008) Bioaccumulation of heavy metals by the aquatic plants Potamogeton pectinatus L. and Potamogeton malaianus Miq. And their potential use for contamination indicators and in wastewater treatment. Sci Tot Environ 392:22–29
Pokorný J, Pechar L, Radová J, Bastl J, Drbal K, Švehla J (1999) Heavy metals in ecosystems of Luznice River and Nadeje fishpond system (Trebon Biosphere Reserve. In: Vymazal J (ed) Nutrient cycling and retention in natural and constructed wetlands. Backhuys Publishers, Leiden, pp 144–155
Ramos L, Hernandez LM, Gonzales MJ (1994) Sequential fraction of copper, lead, cadmium and zinc in soils from or near Donana National Park. J Environ Qual 23:50–57
Remacle J, Muguruza L, Fransolet M (1992) Cadmium removal by a strain Alcaligenes denitrificans isolated from a metal-polluted pond. Water Res 26:923–926
Samecka-Cymerman A, Kempers AJ (2001) Concentration of heavy metals and plant nutrients in water sediments and aquatic macrophytes of anthropogenic lakes (former open cut brown coal mines) differing in stage of acidification. Sci Tot Environ 281:87–98
Samecka-Cymerman A, Kempers AJ (2004) Toxic metals in aquatic plants surviving in surface water polluted by copper mining industry. Ecotoxicol Environ Saf 59:64–69
Samecka-Cymerman A, Stepien D, Kempers AJ (2004) Efficiency in removing pollutants by constructed wetland purification systems in Poland. J Toxicol Environ Health A 67:265–275
Santos-Oliveira J, Fernandes JA, Alves C, Morais J, Urbano P (1999) Metals in sediment and water of free reed (Phragmites australis (Cav.) Trin ex Steudel) stands. Hydrobiologia 415:41–45
Schierup H-H, Larsen VJ (1981) Macrophyte cycling of zinc, copper, lead and cadmium in the littoral zone of a polluted and a non-polluted lakes I. Availability, uptake and translocation of heavy metals in Phragmites australis (Cav.) Trin. Aquat Bot 11:197–210
Scholes L, Shutes RBE, Revitt DM, Forshaw M, Purchase D (1998) The treatment of metals in urban runoff by constructed wetlands. Sci Tot Environ 214:211–219
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Miner Eng 19:105–116
Shomar BH, Müller G, Yahya A (2005) Seasonal variations of chemical composition of water and bottom sediments in the wetland of Wadi Gaza, Gaza Strip. Wetl Ecol Manag 13:419–431
Shutes RB, Ellis JB, Revitt DM, Zhang TT (1993) The use of Typha latifolia for heavy metal pollution control in urban wetlands. In: Moshiri GA (ed) Constructed wetlands for water quality improvement. CRC Press/Lewis Publishers, Boca Raton, pp 407–414
Simpson RL, Good RE, Walker R, Frasco BR (1983) The role of Delaware River freshwater tidal wetlands in the retention of nutrients and heavy metals. J Environ Qual 12:41–48
Singer PC, Stumm W (1970) Acid mine drainage—the rate limiting step. Science 167:1121–1123
Sobolewski A (1996) Metal species indicate the potential of constructed wetlands for long-term treatment of mine drainage. J Ecol Eng 6:259–271
Sobolewski A (1999) A review of processes responsible for metal removal in wetlands treating contaminated mine drainage. Int J Phytoremed 1:19–51
Stumm W, Morgan JJ (1981) Aquatic chemistry. An introduction emphasizing chemical equilibria in natural waters, 2nd edn. Wiley-Interscience, New York
Sundby B, Caetano M, Vale C, Gobeil C, Luther GW, Nuzzio DB (2005) Metal-rich concretions on the roots of salt marsh sediments. Environ Sci Technol 39:2080–2086
Švehla J, Chrastný V, Bastl J, Mikuláš R (2002) Content of some hazardous heavy metals in sediments of selected fishponds in South Bohemia. In: Papáček M (ed) Biodiversity and nature of Novohradské mountains. South Bohemian University and Institute of Entomology, České Budějovice, Czech Republic, pp 53–57
Szymanowska A, Samecka-Cymerman A, Kempers AJ (1999) Heavy metals in three lakes in west Poland. Ecotoxicol Environ Saf 43:21–29
Taylor GJ, Crowder AA (1983) Uptake and accumulation of heavy metals by Typha latifolia in wetlands of the Sudbury, Ontario region. Can J Bot 61:63–73
Teuchies J, de Deckere E, Bervoets L, Meynendonckx J, van Regenmortel S, Blust R, Meire P (2008) Influence of tidal regime on the distribution of trace metals in a contaminated tidal freshwater marsh soil colonized with common reed (Phragmites australis). Environ Pollut 155:20–30
Tonkin JW, Balistrieri LS, Murray JW (2004) Modeling sorption of divalent metal cations on hydrous manganese oxide using the diffuse double layer model. Appl Geochem 19:29–53
U.S. EPA (1994) Determination of trace elements in waters and wastes by inductively coupled plasma-mass spectrometry, Method 200.8. US Environmental Protection Agency
U.S. EPA (1995) Microwave assisted acid digestion of siliceous and organically based matrices including ash, biological tissue, oil, oil contaminated soil, sediment, sludge, and soil, Method 3052. US Environmental Protection Agency
Verloo M, Cottenie A (1972) Stability and behavior of complexes of Cu, Fe, Mn, and Pb with humic substances in soil. Pedologie 22:174–184
VLAREBO (1996) Decision of the Flemish Government of 05/03/96 concerning Flemish regulations with regard to soil remediation. Belgian Government Gazette 05/03/96 (in Dutch)
Von der Heyden CJ, New MG (2004) Sedimentary chemistry: a history of mine contaminant remediation and an assessment of processes and pollution potential. J Geochem Explor 82:35–57
Vymazal J (1995) Constructed wetlands for wastewater treatment in the Czech Republic-state of the art. Water Sci Technol 32(2):357–364
Vymazal J (1996) The use of subsurface-flow constructed wetlands for wastewater treatment in the Czech Republic. Ecol Eng 7:1–14
Vymazal J (2001) Types of constructed wetlands for wastewater treatment: their potential for nutrient removal. In: Vymazal J (ed) Transformations of nutrients in natural and constructed wetlands. Backhuys Publishers, Leiden, pp 1–93
Vymazal J (2002) The use of sub-surface constructed wetlands for wastewater treatment in the Czech Republic: 10 years experience. Ecol Eng 18:633–646
Vymazal J (2003) Distribution of iron, cadmium, nickel and lead in a constructed wetland receiving municipal sewage. In: Vymazal J (ed) Wetlands—nutrients, metals and mass cycling. Backhuys Publishers, Leiden, pp 341–363
Vymazal J (2009) Horizontal sub-surface flow constructed wetlands Ondřejov and Spálené Poříčí in the Czech Republic—15 years of operation. Desalination 246:226–237
Vymazal J, Krása P (2003) Distribution of Mn, Al, Cu and Zn in a constructed wetland receiving municipal sewage. Water Sci Technol 48(5):299–305
Vymazal J, Kröpfelová L (2005) Growth of Phragmites australis and Phalaris arundinacea in constructed wetlands for wastewater treatment in the Czech Republic. Ecol Eng 25:606–621
Wieder RK (1989) A survey of constructed wetlands for acid coal mine drainage treatment in the eastern United States. Wetlands 9:299–315
Wong CSC, Wu SC, Duzgoren-Aydin NS, Aydin A, Wong MH (2007) Trace metal contamination of sediments in an e-waste processing village in China. Environ Pollut 145:434–442
Woulds C, Ngwenya BT (2004) Geochemical processes governing the performance of a constructed wetland treating acid mine drainage, Central Scotland. Appl Geochem 19:1773–1783
Yang Z, Li B, Li G, Wang W (2007) Nutrient elements and heavy metals in the sediment of Baiyangdian and Taihu Lakes: a comparative analysis of pollution trends. Front Agric China 1:203–209
Yao Z, Gao P (2007) Heavy metal research in lacustrine sediment: a review. Chin J Ocean Limnol 25:444–454
Ye ZH, Baker AJM, Wong M-H, Willis AJ (1997) Zinc, lead and cadmium tolerance, uptake and accumulation by Typha latifolia. New Phytol 136:469–480
Ye Z, Baker AJM, Wong M-H, Willis AJ (1998) Zinc, lead and cadmium accumulation and tolerance in Typha latifolia as affected by iron plaque on the root surface. Aquat Bot 61:55–67
Zhulidov AV, Headley JV, Robarts RD, Nikanorov AM, Ischenko AA, Champ MA (1997) Concentrations of Cd, Pb, Zn and Cu in contaminated wetlands of the Russian Arctic. Mar Pollut Bull 35:252–259
Zuidervaart I (1996) Heavy metals in constructed wetlands. Institute of Botany, Třeboň
Acknowledgements
The study was supported by grant no. 206/06/0058 “Monitoring of Heavy Metals and Selected Risk Elements during Wastewater Treatment in Constructed Wetlands” from the Czech Science Foundation and grants no. 2B06023 “Development of Mass and Energy Flows Evaluation in Selected Ecosystems” and no. ZF JU-MSM 6007665806 “Sustainable Methods in Agricultural Operations in Submontane and Mountainous Regions Aimed at Harmonization of Their Production and Extraproduction Functions” from the Ministry of Education, Youth and Sport of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vymazal, J., Švehla, J., Kröpfelová, L. et al. Heavy metals in sediments from constructed wetlands treating municipal wastewater. Biogeochemistry 101, 335–356 (2010). https://doi.org/10.1007/s10533-010-9504-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-010-9504-8