Abstract
A Gram-negative strain DD1, which could use 1,4-dioxane as the sole carbon and energy source, was isolated from the mixture of activated sludge obtained from Qige urban sewage treatment plant. According to the Biolog GNIII detection and the 16S ribosomal DNA (rDNA) sequence, DD1 was identified as Acinetobacter baumannii. Cells of A. baumannii DD1 precultured in 1,4-dioxane could completely degrade 100 mg/L 1,4-dioxane in 42 h with a cell yield of 0.414 mg-protein (mg-1,4-dioxane)−1 and a generation time of 6.75 h, demonstrating that DD1 bears the highest 1,4-dioxane-degrading activity among the described strains. Moreover, DD1 tolerates higher 1,4-dioxane concentration almost up to 1,000 mg/L. The strain could also grow on several benzene homologues including benzene, toluene, ethylbenzene, o-xylene, m-xylene, and phenol. During the degradation process of 1,4-dioxane, the first oxidation was initiated by monooxygenase in DD1. However, the main second monooxygenation intermediate 2-hydroxyethoxyacetic acid was not detected. As replacer, 1,4-dioxene was identified, and other intermediates such as ethylene glycol and oxalic acid were also detected. Based on the analysis of degradation products, a partial degradation pathway was proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The cyclic ether 1,4-dioxane is a solvent widely used in a range of industrial organic products including paints, lacquers, cosmetics, deodorants, and fumigants. As a byproduct, it is presented in many consumer products such as cleaning products, cosmetics, shampoos, and laundry detergents (Sara et al. 2001). It has also been used as a stabilizer for chlorinated organic solvents (e.g., 1,1,1-trichloroethane (TCA) and 1,1-dichloroethylene (DCE)), and it is a natural composition in some food products (e.g., tomatoes, shrimp, and coffee) (Mahendra et al. 2013). It is reported that about 41 and 65 tons of 1,4-dioxane in the USA and Japan, respectively, were released into surface water from manufacturing and processing facilities annually (Sei et al. 2013; Agency for Toxic Substances and Disease Registry (ATSDR) 2012). Similar to other ethers such as methyl tert-butyl ether (Sun et al. 2012 b), 1,4-dioxane also has a high water solubility, low logoctanol-water partition coefficient, and low Henry’s law constant (Sei et al. 2013) and, thus, is highly mobile in water and can persist for a long time in the environment once released. Currently, worldwide, 1,4-dioxane is detected in surface waters, groundwater, and some drinking water sources, even in the arctic groundwater (Li et al. 2013). The concentration of 1,4-dioxane is up to 260 μg/L in surface water and 220 mg/L in groundwater in the USA; meanwhile, it is 46 μg/L in surface water and 2,000 μg/L in landfill leachate in Japan (Sei et al. 2013). In addition, 1,4-dioxane has widely been detected in landfill in China (Lu and Xia 2003).
Due to the acute and chronic toxicity of 1,4-dioxane, as well as its suspected carcinogenicity (Kano et al. 2010), increasing public concern over its presence in aquatic environments has emerged. A great many methods have been investigated to enhance 1,4-dioxane degradation including conventional physical and chemical methods, advanced chemical oxidation, and photocatalytic processes (Coleman et al. 2007; Son et al. 2008). However, these processes require substantial infrastructure and are consequently associated with extremely high treatment costs, making them impractical for the in situ remediation of vast contaminated sites (Kim et al. 2009). Microorganism treatment as a new promising alternative remediation technology has been widely applied for its economic and ecological advantages, thus exhibiting a potential for 1,4-dioxane degradation. In 1991, the first organism that grew on 1,4-dioxane was isolated by Bernhardt and Diekmann 1991 called Rhodococcus rubber strain 219. Several studies have shown that a microorganism plays a key role in bioremediation, such as Mycobacterium vaccae JOB, Pseudonocardia strain K1, Pseudonocardia sp. ENV478, and Graphium sp. (Burback and Perry 1993; Kohlweyer et al. 2000; Vainberg et al. 2006; Kristin et al. 2009). However, those organisms could only co-metabolize 1,4-dioxane degradation with the growth-supporting substrates such as tetrahydrofuran (THF), methane, propane, toluene, or ethanol and could not grow with 1,4-dioxane as a sole carbon source. It would not only increase the cost of practical application but also would pollute the environment. What was worse, those strains had difficulty in mineralizing 1,4-dioxane (Vainberg et al. 2006; Kristin et al. 2009). Thus, some organisms with the ability to use 1,4-dioxane as a sole carbon and energy source need to be further discovered and evaluated. So far, only a few aerobic microorganisms have the said ability including Rhodococcus 219, Pseudonocardia dioxanivorans CB1190, the fungus Cordyceps sinensis, Mycobacterium sp. PH-06, and Afipia sp. D1 (Bernhardt and Diekmann 1991; Parales et al. 1994; Nakamiya et al. 2005; Kim et al. 2009; Sei et al. 2013). Regrettably, those organisms usually have low cell yield with the highest cell yield of 0.223 mg-protein (mg-1,4-dioxane)−1 from the Afipia sp. D1 (Sei et al. 2013). The efficiency of resource utilization during growth is the significant factor for large-scale application. So, it is still urgent to get an organism with a higher efficiency to use 1,4-dioxane to meet the demand of application. Additionally, it is known to all that a thorough understanding of the biotransformation process can help to achieve an effective control of 1,4-dioxane bioremediation; so, further investigation of the 1,4-dioxane pathway is necessary.
Thus, a Gram-negative strain DD1 was isolated and identified in this study to effectively utilize 1,4-dioxane as the sole carbon and energy source. In addition, the substrate tolerance and the biodegradation of 1,4-dioxane were also studied. Finally, the 1,4-dioxane-degrading pathway by the bacterium was deduced based on gas chromatography/mass spectroscopy (GC/MS) analysis.
2 Materials and Methods
2.1 Reagents and Medium
1,4-Dioxane (99 % purity) was purchased from Aladdin Company (Shanghai, China). All other reagents used in this study were of analytical reagent grade and purchased from Huipu Company (Hangzhou, China). R2A liquid medium with pH 7.2 consisted of (per liter of distilled water) 0.5 g yeast extract, 0.5 g tryptone, 0.5 g casamino acid, 0.5 g glucose, 0.5 g soluble starch, 0.3 g sodium pyruvate, 0.3 g K2HPO4, and 0.05 g MgSO4·7H2O. Mineral salt medium (MSM) containing 1,4-dioxane had the following composition: 3.5 g Na2HPO4·2H2O, 1 g KH2PO4, 0.5 g (NH4)2SO4, 0.1 g MgCl2·6H2O, 50 mg Ca(NO3)2·4H2O, and 1 mL of trace elements solution in 1 L distilled water and adjusted to pH 7.0. The trace element solution contained the following (per liter): 1.0 g FeSO4·7H2O, 0.02 g CuSO4·5H2O, 0.014 g H3BO3, 0.10 g MnSO4·4H2O, 0.10 g ZnSO4·7H2O, 0.02 g Na2MoO4·2H2O, and 0.02 g CoCl2·6H2O. The concentration of 1,4-dioxane added to the MSM was set based on the requirement of each experiment. All solid medium contained 2.0 % agar.
2.2 Isolation of 1,4-Dioxane Degradation Organisms
The activated sludge sample was obtained from Qige urban sewage treatment plant in Zhejiang, China. The sample was cultured in 250 mL bottles with 50 mL MSM at 30 °C and 130 rpm to enrich the 1,4-dioxane-degrading microorganisms with 100 mg/L 1,4-dioxane for about 3 months. Then, degradation of 1,4-dioxane was observed, and 5 mL of the culture was transferred to the same new medium. After repeating the same procedure three times to enrich the 1,4-dioxane degradation microorganisms, the culture was transferred to the solid R2A medium. After incubation at 30 °C for 2–4 days, a single colony was selected and transferred to MSM with 1,4-dioxane as the sole carbon and energy source for testing the 1,4-dioxane-degrading ability.
2.3 Identification and Characterization of 1,4-Dioxane-Degrading Bacterium
After five or more generations, the 1,4-dioxane-degrading bacterium was confirmed to be purified by a light microscope. Carbon substrate utilization and chemosensitivity were examined by the Biolog MicroStation System/MicroLogTM (GNIII; Biolog Hayward, CA, USA).
Chromosomal DNA of the bacterial isolate was extracted according to the instructions from the Bacterial DNA Extraction Kit (Spin-column) purchased from Bioteke Corporation (Beijing, China). The 16S ribosomal DNA (rDNA) gene were PCR amplified with primers 27F and 1492R. The PCR conditions were as follows: 5 min at 94 °C; 45 cycles of denaturation for 45 s at 94 °C, annealing for 40 s at 55 °C, and extension for 90 s at 72 °C; and one final step of 5 min at 72 °C. The purified PCR products containing the 16S rDNA were performed according to the instructions from Shanghai Sangon Biotech Corporation (Shanghai, China). The obtained 16S rDNA sequence was aligned to sequences in GenBank using the BLAST program. The aligned 16S rDNA sequences of the related species were retrieved from the NCBI nucleotide database. The program Clustal X (version 1.8) with default parameters was run for multiple sequence alignment. Phylogenetic and distance analysis of the aligned sequences was performed by the program MEGA (version 5.0).
2.4 Biodegradation of 1,4-Dioxane by DD1
The strain DD1 was respectively grown in 50 mL of R2A and MSM (100 mg/L 1,4-dioxane) in 250 mL bottles and incubated at 30 °C and 130 rpm. Cells of strain DD1 were harvested at the exponential growth phase by centrifugation (22,000×g) for 10 min. Cells were washed twice with 0.85 % (w/v) NaCl and resuspended in MSM for further use.
Incubation with R2A-grown cell suspensions was conducted in order to find out whether the 1,4-dioxane-degrading ability of strain DD1 cells was induced by 1,4-dioxane in the culture medium. In this experiment, each 250 mL bottle was supplemented with 72.5 mg/L 1,4-dioxane, 50 mL of MSM, and 60 mg/L of 1,4-dioxane-grown or R2A-grown cell suspensions.
Acetylene is an inhibitor of monooxygenase activity. To investigate the effect of acetylene on 1,4-dioxane degradation, R2A-grown cell suspensions were exposed to 5 % acetylene in the headspace (Mahendra and Alvarez-Cohen 2006). In this experiment, each 250 mL bottle was supplemented with 72.5 mg/L 1,4-dioxane, 50 mL MSM, and 60 mg/L R2A-grown cell suspensions. Samples were removed for analysis of the residual 1,4-dioxane at designated intervals during degradation. Each experiment was performed in triplicate and included abiotic controls.
2.5 Biological Tolerance of 1,4-Dioxane by DD1
Batch experiments of DD1 were taken in 250 mL bottles with 50 mL MSM and the initial concentrations of the cells were 8 mg/L. 1,4-Dioxane was added as the sole carbon. Cultures were incubated at 30 °C and 130 rpm, and the initial 1,4-dioxane concentrations from 100 to 1000 mg/L were studied. Bacterial growth was measured by absorbance at OD600. Samples were removed for analysis of the residual 1,4-dioxane at designated intervals during degradation. Each experiment was performed in triplicate and included abiotic controls.
2.6 Substrate Range of DD1
To examine the ability of DD1 to degrade different substrates, batch experiments of DD1 were taken in 250 mL bottles with 50 mL MSM, and the initial concentrations of the cells were 8 mg/L. Some important compounds were selected, including cyclic ethers, chlorinated solvents, benzene homologues, alkanes, and other substrates. The initial concentrations of each substrate were 1 mM, and samples were collected and analyzed every 12 h in 120 h. Each experiment was performed in triplicate and included abiotic controls.
2.7 Analytical Methods
Cultures were centrifuged at 22,000×g for 10 min, and the supernatant was analyzed for 1,4-dioxane concentration by gas chromatography (Agilent 7890A) equipped with a silica HP-INNOWAX capillary column (30 m × 0.32 mm × 0.5 μm, J&W Scientific, USA) and a flame ionization detector. The injector and temperatures were set at 250 and 300 °C, respectively. The temperature program was 70 °C as initial temperature, increased by 20 °C/min to 180 °C, and kept for 1 min.
To analyze the intermediates during 1,4-dioxane biodegradation, cultures were centrifuged at 22,091×g for 10 min, and the supernatant was subsequently extracted with ethyl acetate (V sample:V ethyl acetate = 1:1) by vigorously shaking for 30 min. After standing for 10 min, the organic layer was collected and then evaporated to dryness under N2 stream. The dry residue was derivatized by the addition of 50 μL pyridine and 100 μL bis-(trimethyl-silyl)-trifluoroacetamide (BSTFA), which were preheated in a heating block at 65 °C for 30 min. After derivatization, the extracts were ready for injecting into the GC/MS (Zhou et al. 2011).
The GC/MS analysis of biodegradation intermediates was performed, using a GC (Agilent 6890) equipped with an inert mass selective detector (Agilent 5973), on a silica HP-5 capillary column (30 m × 0.25 m × 1 μm, J&W Scientific, USA) with a splitless mode using helium as carrier gas at a flow rate of 1.0 mL/min. The MS was operated in full scan or selected ion monitoring mode with positive ionization by electron impact. The inlet and MS transfer line temperatures were maintained at 250 °C, and the ion source temperature was 250 °C. The following GC oven temperature was applied: 40 °C as initial temperature for 5 min, increased by 5 °C/min to 150 °C, then programmed to raise to 200 °C at the speed of 10 °C/min, and maintained for 3 min. The intermediates were identified on the basis of mass spectra using the mass spectral library NIST08.L.
Biomass concentrations (expressed in mg dry weight/L) were measured by optical density (OD) at 600 nm with a UV spectrometer. The OD measurements were converted to dry weight concentrations in strain DD1 by an established calibration curve.
3 Result and Discussion
3.1 Isolation of 1,4-Dioxane-Degrading Strains
Due to the particularity structure, 1,4-dioxane is recalcitrant to biodegradation and remains persistent in the environment. Since the degradation rate is the bottleneck in 1,4-dioxane bioremediation process, isolating a strain with high 1,4-dioxane degradation activity is the key to solve the 1,4-dioxane contamination problem. After 3 months of enrichment, several pure strains were isolated from the sludge sample obtained from Qige urban sewage treatment plant in Zhejiang, China. Additionally, strain DD1 was chosen as the test bacterial strain for future study because of its relatively higher degrading efficiency. Now, strain DD1 has been deposited in the China Center for Type Culture Collection (CCTCC), Wuhan, China, under accession no. CCTCC M 2013560.
3.2 Characterization and Identification of Strain DD1
Microscopic observation demonstrated that the strain DD1 was a Gram-negative, short rod, aerobic, and no-spore bacterium. The strain grew at temperatures from 20 to 40 °C and at pH values from 5.0 to 8.0. Oxidase reaction and contact enzyme reaction for strain DD1 were negative, whereas the liquefaction of gelatin, nitrate reduction, and citric acid reduction were positive. These data indicated that strain DD1 resembled a member of Acinetobacter genus.
A Biolog GNIII was employed to determine the capacity of strain DD1 in substrate utilization. As shown in Table 1, a total of 30 carbon substrates were easily utilized. In addition, 12 of 23 substrates displayed no inhibition on DD1 growth after 21 h of incubation. According to Biolog GNIII identification, the degradation profile was similar to that of Acinetobacter genus.
The partial 16S rDNA of DD1 was sequenced and deposited in GenBank. After alignment with other 16S rDNA sequences in GenBank, it showed a high degree of similarity (100 %) to Acinetobacter baumannii AQ-3 (JF751054.1), and a phylogenetic tree was constructed in Fig. 1. The result of this phylogenetic analysis was consistent with that of the phenotypic test.
Based on the results of the physiological and biochemical characterization, 16S rDNA sequencing, and the phylogenetic tree, strain DD1 was identified as A. baumannii, and named A. baumannii DD1. In addition, the 16S rDNA gene sequences of strain DD1 were deposited in the National Center for Biotechnology Information (NCBI) GenBank with accession no. KF713537.
Some other reports, which focused on rapid degradation of environmental pollutants by Acinetobacter sp. TW, showed that 1 g/L nicotine can be degraded within 12 h to an undetectable level (Wang et al. 2011). Acinetobacter sp. SJ-15 is capable of growing with phenol and n-hexadecane. It can completely degrade phenol (400 mg/L) and n-hexadecane (400 mg/L) in 24 and 60 h, respectively (Sun et al. 2012a). However, this is the first report of a bacterium from the Acinetobacter genus capable of degrading 1,4-dioxane.
3.3 Biodegradation of 1,4-Dioxane by DD1
As shown in Fig. 2, a dynamic curve was observed under the following culture conditions: pH 7, 30 °C, and 100 mg/L 1,4-dioxane. The 1,4-dioxane was completely degraded by the isolate DD1 in 42 h with the cell yield of 0.414 mg-protein (mg-1,4-dioxane)−1 and the generation time of 6.75 h. Indeed, several studies have been conducted to screen the bacteria capable of utilizing 1,4-dioxane as the sole carbon and energy source. For example, P. dioxanivorans CB1190, isolated from a 1,4-dioxane contaminated sludge using 1,4-dioxane as the sole carbon and energy source, had a generation time of 30 h (Parales et al. 1994). Also, throughout the previous investigations, the Afipia sp. had the shortest generation time of about 18 h (Sei et al. 2013). Satisfactorily, A. baumannii DD1 had a much shorter generation time of 6.75 h than any other reported studies.
Typical profile of 1,4-dioxane degradation and concomitant growth of strain DD1. The initial culture density and 1,4-dioxane concentration were 8 and 100 mg/L, respectively. Symbols: biomass (filled square) and 1,4-dioxane concentration (filled triangle). Error bars indicate the standard deviation of three replicates
Achieving successful growth of the pollutant-degrading culture is a key to bioaugmentation. To date, the experimental cell yield on 1,4-dioxane all appears to be lower than 0.223 mg-protein (mg-1,4-dioxane)−1 (Sei et al. 2013). Based on this work, the cell yield on 1.4-dioxane from DD1 was 0.414 mg-protein (mg-1,4-dioxane)−1 with initial 1,4-dioxane concentration of 100 mg/L, almost twofold higher than Afipia sp. D1. To evaluate the value of cell yield on 1,4-dioxane and energy loss during the metabolism process, the energy discrepancy index (δ e), defined as the ratio of the theoretical to the experimental cell yield (Fortin et al. 2001), is introduced in this work. The index value of 1.10 (greater than 1) indicated that some energy share might be lost during the 1,4-dioxane degradation by DD1. Based on the analogy of the results shown by Fortin et al. (2001) for degrading MTBE which also had a carbon ether bond, the cleavage of carbon ether bond for 1,4-dioxane perhaps was the main cause of energy discrepancy index.
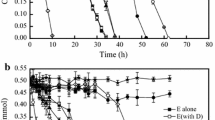
It is noteworthy that the biodegradation of 1,4-dioxane by 1,4-dioxane-grown cells was faster than that by R2A-grown cells under the same incubation conditions (Fig. 3). Prolonged incubation of rich medium-grown cells with 1,4-dioxane resulted in the complete degradation of 1,4-dioxane within 75 h, while that of 1,4-dioxane-grown cells resulted in a complete degradation within 25 h without any lag phase. This result showed that the 1,4-dioxane degradation enzyme could be induced. Similar to strain DD1, P. dioxanivorans CB1190 (Parales et al. 1994) also had inducible 1,4-dioxane degradation enzymes, whereas strains D11 and D17 had constitutive enzymes (Sei et al. 2013). Thus, it implied that degradation mechanisms varied with different kinds of 1,4-dioxane-degrading bacteria.
Biodegradation of 1,4-dioxane by R2A-grown cell suspensions and 1,4-dioxane-grown cell suspensions. The initial culture density and 1,4-dioxane concentration were 60 and 72.5 mg/L, respectively. Symbols: 1,4-dioxane concentration by 1,4-dioxane-grown cells (filled square), 1,4-dioxane concentration by R2A-grown cells (filled circle), and 1,4-dioxane concentration by R2A-grown cells with 5 % acetylene (filled triangle). Error bars indicate the standard deviation of three replicates
Also shown in Fig. 3, the 1,4-dioxane biodegradation was completely inhibited in the presence of acetylene, a known monooxygenase inhibitor, supporting the hypothesis that monooxygenase (MMO) is responsible for catalyzing the initial oxidation of 1,4-dioxane in strain DD1. Similar phenomena were reported in the dioxane degradation processes by P. dioxanivorans CB1190 and Pseudonocardia benzenivorans B5 (Mahendra and Alvarez-Cohen 2006).
3.4 Tolerance of 1,4-Dioxane by DD1
The substrate tolerance of DD1 was investigated at different 1,4-dioxane concentrations from 100 to 1,000 mg/L, and the results are shown in Fig. 4. When the 1,4-dioxane concentration was under 400 mg/L, the substrate was degraded quickly by strain DD1 within 84 h. The values of the average degradation rate increased as 1,4-dioxane concentration increased, and the max value was 4.76 mg/L/h when the concentration was up to 400 mg/L. While the concentration was over 400 mg/L, the average degradation rates were reduced. The reduction of average degradation rates in the case of high substrate concentration was probably due to the toxicity of 1,4-dioxane and metabolites (Kano et al. 2010). Further increasing 1,4-dioxane concentration to 1,000 mg/L, strain DD1 exhibited an excellent substrate tolerance and completely degraded 1,4-dioxane within 264 h. As reported, strains Afipia sp. D1 and P. dioxanivorans CB1190 had 1,4-dioxane tolerance of 450 and 880 mg/L, respectively. To date, strain Mycobacterium sp. PH-06 had the highest 1,4-dioxane tolerance of 1,000 mg/L 1,4-dioxane, but it could not completely degrade such high concentration even in 360 h. In this sense, strain DD1 was superior than PH-06, bearing the highest 1,4-dioxane tolerance with higher degrading activity. As it is known to all, low Pow is defined as the common logarithm of the partition coefficient (Pow) of each organic solvent between n-octanol and water, and an organic solvent with lower log Pow value is more toxic for microorganisms (Inoue and Horikoshi 1989). Additionally, 1,4-dioxane was a solvent widely used in a range of industrial organic products and its log Pow was −0.27. As reported by Inoue and Horikoshi (1991), the tolerance of Acinetobacter to organic solvent was better than most of the microorganisms, which was perhaps a reason for A. baumannii DD1 to have a better tolerance to 1,4-dioxane. Another reason for the outstanding tolerance of DD1 was possibly that as a Gram-negative organism, its outer membrane contained more lipopolysaccharides, which had a function to chelate with divalent metal ions to resist organic solvent (Inoue and Horikoshi 1991).
1,4-Dioxane degradation by DD1 in the presence of different substrate concentrations. The initial culture density in each bottle was 8 mg/L. The symbols indicate concentrations of 100 mg/L (filled square), 200 mg/L (filled circle), 300 mg/L (filled triangle), 400 mg/L (filled inverted triangle), 500 mg/L (filled diamond), 1,000 mg/L (filled star), abiotic control (times symbol). Error bars indicate the standard deviation of three replicates
3.5 Substrate Spectrum of DD1
As shown in Table 2, strain DD1 exhibited a great versatility in utilizing a variety of substrates including n-hexane, alcohol, n-butyl alcohol, and phenol, aside from 1,4-dioxane. Furthermore, growth was also observed on 1,3-dioxane and THF, similar with other 1,4-dioxane degraders (Kohlweyer et al. 2000; Sara et al. 2001; Kim et al. 2009). Regrettably, TCA, DCE, and trichloromethane, which generally co-existed with 1,4-dioxane in groundwater, could not be used by DD1, even with 1,4-dioxane, but supported the growth of other 1,4-dioxane degraders in the presence of 1,4-dioxane (Mahendra et al. 2013).
It is worth noting that benzene, which has never been found to be metabolized by 1,4-dioxane-degrading strain till now, could be utilized as the sole carbon and energy source by A. baumannii DD1. Interestingly, a diauxic biodegradation curve occurred in the cells of DD1 precultured by 1,4-dioxane showing a phenomenon that the degradation of 1,4-dioxane was not started until benzene was completely degraded (data not shown). It implied that benzene was the preferential utilization substrate for DD1. Furthermore, the benzene homologues, such as toluene, ethylbenzene, o-xylene, and p-xylene, could also support the growth of DD1 strain as the sole carbon and energy source, possibly because benzene homologues were degraded by the enzyme similar with that of benzene (Zhou et al. 2011), while m-xylene, as an exception, could not support the growth of DD1 strain as the carbon and energy source, possibly for the spatial structure leading to their resistance to biodegradation (Zhou et al. 2011). Additionally, previous studies showed that benzene and its homologue always co-existed with 1,4-dioxane in industrial process (Anil et al. 2008) and gas service stations (Yimrungruang et al. 2008); so, the substrate interactions during the biodegradation of benzene homologues and 1,4-dioxane should be given increased attention and careful investigations in the future.
3.6 Probable Metabolic Pathway of 1,4-Dioxane
Figure 5a shows the chromatogram of metabolite I obtained from GC/MS. The retention time of one peak was 11.18 min, which is shown by the MS fragmentation pattern in Fig. 5b, identified by MS as oxalic acid in the trimethylsilated (TMS) form. Another metabolite II peak with a retention time of 11.30 min was achieved. This metabolite had an M+ at m/z 205 and fragmentation ions at m/z 191 [M+−45, –CH2– loss], 73 [M+−132, –C5H13O2Si loss], and 131 [M+−74, loss of C2H6O2 from the m/z 191 ion] (Fig. 5c). Based on the fragmentation pattern, metabolite II was identified as ethylene glycol in the TMS form. During the biodegradation of 1,4-dioxane by the fungus C. sinensis strain A (Nakamiya et al. 2005), ethylene glycolic and oxalic acid were also detected as intermediates, and a degradation pathway was proposed based on those detections.
As shown in Fig. 5d, there were two peaks that appeared in the chromatogram by purge-and-trap coupled with gas chromatography. The first peak with a retention time of 2.358 was identified as 1,4-dioxene since it provided an M+ at m/z 86 by GC/MS, and the other one was the substrate 1,4-dioxane. In fact, 1,4-dioxene was detected during the prophase and metaphase of 1,4-dioxane degradation with a quite stable concentration. Until the later stage, 1,4-dioxene concentration declined to an undetectable level (data not shown). It implied that 1,4-dioxene was an important intermediate during the biodegradation of 1,4-dioxane by DD1.
There were several researches about the intermediates of dioxane degradation. Parales et al. (1994) firstly detected 2-hydroxyethoxyacetic acid (HEAA) as the major degradation product during the degradation of 1,4-dioxane by P. dioxanivorans CB1190. Vainberg et al. (2006) also observed the accumulation of HEAA in the degradation process by Pseudonocardia sp. strain ENV478 and proposed 1,4-dioxane-2-ol or 2-hydroxyethoxy-2-acetaldehyde as the first product. Then, 1,4-dioxane-2-ol was determined as the first product through monooxygenation in Mycobacterium sp. PH-06 (Kim et al. 2009). According to the result that exposure to acetylene gas inhibited dioxane degradation (shown in Fig. 3), the biodegradation of 1,4-dioxane was initiated by monooxygenase and perhaps also occurred through monooxygenation to form 1,4-dioxane-2-ol by DD1. However, during further biodegradation, HEAA was not detected but 1,4-dioxene, suggesting that a reductive reaction might be involved in 1,4-dioxane degradation by DD1. Although the reductive reaction is not the typical behavior in aerobic bacteria, such reactions were ever precedented. For example, the reductive dehalogenation of 3,4-dichloroaniline occurs under aerobic condition by Acinetobacter baylyi strain GFJ2 (Hongsawat and Vangnaib 2011). The detection of 1,4-dioxene implied a new pathway in 1,4-dioxane degradation by A. baumannii DD1. The mechanism and the enzyme involved in the reductive reaction in DD1 have yet to be investigated. On the basis of GC and GC/MS analysis of metabolites, the potential for 1,4-dioxane-degrading pathway by A. baumannii DD1 is shown in Fig. 6.
4 Conclusion
A. baumannii DD1 was isolated as a bacterium capable of efficient 1,4-dioxane biodegradation. We confirm for the first time that 1,4-dioxane-degrading bacterium from the Acinetobacter genus has the highest cell yield of 0.414 mg-protein (mg-1,4-dioxane)−1 and the shortest generation time of 6.75 h. Strain DD1 had the highest tolerance up to 1,000 mg/L. Besides 1,4-dioxane, DD1 could also degrade other contaminants such as 1,3-dioxane, THF, phenol, toluene, and benzene as the sole carbon and energy sources. Additionally, this study also presented a novel pathway for dioxane biodegradation. However, the mechanism and the enzyme involved in this reaction require further investigation.
References
Agency for Toxic Substances and Disease Registry (ATSDR). (2012). Toxicological profile for 1,4-dioxane. United States Department of Health and Human Services, Public Health Service, Atlanta, GA. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=955&tid=199. Accessed 1 Oct 2012
Anil, K., Nain, P. C., Jata, D. P., & Swarita, G. (2008). Densities, refractive indices, and excess properties of binary mixtures of 1,4-dioxane with benzene, toluene, o-xylene, m-xylene, p-xylene, and mesitylene at temperatures from (288.15 to 318.15) K. Journal of Chemical and Engineering Data, 53, 2654–2665.
Bernhardt, D., & Diekmann, H. (1991). Degradation of dioxane, tetrahydrofuran and other cyclic ethers by an environmental Rhodococcus strain. Applied Microbiology and Biotechnology, 36, 120–123.
Burback, B. L., & Perry, J. J. (1993). Biodegradation and biotransformation of groundwater pollutant mixtures by Mycobacterium vaccae. Applied and Environmental Microbiology, 59, 1025–1029.
Coleman, H. M., Vimonses, V., & Leslie, G. (2007). Degradation of 1,4-dioxane in water using TiO2 based photocatalytic and H2O2/UV processes. Journal of Hazardous Materials, 146, 496–501.
Fortin, N. Y., Morales, M., Nakagawa, Y., Focht, D. D., & Deshusses, M. A. (2001). Methyltert-butyl ether (MTBE) degradation by a microbial consortium. Environmental Microbiology, 3, 407–416.
Hongsawat, P., & Vangnaib, A. S. (2011). Biodegradation pathways of chloroanilines by Acinetobacter baylyi strain GFJ2. Journal of Hazardous Materials, 186, 1300–1307.
Inoue, A., & Horikoshi, K. (1989). A Pseudomonas thrives in high concentration of toluene. Nature, 338, 264–266.
Inoue, A., & Horikoshi, K. (1991). Estimation of solvent-tolerance of bacteria by the solvent parameter log P. Journal of Fermentation and Bioengineering, 71, 194–196.
Kano, H., Umeda, Y., Kasai, T., Sasaki, T., Matsumoto, M., Yamazaki, K., Nagano, K., Arito, H., & Fukushima, S. (2010). Carcinogenicity studies of 1,4-dioxane administered in drinking-water to rats and mice for 2 years. Food and Chemical Toxicology, 47, 2776–2784.
Kim, Y. M., Jeon, J. R., Murugesan, K., Kim, E. J., & Chang, Y. S. (2009). Biodegradation of 1,4-dioxane and transformation of related cyclic compounds by a newly isolated Mycobacterium sp. PH-06. Biodegradation, 20, 511–519.
Kohlweyer, U., Thiemer, B., Schrader, T., & Andreesen, J. R. (2000). Tetrahydrofuran degradation by a newly isolated culture of Pseudonocardia sp. strain K1. FEMS Microbiology Letters, 186, 301–306.
Kristin, S., Lynda, C., & Michael, H. (2009). Metabolism and cometabolism of cyclic ethers by a filamentous fungus, a Graphium sp. Applied and Environmental Microbiology, 75, 5514–5522.
Li, M. Y., Mathieu, J., Yang, Y., Fiorenza, S., Deng, Y., He, Z. L., Zhou, J. Z., & Alvarez, P. J. J. (2013). Widespread distribution of soluble di-Iron monooxygenase (SDIMO) genes in arctic groundwater impacted by 1,4-dioxane. Environmental Science and Technology, 47, 9950–9958.
Lu, D. H., & Xia, A. J. (2003). Study of comment method uncovering area for harmful material caused by treatment of waste covering. Chinese Journal of Environmental Management, 22, 39–40.
Mahendra, S., & Alvarez-Cohen, L. (2006). Kinetics of 1,4-dioxane biodegradation by monooxygenase-expressing bacteria. Environmental Science and Technology, 40, 5435–5442.
Mahendra, S., Ariel, G., & Alvarez-Cohen, L. (2013). The impact of chlorinated solvent co-contaminants on the biodegradation kinetics of 1,4-dioxane. Chemosphere, 91, 88–92.
Nakamiya, K., Hashimoto, S., Ito, H., Edmonds, J. S., & Morita, M. (2005). Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus. Applied and Environmental Microbiology, 71, 1254–1258.
Parales, R. E., Adamus, J. E., White, N., & May, H. D. (1994). Degradation of 1,4-dioxane by an Actinomycete in pure culture. Applied and Environmental Microbiology, 60, 4527–4530.
Sara, L. K., Eric, W. A., Milind, D., Jerald, L. S., & Pedro, J. J. A. (2001). Biodegradation of 1,4-dioxane in planted and unplanted soil: effect of bioaugmentation with amycolata sp. CB1190. Water Research, 35, 3791–3800.
Sei, K., Miyagaki, K., Kakinoki, T., Fukugasako, K., Inoue, D., & Ike, M. (2013). Isolation and characterization of bacterial strains that have high ability to degrade 1,4-dioxane as a sole carbon and energy source. Biodegradation, 24, 665–674.
Son, H. S., Im, J. K., & Zoh, K. D. (2008). A Fenton-like degradation mechanism for 1,4-dioxane using zero-valent iron (FeO) and UV light. Water Research, 43, 1457–1463.
Sun, J. Q., Xu, L., Tang, Y. Q., Chen, F. M., & Wua, X. L. (2012a). Simultaneous degradation of phenol and n-hexadecane by Acinetobacter strains. Bioresource Technology, 123, 664–668.
Sun, W. M., Sun, X. X., & Cupples, A. M. (2012b). Anaerobic MTBE degrading microorganisms identified in wastewater treatment plant samples using stable isotope probing. Applied and Environmental Microbiology, 78, 2973–2980.
Vainberg, S., McClay, K., Masuda, H., Root, D., Condee, C., Gerben, J. Z., & Robert, J. S. (2006). Biodegradation of ether pollutants by Pseudonocardia sp. strain ENV478. Applied and Environmental Microbiology, 72, 5218–5244.
Wang, M. Z., Yang, G. Q., Wang, X., Yao, Y. L., Min, H., & Lu, Z. M. (2011). Nicotine degradation by two novel bacterial isolates of Acinetobacter sp. TW and Sphingomonas sp. TY and their responses in the presence of neonicotinoid insecticides. World Journal of Microbiology and Biotechnology, 27, 1633–1640.
Yimrungruang, D. D., Cheevaporn, V., Boonphakdeeb, T., Watchalayann, P., & Helander, H. F. (2008). Characterization and health risk assessment of volatile organic compounds in gas service station workers. EnvironmentAsia, 2, 21–29.
Zhou, Y. Y., Chen, D. Z., Zhu, R. Y., & Chen, J. M. (2011). Substrate interactions during the biodegradation of BTEX and THF mixtures by Pseudomonas oleovorans DT4. Bioresource Technology, 102, 6644–6649.
Acknowledgments
This work was financially supported by the Fundamental Research Funds for Zhejiang Gongshang University [Grant No. X13-14 and No. X13-01].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, H., Shen, D., Li, N. et al. Biodegradation of 1,4-Dioxane by a Novel Strain and Its Biodegradation Pathway. Water Air Soil Pollut 225, 2135 (2014). https://doi.org/10.1007/s11270-014-2135-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2135-2