Abstract
1,4-Dioxane is a highly toxic and carcinogenic pollutant found worldwide in groundwater and soil environments. Several microorganisms have been isolated by their ability to grow on 1,4-dioxane; however, low 1,4-dioxane tolerance and slow degradation kinetics remain obstacles for their use in 1,4-dioxane bioremediation. We report here the isolation and characterization of a new strain, Xanthobacter sp. YN2, capable of highly efficient 1,4-dioxane degradation. High degradation efficiency and high tolerance to 1,4-dioxane make this new strain an ideal candidate for the biodegradation of 1,4-dioxane in various treatment facilities. The maximum degradation rate of 1,4-dioxane was found to be 1.10 mg-1,4-dioxane/h mg-protein. Furthermore, Xanthobacter sp. YN2 was shown to grow in the presence of higher than 3000 mg/L 1,4-dioxane with little to no degradation inhibition. In addition, Xanthobacter sp. YN2 could grow on and degrade 1,4-dioxane at pH ranges 5 to 8 and temperatures between 20 and 40 °C. Xanthobacter sp. YN2 was also found to be able to grow on a variety of other substrates including several analogs of 1,4-dioxane. Genome sequence analyses revealed the presence of two soluble di-iron monooxygenase (SDIMO) gene clusters, and regulation studies determined that all of the genes in these two clusters were upregulated in the presence of 1,4-dioxane. This study provides insights into the bacterial stress response and the highly efficient biodegradation of 1,4-dioxane as well as the identification of a novel Group-2 SDIMO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
1,4-Dioxane has been listed as a Group 2B carcinogen since 1999 by the International Agency for Research on Cancer (IARC). 1,4-Dioxane is highly water soluble while its volatilization from water occurs slowly [1, 2]. This high level of water solubility has led to its wide use as an industrial solvent. 1,4-Dioxane is also an unwanted byproduct in the industrial manufacture of food, medicine, and personal care products [3]. High-industrial use has led to 1,4-dioxane pollution of many aquatic environments [4]. High solubility and low evaporation rates complicate remediation efforts to remove 1,4-dioxane from these environments, thereby increasing the threat to public safety [2, 5,6,7,8,9]. One of the main sources of 1,4-dioxane entering the environment is via industrial sewage. Wastewater treatment plants (WWTPs) employing the use of biodegradation processes would be a very desirable and cost-effective method to deal with 1,4-dioxane contamination [10]. A major drawback in the use of bioremediation, in this instance, is the lack of highly efficient 1,4-dioxane microbial degraders being suitable for application.
Kinetic studies of the isolates have demonstrated that they may not be ideal for environmental applications. The total cell yields of Gram-positive 1,4-dioxane degraders tended to be quite low when compared to Gram negatives, thereby leading to lower rates of 1,4-dioxane degradation. To date, several microorganisms capable of growing on 1,4-dioxane have been reported, yet the number of Gram-negative isolates is quite small [11,12,13,14,15,16]. To accelerate growth rates and increase cell yields, additional nutrients or analogs of 1,4-dioxane are necessities in bioremediation applications using these strains [16,17,18,19]. These additives have the undesirable effect of increasing costs and the possibility of introducing additional environmental pollution if they are not completely mineralized [16, 17, 20]. Therefore, investigations into the isolation and characterization of 1,4-dioxane degraders with higher growth rates and cell yields are critical to efficient and safe biodegradation of 1,4-dioxane.
Soluble di-iron monooxygenase (SDIMO) has been shown to be the key genes to the degradation of 1,4-dioxane [21]. Enrichment of SDIMO genes at 1,4-dioxane-contaminated sites has been shown to be directly related to bacterial 1,4-dioxane degradation activity [22, 23]. To date, only two SDIMOs have been confirmed to be involved in metabolic degradation of 1,4-dioxane: Group-5 SDIMO gene cluster thmADBC [24] and Group-6 SDIMO gene cluster prmABCD [25, 26]. Thus, to determine the importance of SDIMOs in 1,4-dioxane, biodegradation requires further research.
In this study, Xanthobacter sp. YN2 was isolated from sludge of a municipal WWTP for its ability to grow on 1,4-dioxane. Bacterial characteristics and kinetics of degradation and growth in the presence of various concentrations of 1,4-dioxane as well as under various culture conditions were investigated. Genomic analysis revealed a gene cluster on the chromosome that was predicted to encode a novel Group-2 SDIMO, which was phylogenetically distant from other previously reported SDIMOs involved in 1,4-dioxane degradation. Regulation studies showed that all the genes in this new SDIMO gene cluster were constitutive.
Materials and Methods
Reagents and Culture Media
1,4-Dioxane, 1,3-dioxane, tetrahydrofuran, ethanol, n-hexane, cyclohexane, toluene, ethyl acetate, and methanol were of analytical grade (J&K Scientific Ltd.). Basal salts medium (BSM) and ammonium mineral salts medium (AMS) were prepared by the method of Parales et al. [18]. For solid media, 1.8% (wt/vol) Nobel agar was added. All experiments that involved culture media supplemented with 1,4-dioxane were carried out in headspace bottles sealed with Teflon to limit volatilization.
Analysis of 1,4-Dioxane and Total Oxidizable Carbon (TOC)
Cultures were filtered through nylon filters (0.45 μm pore size), and 1 μL of each sample was analyzed with an Agilent 7890 gas chromatograph (GC) equipped with an INNOWH4 column (30 m × 0.53 mm × 1.0 μm) and a flame ionization detector (FID). The initial column temperature was 45 °C, which was maintained for 3 min, then raised to 70 °C at a rate of 15 °C /min. The temperatures of the inlet and the detector were 250 °C and 300 °C, respectively. The carrier gas was hydrogen/air (400/40), and the flow rate was 6 mL/min. Total oxidizable carbon (TOC) of filtered samples was analyzed with an Analytikjena Multi N/S 2100S TOC analyzer.
Enrichment, Isolation, and Identification of Xanthobacter sp. YN2
The enrichment was started with active sludge from a secondary sedimentation basin of a WWTP in Harbin, China. Sludge (5 g) was added to 100 mL BSM containing 200 mg/L 1,4-dioxane and incubated at 30 °C. Dilutions were carried out weekly by transferring 20% of each culture into fresh BSM with 200 mg/L 1,4-dioxane until 1,4-dioxane degradation was observed (approximately 3 months). Once the 1,4-dioxane was depleted, dilutions into fresh AMS containing 200 mg/L 1,4-dioxane were performed, approximately every few days. When the rate of 1,4-dioxane degradation stabilized, the culture was diluted and spread onto AMS plates containing 200 mg/L 1,4-dioxane to isolate 1,4-dioxane degrading single colonies.
The isolate was identified by 16S rRNA gene sequence analysis. The sequence of the 16S rRNA gene was determined by Sangon Biotech Co., Ltd. (Shanghai, China). Bacteria genome was extracted using Ezup column bacteria genomic DNA purification kit (SK8255) and used as the template for PCR. Primer 7F (5′-CAGAGTTTGATCCTGGCT-3′) and primer 1540R (5′-AGGAGGTGATCCAGCCGCA-3′) were used for sequencing. The thermal profile was as follow: initial denaturation at 94 °C for 4 min; 30 cycles of denaturation at 94 °C for 45 s; annealing at 55 °C for 45 s; extension at 72 °C for 1 min; and final extension at 72 °C for 10 min. The 16 S rRNA gene sequence was deposited in GenBank under accession number MK256301.
Determination of Optimal Growth Conditions
Four different factors involved in growth and degradation of 1,4-dioxane were tested: temperature, pH, aeration, and initial OD660 (optical density at 660 nm). All experiments were carried out in 50 mL Teflon sealed vials filled with 10 mL of AMS containing 200 mg/L 1,4-dioxane. Experiments were carried out in duplicate and repeated three times.
Unless otherwise stated, the following experiments were carried out at 30 °C, pH 7.0, with initial OD660 = 0.007 and shaken at 160 rpm. To determine the optimum growth temperature, cultures were incubated at 10, 20, 30, or 40 °C. The optimum pH for growth was determined by growth as described above except that the pH of the media was adjusted to 5.0, 6.0, 7.0, or 8.0 with 1 M HCl or 1 M NaOH if necessary before inoculation, and the pH of each culture was checked at the end of growth. Optimum aeration was determined by growing cultures as above with varying shaking speeds (120, 140, 160, or 180 rpm). To determine the optimum initial OD660 for degradation and growth, cultures were grown as above but started with an initial OD660 of 0.001, 0.004, 0.007, or 0.011.
1,4-Dioxane Tolerance
In order to examine tolerance levels for 1,4-dioxane, Xanthobacter sp. YN2 was cultivated in AMS containing 1000 mg/L 1,4-dioxane until mid-exponential phase. This culture was then used to inoculate fresh AMS containing various 1,4-dioxane concentrations (3, 5, 8, 10, 20, 30, 40, 50, and 100 g/L) and grown under the optimum conditions determined above. Three replicate cultures were grown for each concentration and uninoculated vials served as negative controls. After 14 days, viability was determined by plating onto AMS plates containing 1,000 mg/L 1,4-dioxane.
Growth on and Degradation of 1,4-Dioxane
Cultures were carried out in duplicate 50 mL headspace vials, filled with 10 mL of AMS containing 1,4-dioxane under optimum growth conditions. In addition, three sets of controls were performed. To determine the effect of volatilization, culture vials with no added bacteria were used. To test for bacterial growth on possible contaminants, cultures with no carbon source were used. To eliminate the effect of bacterial absorption of 1,4-dioxane, autoclaved bacteria (OD660 ≈ 0.3) were also used as controls. All of the growth cultures and controls were set up in duplicate and repeated three times. OD660, 1,4-dioxane concentration and TOC were monitored during incubation.
Preparation of Resting Cells
Cells were cultivated in AMS with 1000 mg/L 1,4-dioxane and harvested by centrifugation when cell growth reached mid-exponential phase. Harvested cells were washed twice with 0.02 M phosphate buffer (PBS, pH 7) and then resuspended in 0.02 M PBS (pH 7) to an OD660 ≈ 0.2.
Utilization Experiments for Various Substrates
Resting cells (0.5 mL) of Xanthobacter sp. YN2 were inoculated into 20 mL AMS and grown as above in the presence of 200 mg/L 1,4–dioxane, 1,3-dioxane, tetrahydrofuran, ethanol, n-hexane, cyclohexane, toluene, ethyl acetate, or methanol. Utilization of substrates was determined by growth (measurement of OD660). Generation times were determined from the slope of OD660 plotted against time (semi-log) during exponential phase. All experiments were carried out in duplicate. Growth on a variety of other carbon sources was investigated using GEN3 microplates (Biolog) and analyzed using Biolog’s Microbial Identification System software.
Total DNA Extraction and Genome Sequencing
Xanthobacter sp. YN2 was grown as above on 1,4-dioxane. Cells were harvested by centrifugation and total DNA was extracted using the DNA extraction kit for bacteria (Shanghai Lifefeng Biotechnology Co., Ltd). The Xanthobacter sp. YN2 genome was sequenced by Woosen Biotechnology (China) on the PacBio platform. A de novo assembly was generated using Canu 1.8 [27]. The resulting contigs were then submitted to Genbank under accession number CP063362-CP063366 and annotated using RAST 2.0 [28].
RNA Extraction and Quantitative Real-Time RT-PCR
Total RNA was extracted from cultures grown at 30 °C on liquid AMS with 5 mM succinate or 1000 mg/L 1,4-dioxane as the exclusive carbon source.
RNA was extracted using the Maxigen HiPure Total RNA Mini Kit according to the manufacturer’s instructions (including the optional DNase treatment). Reverse transcription was performed with FastQuant RT Kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions.
Quantitative real-time reverse transcriptase-PCR (RT-qPCR) analyses were performed by amplification of the cDNA samples from above using the Bestar SybrGreen qPCR Mastermix, according to the manufacturer’s instructions. Primer sequences are listed in Table S1. Thermocycling conditions were as follows: 2 min at 95 °C, followed by 45 cycles of 10 s at 95 °C, 1 min at 60 °C, followed by melting curve analysis. Expression of the 16S rRNA gene was used as the reference gene to normalize tested genes. The ΔΔCt method with the 16S rRNA gene as the reference was used to determine relative abundance of target transcripts.
Accession Numbers
The 16S rRNA gene sequence has been deposited in GenBank under the accession number of MK256301. The genome sequence has been submitted to GenBank under accession numbers CP063362-CP063366. The strain, Xanthobacter sp. YN2, has been deposited in the China General Microbiological Culture Collection Center (CGMCC) under the number CGMCC No. 14610. The strain is also being deposited at the American Type Culture Collection (ATCC), but a culture number is not available at this time.
Results
Evaluation of 1,4-Dioxane-Degrading Strain
Using standard enrichment techniques, we were able to obtain a single isolate capable of growing on 1,4-dioxane as sole carbon source. No growth or degradation occurred in autoclaved controls or carbon-free controls, and no decrease of 1,4-dioxane or increase in OD660 occurred in abiotic controls (data not shown). Blast result based on the 16S rRNA gene showed that the isolate is most related to Xanthobacter sp., and as such was labeled Xanthobacter sp. YN2. Experiments were carried out to determine optimum growth conditions for Xanthobacter sp. YN2 (Fig. 1). The optimum growth conditions were determined to be 30 °C, pH 7.0, and a shaking speed of 180 rpm. An initial OD660 of approximately 0.007 was shown to result in the shortest lag phase (Fig. 1). The growth of YN2 on 1,4-dioxane as the sole carbon source under the optimum conditions is as demonstrated in Fig. 2.
Effects of incubating temperature (a), inoculation amount (b), rotation speed of shaking (c), and pH (d) on growth and degradation of YN2 with 1,4-dioxane as sole carbon source. Columns represent degradation ratio of 1,4-dioxane; open circles represent OD660. Created using ORIGIN 2018. The error bars represent the range of triplicates
Substrate Range of YN2
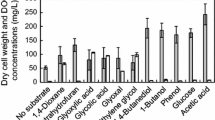
YN2 demonstrated the ability to grow on many substrates. Generation times of YN2 grown on different substrates are shown in Table 1 to display growth rates. YN2 grew on analogs of 1,4-dioxane, including 1,4-dioxene, 1,3-dioxane, and tetrahydrofuran and also on ethanol, methanol, and ethyl acetate. However, YN2 was unable to grow on 1,4-dioxane-ol, n-hexane, cyclohexane, or toluene. The absence of a lag phase in both growth and degradation of 1,4-dioxane after growth on non-inducing substrates such as succinate, pyruvate, acetate, and citrate indicated that enzymes involved in degradation of 1,4-dioxane are constitutive.
Growth Kinetics
YN2 was capable of growth in the presence of more than 3000 mg/L of 1,4-dioxane and remained viable even when the concentration approached 50 g/L; however, growth rates at these higher concentrations were much slower (data not shown).
Growth kinetics of YN2 on 1,4-dioxane were determined. Cells of YN2 were cultured with 1000 mg/L 1,4-dioxane until mid-exponential phase. This culture was then used to inoculate fresh AMS supplemented with different concentrations of 1,4-dioxane and cultivated under optimum growth conditions. The OD660 of the cultures were monitored every 4 h. The growth kinetics of YN2 were well described by Monod kinetics as seen in Fig. 3a. The maximum specific growth rate (μmax) was calculated to be 0.025/h with the highest cell yield determined as 0.27 mg-protein/mg-1,4-dioxane.
Growth kinetics (a) and degradation kinetics (b) of YN2 on 1,4-dioxane. Open circles represent specific growth rates; solid circles represent specific degradation rates; the dotted line represents nonlinear curve fitting result of specific growth rates (a) or specific degradation rates (b) at each concentration using Monod equation by minimizing the least absolute residuals. Created using ORIGIN 2018. The error bars represent the range of triplicates
No growth inhibition was seen when YN2 was grown with high 1,4-dioxane concentrations up to 1000 mg/L of 1,4-dioxane. When grown at these high 1,4-dioxane levels, the specific growth rate continued to increase with the substrate concentration; however, when the concentration of 1,4-dioxane was above 1000 mg/L, the specific growth rate of YN2 continued to increase but at a reduced level.
Degradation Kinetics
The relationship between the degradation rate and the concentration of 1,4-dioxane was studied using resting cells. As expected, no increase in biomass was observed throughout these experiments. In order to compare with other reported 1,4-dioxane degraders, the experiments were performed at concentrations between 100 and 1000 mg/L. The degradation kinetics for YN2 were well described by Monod kinetics. The maximum specific degradation rate (kmax) and half-saturation concentration (Ks) were calculated as 1.10 mg-1,4-dioxane/h mg-protein and 410.91 mg/L, respectively (Fig. 3b).
Genome Sequence Analysis
The genome of YN2 was found to have a size of 6,650,818 bp with a 67.95% G + C content spread over 6 contigs. N50 and N90 values were 5,964,455 bp and 312,888 bp, respectively. The genome consisted of 5 replicons, including the chromosome (6.2 Mb), plasmid 1 (circular, 147 kb), plasmid 2 (circular, 144 kb), plasmid 3 (circular, 51 kb), and plasmid 4 (linear, 30 kb). Automated annotation by RAST identified 6,585 protein-coding sequences and 58 RNA genes in the YN2 genome. The genome annotation revealed two SDIMO gene clusters (Fig. 4) that may be involved in 1,4-dioxane metabolism, both located in the chromosome, each encoding six protein components: a monooxygenase α, β and γ subunit, a ferredoxin, a coupling/effector protein, and a reductase. The sequences of the two clusters had extremely high-sequence homology to each other and are located distantly in the chromosome. Blast results showed that the protein sequences of the two gene clusters had high similarity with toluene monooxygenase genes of Sinirhodobacter hungdaonensis (84%) [29], Bradyrhizobium sp. ORS 375 (84%) [30] and Pseudooceanicola lipolyticus (84%) [31]. However, YN2 was not capable of utilizing toluene, but it could grow on tetrahydrofuran. Thus, the YN2 open-reading frames were designated as thmABCDEF.
Two SDIMO gene clusters of YN2 and phylogenetic tree of ThmA of YN2 with α subunit genes of other SDIMOs and their classification. Numbers below gene designations indicate the gene lengths in bp. Numbers above and below hash marks at the terminals of the clusters represent the locations within the chromosome of YN2. Phylogenetic tree of ThmA of YN2 with α subunit genes of other SDIMOs and their classification. Each group is marked with a different color, with their names listed on the right. Blocks on the right side are schemes of the whole clusters encoding each α subunit, with black blocks representing α subunit genes, and gray blocks for the remaining genes. The numbers below the blocks indicate the lengths (bp) of the genes. YN2 ThmA is marked with the black circle; toluene monooxygenases are marked with black triangles. Created using EVOLVIEW v2 [52]
Upregulation of thmABCDEF by 1,4-dioxane
The SDIMO gene clusters were shown to be expressed when cells were grown on either 1,4-dioxane or succinate, indicating that the genes are constitutively expressed. In addition, all six genes were significantly upregulated by 1,4-dioxane when compared to succinate (Fig. 5). Since the sequences of the two gene clusters are highly similar to each other, little to no sequence differences existed between the two gene clusters; therefore, their expression products were not distinguishable at present.
Previous studies have grouped SDIMOs into six groups based on sequence similarity and substrate range [32,33,34]. Sequence comparisons based on pairwise identity place the SDIMOs of YN2 into Group-2. According to the phylogenetic tree of amino acid sequences of α subunits of reported SDIMOs (Fig. 4), ThmA of YN2 is distant from PrmA of Mycobacterium sp. PH-06 and ThmA of Pseudonocardia dioxanivorans CB1190. Among SDIMOs of Group-2, ThmABCDEF is the first that appears to be involved in the metabolism of 1,4-dioxane.
Discussion
The 1,4-dioxane-specific degradation rate of YN2 (1.1 mg-1,4-dioxane/h mg-protein) is the highest of all Gram-negative 1,4-dioxane-degrading bacteria reported to date. It is only second to P. dioxanivorans CB1190 (0.92–1.98 mg-1,4-dioxane/h mg-protein), the highest of Gram-positive 1,4-dioxane degraders so far reported (Table 2). However, the half-saturation constant of YN2 is also very high, suggesting that the strain prefers higher concentrations of 1,4-dioxane and may not perform well at low dioxane concentrations. At a 1,4-dioxane concentration of 100 mg/L, there are 4 degraders showing higher specific degradation rates than YN2; with 1,4-dioxane concentrations higher than 100 mg/L, only CB1190 and PH-06 possess higher specific degradation rates than YN2 (Fig. S1). Nonetheless, the actual performance of CB1190 and PH-06 is severely hindered by their low cell yield [18, 25, 35]. Comparatively, the growth rate of YN2 (as measured by cell yield) is relatively high (Table 2). Cell yield may play a major factor in the biotreatment of pollutants [12]. Utilization of 1,4-dioxane is generally concomitant with growth of degraders [36, 37]. Typically, Gram-negative organisms are easier to grow and have higher cell yields than those of Gram-positive organisms as shown in Table 2. Among all reported 1,4-dioxane degrading isolates, Gram-positive CB1190 has the highest kmax of 0.92–1.98 mg-1,4-dioxane/h mg-protein [18, 38]. However, the cell yield of CB1190 is relatively low (0.09 mg-protein/mg-1,4-dioxane) [38]. To achieve higher cell yield, additional substrates are often required to enhance the growth of degraders, which increases the cost of bioremediation efforts [15]. Acinetobacter baumannii DD1 is reported to have a high cell yield, but its degradation performance is relatively poor (11 days to degrade concentrations of 1000 mg/L 1,4-dioxane starting with low biomass) [13]. To date, YN2 is the only 1,4-dioxane-degrading isolate with both a high cell yield and a high 1,4-dioxane degradation rate, demonstrating that YN2 is a good choice for 1,4-dioxane bioremediation.
YN2 was shown to have the unique ability to maintain its degradation rate at extremely high concentration levels of 1,4-dioxane. For example, YN2 was shown to completely degrade 1000 mg/L 1,4-dioxane in 40 h, whereas in similar experiments, Pseudonocardia sp. N23 takes 108 h and Mycobacterium sp. PH-06 takes 15 days to achieve the same result (which also started with 1000 mg/L 1,4-dioxane and relatively low biomass) [35, 39]. This is unique among previously reported 1,4-dioxane degrading isolates. The specific growth rate of YN2 was positively correlated to 1,4-dioxane concentrations up to 3000 mg/L, which is already much higher than the tolerance of most reported degraders (Table 2). Since transformation products of 1,4-dioxane may be toxic to the monooxygenase enzyme and/or the cells [38], this indicates that YN2 may produce non-toxic intermediates or not accumulate them at high enough levels to be toxic. The mechanisms underlying the unique high tolerance of YN2 to 1,4-dioxane is unknown at this time and will require further research. Possessing an extremely high tolerance to high levels of 1,4-dioxane makes YN2 an appropriate choice for field applications in bioremediation of large scale industrial spills.
Genomic analysis of YN2 indicated it belongs to the genus Xanthobacter. Another 1,4-dioxane degrading Xanthobacter, X. flavus DT8, was also isolated in China [36]. Both were isolated from wastewater treatment plants located about 1,700 km away from each other, suggesting that Xanthobacter may be an important 1,4-dioxane degrading genus present in wastewater treatment plants. Interestingly, although both strains belong to the Xanthobacter genus, YN2 could grow at extremely high 1,4-dioxane levels with no apparent decrease in growth rate, whereas the growth rate of X. flavus DT8 decreases significantly as the concentration of 1,4-dioxane was raised above 50 mg/L due to serious substrate inhibition [36]. 1,4-Dioxene is a product of metabolic degradation of 1,4-dioxane by DT8 [36]. YN2 was unable to grow on 1,4-dioxene suggesting that YN2 has a different dioxane degradation pathway than DT8.
We report here the substrate specificity of YN2 which is found to be similar but not identical to other 1,4-dioxane degrading strains (Table 3). Monooxygenases have been confirmed to participate in degradation of 1,4-dioxane in P. dioxanivorans CB1190 [24] and Mycobacterium sp. PH-06 [25, 40], which are both Gram-positive organisms. However, the detailed functions of monooxygenases and other related enzymes are still uncertain. 1,4-Dioxene was detected as a product during metabolic degradation of 1,4-dioxane by X. flavus DT8 [36], but it is not found in cultures of any other 1,4-dioxane degrader, and it could not be utilized by YN2. In addition, 1,4-dioxane-ol has been reported to be a degradation product of some strains [35, 41]. YN2 was shown not to grow on 1,4-dioxane-ol, suggesting that this compound is not an intermediate of YN2.
As mentioned above, monooxygenases have been reported to play an essential role in 1,4-dioxane degradation by metabolism in P. dioxanivorans CB1190 [41] and Mycobacterium sp. PH-06 [35]. The genome sequence of YN2 revealed the unique presence of two chromosomally encoded soluble di-iron monooxygenase (SDIMO) gene clusters of Group-2. Group-2 SDIMOs not only predominantly function as aromatic monooxygenases but also exhibit great variance in substrate range [42]. Currently, Group-2 SDIMOs include phenol, toluene, benzene and alkene monooxygenases [32, 43,44,45]. A great majority of Group-2 members presently reported are toluene monooxygenases, as is shown in Fig. 4, and most of them are able to degrade 1,4-dioxane by cometabolism. Group-2 toluene monooxygenases of Azoarcus sp. DD4 [45,46,47], Pseudomonas mendocina KR1, Pseudomonas pickettii PKO1, and Burkholderia cepacia AA1 are able to oxidize 1,4-dioxane after induction by toluene [38]. Similarly, Group-1 toluene monooxygenase of Burkholderia cepacia G4 [38] can also oxidize 1,4-dioxane. In spite of being similar to toluene monooxygenases of DD4, KR1, and G4, the Group-2 toluene monooxygenase of Pseudomonas stutzeri OX1 cannot oxidize 1,4-dioxane [45].
Although SDIMOs groups are defined more by operon structure and composition than by the substrate range or the phylogenetic classification of the respective bacteria [32], there seems to be a phylogenetic pattern of characteristics among the strains. For example, Xanthobacter sp. Py2 belongs to the same genus of YN2, and it expresses a Group-2 SDIMO that can metabolize alkene [44]. Similarly as YN2, Py2 does not grow on toluene [44]. Interestingly, another strain of the same genus of YN2, Xanthobacter sp. Strain ENV481, is not able to grow on or degrade 1,4-dioxane, but it can grow on a common product of 1,4-dioxane degradation, 2-hydroxyethoxyacetic acid (2HEAA) [24, 48]. For other species, a strain may possess several SDIMOs of the same or different groups [25, 45, 49]. It is not rare to find more than one SDIMO of the same group in the genome of a strain with high pairwise sequence identity [32]. To our knowledge, ThmABCDEF of YN2 is the first Group-2 SDIMO reported to hypothetically degrade 1,4-dioxane by metabolism.
Group-2 SDIMOs play a very important role in bioremediation for 1,4-dioxane [38, 45,46,47]. The process can happen cometabolically or metabolically, depending on the SDIMOs involved. 1,4-Dioxane degradation by Group-2 SDIMOs via cometabolism is the most reported [38, 45,46,47]. However, cells cannot benefit from the reaction of cometabolism, and competition against growth substrates can hinder cell growth, which reduces the efficiency of cometabolic degraders with more favorable kinetic properties and higher affinity for the substrate [50]. The expression of SDIMOs can be constitutive or inducible. It is essential to maintain the activity of desired enzyme during bioremediation [42], so amendment of toluene or other analogs are unavoidable in application of Group-2 SDIMOs that are cometabolic or inducible [45]. RNA analyses demonstrate that expression of the YN2 SDIMO gene clusters is constitutive. It is in agreement with the observation that YN2 did not need to be pregrown on 1,4-dioxane to have maximum 1,4-dioxane degrading activity. Therefore, ThmABCDEF of YN2 as a constitutively expressed SDIMO is the optimum for bioremediation of 1,4-dioxane. Also, many SDIMO gene clusters in other 1,4-dioxane degrading organisms are found on plasmids, which leads to higher risk of gene loss [25, 51]. In comparison, thmABCDEF is located in the chromosome, indicating that degradation ability loss caused by plasmid curing would not happen during field application of YN2.
This study demonstrates that the unique characteristics of YN2 (high growth rate on 1,4-dioxane, high 1,4-dioxane degradation rate, and tolerance to high concentrations of 1,4-dioxane) make it an ideal candidate for use in the bioremediation of 1,4-dioxane. This study also sets a solid foundation for future studies exploring the elucidation of the entire 1,4-dioxane degradation pathway in YN2 and other 1,4-dioxane degrading organisms.
Conclusion
YN2 has the ability to grow on and degrade 1,4-dioxane at higher concentrations than 3000 mg/L with no substrate inhibition and is also capable of degrading several 1,4-dioxane analogs metabolically. Of particular note is that the tolerance of YN2 to 1,4-dioxane (50 g/L) far exceeds those of all other reported degraders, and is six times higher than the highest tolerance reported previously. Furthermore, both degradation and growth kinetics of YN2 are well described by Monod kinetics, and the strain exhibits higher degradation ability compared to other reported 1,4-dioxane degraders, by means of its high cell yield (0.27 mg-protein/mg-1,4-dioxane) and maximum specific 1,4-dioxane degradation rates (1.10 mg-1,4-dioxane/h·mg-protein), indicating that YN2 is suitable for bioremediation applications. This is also the first report of constitutive Group-2 SDIMO gene clusters upregulated by 1,4-dioxane.
References
Stepien DK, Diehl P, Helm J, Thoms A, Püttmann W (2014) Fate of 1,4-dioxane in the aquatic environment: from sewage to drinking water. Water Res 48(1):406–419. https://doi.org/10.1016/j.watres.2013.09.057
Hinchee RE, Dahlen PR, Johnson PC, Burris DR (2018) 1,4-dioxane soil remediation using enhanced soil vapor extraction: I field demonstration. Ground Water Monit Remediat 38(2):40–48. https://doi.org/10.1111/gwmr.12264
Chiang SY, Anderson R, Wilken M, Walecka-Hutchison C (2016) Practical perspectives of 1,4-dioxane investigation and remediation. Remediation 27(1):7–27. https://doi.org/10.1002/rem.21494
Abe A (1999) Distribution of 1, 4-dioxane in relation to possible sources in the water environment. Sci Total Environ 227(1):41–47
Karges U, Becker J, Puettmann W (2018) 1,4-dioxane pollution at contaminated groundwater sites in western Germany and its distribution within a TCE plume. Sci Total Environ 619:712–720. https://doi.org/10.1016/j.scitotenv.2017.11.043
da Silva MLB, Woroszylo C, Castillo NF, Adamson DT, Alvarez PJ (2018) Associating potential 1, 4-dioxane biodegradation activity with groundwater geochemical parameters at four different contaminated sites. J Environ Manag 206:60–64. https://doi.org/10.1016/j.jenvman.2017.10.031
Adamson DT, Mahendra S, Walker KL, Rauch SR, Sengupta S, Newell CJ (2014) A multisite survey to identify the scale of the 1,4-dioxane problem at contaminated groundwater sites. Environ Sci Technol Lett 1(5):254–258. https://doi.org/10.1021/ez500092u
Mohr T, Stickney J, Diguiseppi W (2010) Environmental investigation and remediation: 1,4-dioxane and other solvent stabilizers. CRC Press, Boca Raton
McElroy AC, Hyman MR, Knappe DR (2019) 1,4-dioxane in drinking water: emerging for forty years and still unregulated. Curr Opin Environ Sci Health. https://doi.org/10.1016/j.coesh.2019.01.003
Steffan RJ, McClay KR, Masuda H, Zylstra GJ (2007) ER-1422: Biodegradation of 1, 4-dioxane. Shaw Environmental Inc, Lawrenceville NJ
Isaka K, Udagawa M, Kimura Y, Sei K, Ike M (2015) Biological wastewater treatment of 1,4-dioxane using polyethylene glycol gel carriers entrapping afipia sp. D1. J Biosci Bioeng 121(2):203–208. https://doi.org/10.1016/j.jbiosc.2015.06.006
Sei K, Miyagaki K, Kakinoki T, Fukugasako K, Inoue D, Ike M (2013) Isolation and characterization of bacterial strains that have high ability to degrade 1,4-dioxane as a sole carbon and energy source. Biodegradation 24(5):665–674. https://doi.org/10.1007/s10532-012-9614-1
Huang H (2015) Study on the characteristics and quorum sensing of 1,4-dioxane degradation by A. Baumannii DD1. Zhejiang Gongshang University,
Jin XJ, Chen DZ, Zhu RY, Chen J, Chen JM (2012) Characteristics of 1,4-dioxane degradation by xanthobacter flavus DT8. Environ Sci 33(5):1657–1662
Pugazhendi A, Banu JR, Dhavamani J, Yeom IT (2015) Biodegradation of 1,4-dioxane by rhodanobacter AYS5 and the role of additional substrates. Ann Microbiol 65(4):2201–2208. https://doi.org/10.1007/s13213-015-1060-y
Sun B, Ko K, Ramsay JA (2011) Biodegradation of 1,4-dioxane by a flavobacterium. Biodegradation 22(3):651–659. https://doi.org/10.1007/s10532-010-9438-9
Zenker MJ, Borden RC, Barlaz MA (2000) Mineralization of 1,4-dioxane in the presence of a structural analog. Biodegradation 11(4):239–246. https://doi.org/10.1023/A:1011156924700
Parales R, Adamus J, White N, May H (1994) Degradation of 1, 4-dioxane by an actinomycete in pure culture. Appl Environ Microbiol 60(12):4527–4530. https://doi.org/10.1016/0922-338X(95)92742-U
Rolston HM, Hyman MR, Semprini L (2019) Aerobic cometabolism of 1,4-dioxane by isobutane-utilizing microorganisms including rhodococcus rhodochrous strain 21198 in aquifer microcosms: experimental and modeling study. Sci Total Environ 694:133688. https://doi.org/10.1016/j.scitotenv.2019.133688
Skinner K, Cuiffetti L, Hyman M (2009) Metabolism and cometabolism of cyclic ethers by a filamentous fungus, a graphium sp. Appl Environ Microbiol 75(17):5514–5522. https://doi.org/10.1128/aem.00078-09
Masuda H, Mcclay K, Steffan RJ, Zylstra GJ (2012) Biodegradation of tetrahydrofuran and 1,4-dioxane by soluble diiron monooxygenase in pseudonocardia sp. strain ENV478. J Mol Microbiol Biotechnol 22(5):312–316. https://doi.org/10.1159/000343817
Li M, Mathieu J, Liu Y, Van Orden ET, Yang Y, Fiorenza S, Alvarez PJJ (2014) The abundance of Tetrahydrofuran/Dioxane monooxygenase Genes (thmA/dxmA) and 1,4-dioxane degradation activity are significantly correlated at various impacted aquifers. Environ Sci Technol Lett 1(1):122–127. https://doi.org/10.1021/ez400176h
Li M, Mathieu J, Yang Y, Fiorenza S, Deng Y, He Z, Zhou J, Alvarez PJJ (2013) Widespread distribution of soluble Di-Iron monooxygenase (SDIMO) genes in arctic groundwater impacted by 1,4-dioxane. Environ Sci Technol 47(17):9950–9958. https://doi.org/10.1021/es402228x
Sales CM, Grostern A, Parales JV, Parales RE, Alvarez-Cohen L (2013) Oxidation of the cyclic ethers 1,4-dioxane and tetrahydrofuran by a monooxygenase in two pseudonocardia species. Appl Environ Microbiol 79(24):7702–7708. https://doi.org/10.1128/AEM.02418-13
He Y, Mathieu J, Yang Y, Yu P, Silva MLBD, Alvarez PJJ (2017) 1,4-dioxane biodegradation by mycobacterium dioxanotrophicus PH-06 is associated with a group-6 soluble Di-iron monooxygenase. Environ Sci Technol Lett 4(11):494–499. https://doi.org/10.1021/acs.estlett.7b00456
He Y, Wei K, Si K, Mathieu J, Li M, Pjj A (2017) Whole-Genome sequence of the 1,4-dioxane-degrading bacterium mycobacterium dioxanotrophicus PH-06. Genome Announc 5(35):e00625-e1617. https://doi.org/10.1128/genomeA.00625-17
Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM (2017) Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res 27(5):722. https://doi.org/10.1101/gr.215087.116
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O (2008) The RAST server: rapid annotations using subsystems technology. BMC Genomics 9(1):75. https://doi.org/10.1186/1471-2164-9-75
Xu GT, Piao C, Chang JP, Guo LM, Yang XQ, Li Y (2019) Sinorhodobacter populi sp. nov, isolated from the symptomatic bark tissue of populus× euramericana canker. Int J Syst Evol Microbiol 69(4):1220–1224. https://doi.org/10.1099/ijs.0.066068-0
Mornico D, Miché L, Béna G, Nouwen N, Verméglio A, Vallenet D, Smith AA, Giraud E, Médigue C, Moulin L (2012) Comparative genomics of aeschynomene symbionts: insights into the ecological lifestyle of nod-independent photosynthetic bradyrhizobia. Genes 3(1):35–61. https://doi.org/10.3390/genes3010035
Huang MM, Guo LL, Wu YH, Lai QL, Shao ZZ, Wang CS, Wu M, Xu XW (2017) Pseudooceanicola lipolyticus sp. nov., a marine alphaproteobacterium, reclassification of oceanicola flagellatus as pseudooceanicola flagellatus comb. nov. and emended description of the genus pseudooceanicola. Int J Syst Evol Microbiol 68(1):409–415. https://doi.org/10.1099/ijsem.0.002521
Notomista E, Lahm A, Di Donato A, Tramontano A (2003) Evolution of bacterial and archaeal multicomponent monooxygenases. J Mol Evol 56(4):435–445. https://doi.org/10.1007/s00239-002-2414-1
Coleman NV, Bui NB, Holmes AJ (2006) Soluble di-iron monooxygenase gene diversity in soils, sediments and ethene enrichments. Environ Microbiol 8(7):1228–1239. https://doi.org/10.1111/j.1462-2920.2006.01015.x
Holmes AJ, Coleman NV (2008) Evolutionary ecology and multidisciplinary approaches to prospecting for monooxygenases as biocatalysts. Antonie Van Leeuwenhoek 94(1):75–84. https://doi.org/10.1007/s10482-008-9227-1
Kim Y-M, Jeon J-R, Murugesan K, Kim E-J, Chang Y-S (2009) Biodegradation of 1, 4-dioxane and transformation of related cyclic compounds by a newly isolated mycobacterium sp. PH-06. Biodegradation 20:511–519. https://doi.org/10.1007/s10532-008-9240-0
Chen DZ, Jin XJ, Chen J, Ye JX, Jiang NX, Chen JM (2016) Intermediates and substrate interaction of 1,4-dioxane degradation by the effective metabolizer Xanthobacter flavus DT8. Int Biodeter Biodegrad 106:133–140. https://doi.org/10.1016/j.ibiod.2015.09.018
Nakamiya K, Hashimoto S, Ito H, Edmonds JS, Morita M (2005) Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus. Appl Environ Microbiol 71(3):1254–1258. https://doi.org/10.1128/AEM.71.3.1254-1258.2005
Mahendra S, Alvarez-Cohen L (2006) Kinetics of 1, 4-dioxane biodegradation by monooxygenase-expressing bacteria. Environ Sci Technol 40(17):5435–5442. https://doi.org/10.1021/es060714v
Yamamoto N, Saito Y, Inoue D, Sei K, Ike M (2018) Characterization of newly isolated pseudonocardia sp N23 with high 1,4-dioxane-degrading ability. J Biosci Bioeng 125(5):552–558
Deng D, Li F, Li M (2017) A novel propane monooxygenase initiating degradation of 1,4-dioxane by mycobacterium dioxanotrophicus PH-06. Environ Sci Technol Lett. https://doi.org/10.1021/acs.estlett.7b00504
Mahendra S, Petzold CJ, Baidoo EE, Keasling JD, Alvarez-Cohen L (2007) Identification of the intermediates of in vivo oxidation of 1, 4-dioxane by monooxygenase-containing bacteria. Environ Sci Technol 41(21):7330–7336. https://doi.org/10.1021/es0705745
Singh A, Kuhad R, Ward O (2009) Advances in applied bioremediation. Springer Berl. https://doi.org/10.1007/978-3-540-89621-0
Hino S, Watanabe K, Takahashi N (1998) Phenol hydroxylase cloned from ralstonia eutropha strain E2 exhibits novel kinetic properties. Microbiology 144(7):1765
Zhou NY, Jenkins A, Chan KCC, Leak DJ (1999) The alkene monooxygenase from xanthobacter strain Py2 is closely related to aromatic monooxygenases and catalyzes aromatic monohydroxylation of benzene, toluene, and phenol. Appl Environ Microbiol 65(4):1589
Deng D, Dung P, Li F, Li M (2020) Discovery of an inducible toluene monooxygenase that co-oxidizes 1,4-Dioxane and 1,1-Dichloroethylene in propanotrophic azoarcus sp DD4. Appl Environ Microbiol. https://doi.org/10.1128/AEM.01163-20
Deng D, Li F, Wu C, Mengyan L (2018) Synchronic biotransformation of 1,4-Dioxane and 1,1-Dichloroethylene by a gram-negative propanotroph azoarcus sp DD4. Environ Sci Technol Lett 5(8):526–532
Li F, Deng D, Li M (2019) Distinct catalytic behaviors between two 1,4-dioxane degrading monooxygenases: kinetics, inhibition, and substrate range. Environ Sci Technol. https://doi.org/10.1021/acs.est.9b05671
Mcclay K, Schaefer CE, Vainberg S, Steffan RJ (2007) Biodegradation of Bis(2-Chloroethyl) ether by xanthobacter sp. strain ENV481. Appl Environ Microbiol 73(21):6870–6875
Grostern A, Sales CM, Zhuang WQ, Erbilgin O, Alvarez-Cohen L (2012) Glyoxylate metabolism is a key feature of the metabolic degradation of 1,4-dioxane by pseudonocardiadioxanivorans strain CB1190. Appl Environ Microbiol 78(9):3298–3308
Alvarez-Cohen L, McCarty PL (1991) A cometabolic biotransformation model for halogenated aliphatic compounds exhibiting product toxicity. Environ Sci Technol 25(8):1381–1387. https://doi.org/10.1021/es00020a003
Sales CM, Mahendra S, Grostern A, Parales RE, Goodwin LA, Woyke T, Nolan M, Lapidus A, Chertkov O, Ovchinnikova G (2011) Genome sequence of the 1, 4-dioxane-degrading pseudonocardia dioxanivorans Strain CB1190. J Bacteriol 193(17):4549–4550
He Z, Zhang H, Gao S, Lercher M, Chen W-H, Hu S (2016) Evolview v2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. https://doi.org/10.1093/nar/gkw370
Ramosgarcia AA, Shankar V, Saski CA, Hsiang T, Freedman DL (2018) Draft genome sequence of the 1,4-dioxane-degrading bacterium pseudonocardia dioxanivorans BERK-1. Genome Announc. https://doi.org/10.1128/genomeA.00207-18
Bernhardt D, Diekmann H (1991) Degradation of dioxane, tetrahydrofuran and other cyclic ethers by an environmental rhodococcus strain. Appl Microbiol Biotechnol 36(1):120. https://doi.org/10.1007/BF00164711
Inoue D, Tsunoda T, Yamamoto N, Ike M, Sei K (2018) 1,4-Dioxane degradation characteristics of rhodococcus aetherivorans JCM 14343. Biodegradation 29(3):1–10. https://doi.org/10.1007/s10532-018-9832-2
Acknowledgements
This study was supported by the National Major Science and Technology Projects of China under 2014ZX07201-012-03. The active sludge used in the study was collected from a WWTP (Harbin, China) by permission.
Author information
Authors and Affiliations
Contributions
All the authors contribute to (1) substantial contribution to conception and design or the acquisition and analysis of data, (2) drafting or critically revising the manuscript, and (3) approval of the final submitted version. FM—also contributes to conceptualization, funding acquisition, investigation, and project administration. YW—also contributes to the original draft, investigation, data curation, and visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human and Animal Participants
This research does not involve any human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, F., Wang, Y., Yang, J. et al. Degradation of 1,4-Dioxane by Xanthobacter sp. YN2. Curr Microbiol 78, 992–1005 (2021). https://doi.org/10.1007/s00284-021-02347-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02347-6