Abstract
Assessment of native plants and laboratory-scale phytoextraction tests are fundamental and preliminary steps in checking the feasibility and practice of low-cost and low-impact phytoremediation. In this study, we investigated the absorption of B by plants as a tool to remove boron in sediments from different areas of the Cecina River basin in Tuscany, Italy. The investigation was performed analyzing total and available B fraction in sediment samples as well as the B content in different tissues of native plants colonizing the contaminated areas. In laboratory scale, a phytoextraction screening test was performed. Selected high biomass crops (Brassica juncea, Zea mays, and Helianthus annuus) were evaluated in the most contaminated sample in two consecutive growing cycles. Results from field survey showed no hyperaccumulator native plant was present in the investigated areas although, high accumulation levels were found in native species from Bulera dump (Rumex crispus—259 mg kg−1 and Poa spp—203 mg kg−1). Results from laboratory phytoextraction tests showed a higher ability of B. juncea which removed about 18.5 mg B kg−1 sediment in after the two consecutive growing cycles, representing on the whole 45% of the initial available B fraction. The sediment characteristics affected by the phytoextraction processes were also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Boron is an essential micronutrient for plants and animals and is vital for human health (Parks and Edwards 2005; Goldbach and Wimmer 2007). For instance, recent studies demonstrate the structural role of B in cell walls of higher plants, where the B-binding biomolecules such as the pectic polysaccharide rhamnogalecturonan II cross-linked by a borate bridge, provide stability to the cell wall matrix (O’Neil et al. 2004). However, B contamination in soils, sediments, and water can cause health problems, mainly from food sources, but high boron solubility can also affect drinking water (Litovitz et al. 1988; Murray 1995; O’Sullivan and Taylor 1983). Contamination can also influence the agro-industrial economy, as it can be toxic for crop plants, thus reducing yields (e.g., 17% yield reduction in barley crops, Cartwright et al. 1986).
Boron can be found in the form of boric acid or its salt (borate) in soils, sediments, and groundwater as a consequence of its natural presence in many silicate minerals; however, only borax, colemanite, kernite, and uxelite are mined extensively (Parks and Edwards 2005). Areas with a history of volcanism and geothermal ducts frequently have boron-containing minerals (Smith 2001). Boron can also be released in the environment from anthropogenic activities: e.g., the use of borate-containing fertilizers, herbicides and detergents, glass and ceramic production, release of waste from borate mining processing (Parks and Edwards 2005), etc.
Boric acid and borates dissolved in water can adsorb onto and desorb from the many different solid surfaces found in soil and sediment particles. Boron availability and levels in soil depend on the solubility of the parent rock, physical and chemical characteristics of the soil (e.g., pH, organic matter content, texture, etc.), and on wetting and drying cycles derived from water moving through the B-adsorbed sites. Traditional ex situ treatments for definitively reducing the mobility of inorganic contaminants in soil (including B) are: exposure to high temperature treatments producing a vitrified material, addition of solidifying agents producing a cement-like material and soil washing process that produces contaminated leachate. However, these methods are cost prohibitive when large areas are contaminated (USDA NRCS 2000). Agricultural soils containing high levels of B can be extensively leached with water (an in situ practice) to promote B migration to a particular depth below the root zone (Leyshon and Jame 1993). The application of amendments such as gypsum in sodic soils or Ca(H2PO4)2 in acid soils, which adsorb and reduce B availability (Nable et al. 1997) are also commonly used methods for in situ treatment.
Alternatively, phytoremediation has been used in various environmental studies (Di Gregorio et al. 2006; Tassi et al. 2008; Pedron et al. 2009) as a low cost and environmental friendly technology to reduce contaminants from soils, moreover, it could be used to manage boron contamination in soils (Angin and Turan 2008) and waters (Del-Campo Marin and Oron 2007). A strategy for lowering the B level in the environment is planting tolerant species that can take up and accumulate high B quantities in the upper parts, which can then be harvested and disposed in suitable places, for example in B deficient soil. Robinson et al. (2003, 2007) showed that poplar trees enhanced the evapotranspiration from wood waste while B accumulated in the aerial portions of the trees lowered B levels in a sawdust pile leachate from 1.87 mg L−1 to <0.80 mg L−1 in less than 2 years. Banuelos (1996) and Banuelos et al. (1993) showed in pot and field tests that Brassica juncea, Festuca arundinacea, Lotus corniculatus, and Hibiscus cannibinus reduced available B in soil containing high B levels by as much as 52%. Recently, Angin and Turan (2008) stated the enhancement of B and Pb phytoextraction in pot experiments by Vetiveria zizanioides after addition of humic acid solution in the contaminated soil.
Most of Cecina River basin (Italy) is located in the geothermal zone of Larderello, in the region of Tuscany in west central Italy. For more than 150 years, boric acid was extracted from the geothermal fluids, of which H3BO3 is the minor component, and from B minerals, mainly colemanite imported from western Anatolia, Turkey. The waste produced from both activities (boron-rich exhausted geothermal fluids and residual mud from a boric acid processing plant) spilled into the Possera and Pavone creeks (tributaries of Cecina River) until about 1975. In that period, an underground screen was constructed and waste was disposed of in a controlled landfill site (Bulera dump). Since the 1980s, reinjection of the exhausted underground geothermic fluid brought benefits to the area and improvements in the industrial production of boric acid (Grassi and Squarci 2004), although in certain areas of the basin contamination is still present (Grassi and Squarci 2004; Pennisi et al. 2006).
To evaluate whether phytoextraction strategy would be feasible to reduce B levels in sediments from the Cecina basin field and laboratory studies have been carried out. This study consisted of two distinct phases: to explore native plants colonizing the sampled areas and to perform laboratory phytoextraction tests using the most contaminated sediment and high biomass crop plants. Both phases aimed to assess boron tolerant plant species able to reduce B content from the Cecina basin sediments, investigating its uptake and distribution in different plant tissues. So far, at our knowledge, no studies have been found that survey B phytoextraction from contaminated sediments.
2 Materials and Methods
2.1 Sediment and Native Plant Collection
The number of sediment samples was connected to the vegetal samples existent in the area thus, sediment samples (0-20 cm depth) and native vegetation above it were collected from the Cecina basin in four different areas along the Possera creek (Fig. 1). Sampling areas were selected according to data on B content in sediment and river water previously studied in the work of Pennisi et al. (2006). In the sampling area, near Pomarance (the area along the Possera creek nearest the Cecina River), three subsamples (P1, P2 and P3) were collected. In the sampling area near the Bulera dump two subsamples (BD1 and BD2) were collected and in the area near Larderello (the area along the Possera creek farthest from the Cecina River) one subsample (L) was collected. A control sample (C) of uncontaminated sediment was also collected near Berignone (just upstream of the mouth of the Possera creek into the Cecina river). With the exception of the L sample, all remaining samples were collected in dry or almost dry riverbed areas. In each sampling area, only the most abundant native plants were collected and identified.
2.2 Sediment and Plant Analysis
Sediment samples dried at room temperature (around 2 kg DW) were homogenized by pestle and sieved (2 mm) prior to analysis and the performance of phytoextraction tests. Their main chemical and physical properties (pH, C.E.C., E.C., CaCO3, OM, sand, silt, and clay content) were determined according to procedures in Methods of Soil Analysis (SSSA 1996). Moreover, some parameters of agronomic importance (N, P, K) were analyzed to define the matrix capacity to support germination and plant growth as well as to evaluate any further fertilizer requirements. These data were reported in Table 1 as the mean of three replicate analyses.
Sediments were microwave digested in HNO3/H2O2 (2.5:1, sediment:extractant ratio) using Milestone 1200 (Sorisole, Bergamo, Italy). Total B content in sediments was determined in the acid digested solution using the azomethine-H procedure (Keren 1996) with an UNICAM 500 UV/VIS spectrophotometer (Thermo Spectronic) at 420 nm. When necessary, colored digested solutions were decolorized by shaking for a few minutes with a minimal quantity of charcoal, and filtering through a single-use syringe filter 0.45 μm pore size (Minisart-Sartorius AG, Goettingen, Germany). The available B fraction in sediments was determined by extraction with 0.01 M mannitol + 0.01M CaCl2 solution (1:5, sediment/extract ratio, 16 h shaking time). This method is able to desorb most the adsorbed B at pH around 8-9 and was indicated as a B extractant in either alkaline or calcareous soils, removing both soluble and adsorbed B (Keren 1996). The extraction method gave a clear, colorless extract and the azomethine-H colorimetric procedure was used for the quantification of the available B fraction.
Boron content in collected plants and plants from microcosm tests was determined after thoroughly rinsing with deionized water, separation in roots and shoots, and oven drying at 40°C until constant weight. Fine powdered plant materials were pre-digested overnight in a HNO3/H2O2 acid mixture (2.5:1 ratio) and microwave digested (Keren 1996). Boron in plant extracts was determined using the colorimetric azomethine-H method after decoloration (when necessary) of acidic extracts as previously described.
The concentration of B in the aboveground with respect to its concentration in roots and with respect to the concentration on the sediment below were calculated for each collected plant. The ratio of B concentration in shoots to that in roots, translocation factor (TF), and the ratio of B concentration in shoots to the total sediment concentration, bioconcentration factor (BF) were calculated according to Branquinho et al. (2007) and were shown in Table 2. Data on biomass of native plants were not completely reliable due to the difficulty in the collection of the entire root system of all species. Thus, the comparison of the amount of B (concentration × biomass) among all collected species was not allowed and the data on biomass was omitted. Even if the TFs were calculated without considering the biomass, the obtained results represent the movement of B within the plants and indicate the ability of each native plant to transport B to the aboveground part and, most likely, the existence of tolerance mechanisms to face the high B concentration. In this sense, high values of TFs (and BFs) are useful parameters to a preliminary screening of possible hyperaccumulator plants in the contaminated site and to enlarge data on tolerant plants to B contamination.
2.3 Microcosm Setup
Laboratory scale phytoextraction tests were performed choosing the worst scenario, i.e., using the most contaminated sediment (BD2) that had also the highest available B fraction and an uncontaminated sediment (C) as a control test (Table 2).
Before starting microcosm tests, germination and preliminary growth test (data not shown) were performed to investigate the capacity for seed germination and growth of different cultivars of three high biomass crop species (B. juncea cv. Scala, B. juncea cv. Vitasso, Sinapsis alba cv Torpedo; Helianthus annuus cv. Ercules, H. annuus cv. Coralia; and Zea mays cv. Rubens, Z. mays cv. PR34N43). Seeds of all cultivars were put to germinate in paper wetted with 5 mg l−1 B aqueous solution. Although all cultivars germinated well in B solution, new seeds of all cultivars were put to grow in the selected contaminated sediment. The best performers in terms of number of seeds germinated, speed of germination, and ability to develop roots and leaves as in control soil were selected to set up the so called “definitive” microcosm tests.
For the definitive microcosm tests H. annuus (cv Coralia), Z. mays (cv PR34N43) and B. juncea (cv Scala) were selected to grow in the BD2 sediment and in the uncontaminated sediment as a control test. All microcosms were prepared in four replicates using 50 g of sediment mixed with 150 g of inert silica pellets (B free) in a 250 mL beaker sowing five seeds of H. annuus or Z. mays or 0.3 g of B. juncea. Two sequentially growing cycles were carried out in each pot, sowing the same plant species two times in the respective microcosm. In each growing cycle, plants were allowed to grow for 35 days in controlled conditions of light (200 μmol m−2 s−1 for 14 h/day), temperature (19-24°C for day/night periods), and humidity (70%) in a growth chamber (AS s.p.a., model CCL300BH, Perugia Italy). Microcosms were watered every day with deionized water to maintain soil humidity. After each growing cycle, plants were harvested taking care to remove all roots from sediments, especially after the first cycle. The dry biomass of shoots and roots from each microcosm was recorded after rinsing with deionized water and oven drying at 40°C until constant weight. B content in the different plant tissues and in each microcosm sediment (as the residual available fraction) were determined as described in Section 2.2. Prior to the available B determination, the silica pellets were separated from sediment by sieving (2 mm). After the second growing cycle, the pH of sediments from each microcosm was measured and compared with the initial pH (before any growing plant).
2.4 Statistical Analysis
All statistical analysis was performed using Statistica version 6.0 (Statsoft, Inc). Data sets were transformed where necessary (log10 for B available, Table 2; residual B available, Table 3; root biomass in H. annuus and root biomass in Z. mays, Fig. 2a) and normality was verified using Kolmogorov-Smirnov and Lilliefors tests. Treatment effects were analyzed using one-way analysis of variance. Differences among means were compared and a post hoc analysis of variance was performed using the Fischer LSD test (p < 0.05).
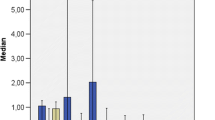
a Biomass (mg MC−1 DW) and b boron content (mg kg−1) in shoots and roots in the two consecutive microcosm tests (I and II). Percentage in b represents the efficiency of B traslocation inside the respective plant. Bars represent the standard deviation of mean values for n = 4 replicate microcosms. Bars with different letters for the same plant tissue are significantly different from each other (P ≤ 0.05) according to Fisher’s LSD test. MC microcosm
3 Results
3.1 Boron Content in Sediment Samples and Native Plants
Very different total B content was found among the different areas where sediment and native plants were collected. Higher values of total B content were found in the BD2 samples followed by that in BD1 samples and those in P area. Total B content in the sediment samples collected in the L area was found very closer to that in the uncontaminated sediment (C; around 17 and 16 mg kg−1, respectively). Moreover, higher B available fractions were found in the Bulera Dump area, where sediment samples BD1 and BD2 showed values significantly different between them and among the other values. B available fraction in these samples represents around 30% and 50% of the respective total B content (Table 2).
Plant samples of the most abundant native species colonizing each area were collected, identified, and analyzed for their B content in the aboveground and belowground tissues to check a probable adapted tolerant and hyperaccumulator species (Table 2). Data on B concentration in shoots, TF, and BF were used here to compare and investigate possible hyperaccumulation of B in the species collected. The highest values were found in Rumex crispus (Bshoots = 259 mg kg−1, TF = 4.6 and BF = 4.5), a plant colonizing the most contaminated area in terms of both total and available B (Bulera Dump). The others herbaceous plants found in the same area showed lower capacity to translocate B to the upper parts and to bioconcentrate it, although Poa spp. had elevated B content in shoots and roots (203 and 154 mg kg−1, respectively). Plants collected in Pomarance area did not show any remarkable B accumulation, even if, among these Brassica napus showed the better ability to translocate B to the upper parts (TF = 4.5). Phragmites australis were found colonizing both Larderello and Berignone (control) areas, where B analysis showed similar values in plants from both areas; similar and low TF (around 0.5), similar and high BC (around 5.5; Table 2).
3.2 Microcosm Tests-Biomass Production
Two consecutive growing cycles (tests I and II) were performed in each microcosm to assess dry weight and B concentration in shoots and roots of selected species (Fig. 2). The biomasses of all plants grown in uncontaminated sediment were higher than the same plant species grown in high available B level. All plants from test I showed visible symptoms of B toxicity as the margins of leaves became yellowish. In general, the second growth produced less pronounced visible toxic symptoms than the first one. This difference was more evident in B. juncea where biomass increased threefold in the aerial part and more than fivefold in the roots at the end of test II. In Z. mays and in H. annuus the difference in biomass between test I and II was not significant except for roots of H. annuus (Fig. 2a).
3.3 Microcosm Tests-B Accumulation in Plant Tissues
Figure 2b shows the B accumulation in shoots and roots in the two consecutive growing tests and compares the accumulation of the same plant species grown in uncontaminated sediment (control). There is an evident higher accumulation of B in the upper part of all plants grown in the contaminated sediment, although greater accumulation was observed in test I. The plants of B. juncea showed the highest B accumulation in shoots (2,911 and 1,800 mg kg-1, respectively for tests I and II) and higher capacity to translocate the element to the upper part as evidenced by a translocation efficiency higher than 98%. The translocation efficiency (percentages on the bars in Fig. 2b) was calculated according to Meers et al. (2004). The same authors defined the translocation efficiency as the fraction, after root absorption that is successfully translocated to the above ground tissues. In our case, it represents the ratio of B content in the shoots to that of the whole plant (shoots + roots). Although Z. mays and H. annuus also showed high translocation efficiency (91-96%) it was lower than that of B. juncea (98-99%). Boron accumulation in shoots of these species ranged from 1,250 to 750 mg kg−1, respectively, for tests I and II in Z. mays, and from 730 to 450 mg kg−1, respectively, for tests I and II in H. annuus.
3.4 Microcosm Tests-B Removal by Plants
The extent of B removal from the contaminated sediment was calculated for each growing cycle (as the product of the dry biomass and the respective accumulation in shoots or roots). It was represented in Fig. 3 as milligram of B extracted by plants (shoots and roots) in a kilogram of sediment. The figure confirms an enormous amount of B removed by shoots, especially by B. juncea, which increased almost twofold in the second growth due to increased biomass. Contrarily, B removal decreased in the second growth of both Z. mays and H. annuus due to the decreased B accumulation in shoots and a small increase in the respective biomass. The total amount of B removed by both shoots and roots was also represented as a percentage of the initial available B fraction in BD2 sediment before any planting (numbers on the bars in Fig. 3). This result underlines that B removed by B. juncea in only two consecutive growing cycles represents more than 45% of the initial available B fraction present in the sediment, while Z. mays and H. annuus removed lower amounts, about 30% and 15%, respectively.
3.5 Sediment Characteristics Affected by the Phytoextraction Process
Plants have biogeochemical mechanisms of adaptation to, and tolerance of, altered growth media. For example, it is known that plant roots exude substances that are involved in the uptake mechanisms, where the composition of these exudates can greatly change under any environmental stress. In general, plants take up trace elements that are in soil solution; however, changes in the pH of the root ambient by various root exudates can significantly increase or reduce the availability of certain elements (Gobran et al. 2001). However, the ability of adaptation and tolerance depends on the intrinsic capacity of each species. The analysis of BD2 sediment pH after the second growth of each plant (Table 3) let us to suppose that the tested plants can influence the rhizosphere pH and the B available fraction. Table 3 shows also the available B fraction in BD2 sediment before any growth and after each planting; it could represent the potential B fraction for the plants’ uptake in subsequent growing cycles. The uptake of B. juncea and Z. mays in two growing cycles produced a decrease in available B fraction from 40 mg kg−1 (before growth) to about 25 and 18 mg kg−1 after tests I and II, respectively. These residual B available fractions after the two consecutive growing cycles of B. juncea and Z. mays represents about 45% of the initial B available fraction. Lower decrease of available fraction was produced by H. annuus, reducing it to about 35 and 22 mg kg−1 after tests I and II, respectively, the last value representing about 54% of the initial available fraction.
It was observed that the presence of plants also influence the pH of sediment (Table 3). The microcosms with H. annuus showed a higher increase of pH (0.35 units) when compared with the value in sediment without plants. In microcosms with Z. mays and B. juncea a lower but significant increase of pH was also observed (0.07 and 0.06 units, respectively).
4 Discussion
Boron desorption from soil colloids is an important factor in B availability because plants primarily take up the B from soil solution. Soil adsorbing surfaces (OM, aluminum and iron oxides, clay, and calcium carbonate), as well as soil properties (texture, moisture, temperature, and especially pH) affected B availability (Goldberg 1997). Generally, we expected low availability to the plants in coarse textured, low OM, and high pH soils. Our results showed large natural differences between the contaminated sediments, generally they had a relatively low OM (1.2-2.2%) and alkaline pH (8 2-8.6) but that samples with relatively high clay content (15.5-17.5%) had also the high available B fraction (BD1 and BD2). These results could probably be explained by the influence of the ensemble of sediment characteristics and by both natural origin and anthropogenic B enrichment. According to literature (Grassi and Squarci 2004; Pennisi et al. 2006), the Cecina basin is still contaminated by an anthropogenic undefined source, particularly in the Larderello area. For this reason, the relatively low total B content in the L sample could be justified by the few adsorption sites in the solid particles of a sandy matrix and the promotion of the desorption process during the water movement through it as the sampled area is subject to watering phenomena.
Excluders and accumulators species can be conveniently distinguished by its BF and TF as they can be >1 in accumulators and <1 in excluders. Moreover, the hyperaccumulation in plants is distinguished by BF and TF > > 1 and having metal(oid) concentration from 2 to 3 orders of magnitude higher than in normal plant species growing on uncontaminated soils (McGrath and Zhao 2003; Yanqun et al. 2005). According to these statements, no hyperaccumulators plants were found to colonizing the explored area of the Cecina basin but almost all collected species are B accumulators (B. napus, R. crispus, Crepis bulbosa, and Poa ssp) while Taraxacum officinale, and P. australis could be considered excluders (Table 2). Even though R. crispus is efficient in the root-to-shoot B transport and accumulation, it is considered a troublesome weed species that could have a severe inconvenience as a possible use as B phytoextractor because it can infest grassland and arable land competing with sown and native pasture species. Zaller (2004) stated that this species could represent an ecological problem, it has the ability to flower several times a year, produces a large numbers of seeds which remain viable in the soil for many years, has the ability to quickly establish from seeds and can re-grow from vegetative fragments left in the soil after cultivation or cutting. The plants of Poa spp also show a relatively high B accumulation, but this species has the inconvenient to produce low biomass, a drawback characteristic for its use for phytoextraction purposes. Another possible candidate for the B phytoextraction purpose could be the B. napus since when it grow in the contaminated environment shows a relatively high B translocation and accumulation in shoots, even in a sediment with lower B available fraction (Table 2). The Brassicaceae species can have an intensive high biomass production (is a crop species compared to the others) and as a dicotyledonous plants requires about four times more B for nutrition and growth than the monocotyledonous (Fleming 1980).
Although no B hyperaccumulator plant was found among the native species investigated, this part of the study indicates the Brassicaceae species as a candidate for B phytoextraction on this sediment. This prompted our study to perform the laboratory phytoextraction tests using dicotyledonous high biomass crop species like B. juncea and H. annuus and a tolerant monocotyledonous such as Z. mays to investigate its ability to phytoextract the element from the contaminated sediment of Cecina basin. The cultivar of crops used in this work was those commonly used by the Italian farmers. In general, the Italian soils are characterized by a pH from neutral to alkaline, especially in the area under study, probably due to the vicinity of the coast (Soil Tuscany Maps 2010; Mazzanti 1966). The basicity of collected sediments (the pH ranged from 8.2 to 8.7, Table 1) could be unfavorable to the optimal growth of selected crops, since their optimal pH ranged from 5.5 to 7.0. Thus, in addition to the toxicity of high B levels in some collected sediments, plants could suffer the deficiency of nutritional elements (such as Fe, Mn, Cu, Zn) and of phosphorous compounds (that could be converted in less available forms) resulting in a reduced optimal availability for crops. These nutritional deficiency could be corrected using an acidic fertilizer, for example K2SO4 or (NH4)SO4, where the untaken anion can reduce the environment pH and increase the availability of nutrients and phosphorous.
The phytoextraction tests were performed in the worst scenario (BD2 sediment) and in uncontaminated sediment. Hypothetically, at the end of each phytoextraction test, all plants were at a similar size and growth stage as they grew in similar conditions (BD2 or C sediment, controlled chamber “environment”, and growing time); thus, the data from different species could be comparable. Control and BD2 sediment differed mainly by its B content, OM and nutrients availability (particularly Nitrogen). By sure, C sediment better support plants’ growth, but the reduced biomass production in BD2 sediment (Fig. 2a) could be also reasonably attributed to the high B level in plants and confirmed by the observation of the characteristic toxic effects due to boron. In fact, a yellowing on margin leaves in all tested plants from contaminated sediment and a less important toxic effect in the second growth (mainly in B juncea) was observed. Thus, this plant, seems more tolerant than the others as test II was performed with a significant reduction in the B available fraction (Table 3) producing a significant increase in biomass in test II respect to test I (Fig. 2a).
Plant tolerance or susceptibility to B is the result of both B availability in the matrix and very different plant physiological processes, which are species specific and could be expressed in a wide range of genetic variations within genotypes of a single species (Nable et al. 1990). Nevertheless, an extremely simplified and similar tolerance mechanism could be shared by most plants as they reduce the uptake of B in roots, although, under conditions of B toxicity high amounts can be found in plants and it seems to be governed by a passive transport, also influenced by the transpiration rate (Nable et al. 1997). The reduction of biomass and the visible yellowing of margin leaves in our plants are thus indexes of B sensitivity and toxicity, the last generally attributed to B immobility and concentration at the end of the transpiration stream (Brown and Shelp 1997).
Our results showed the high ability of tested plants, mainly B. juncea (cv Scala) and Z. mays (cv PR34N43), to extract B from this contaminated sediment with a significant reduction of available B fraction (Fig. 3 and Table 3). The phytoextraction efficiency calculated (Fig. 3) showed that in two growing cycles, Brassica plants were able to remove nearly half of the available fraction initially present in BD2 sediment. These high phytoextraction efficiency is undoubtedly a result connected to the capacity of these plants in tolerate high B content in their upper parts and also connected to the high root density in these experimental conditions. Similar conditions could be tried to achieve in field, testing these herbaceous plant species on a larger scale with specific fertilizers to improve biomass production and sowing superior seeds quantity than the usual in normal crops to promote high root density.
It is known that the secretion of root exudates or other associated compounds (mucilage, HCO -3 , H+) as well as the interactions with the microorganisms can alter the bioavailability of nutrients and the pH of the rhizosphere (Gobran et al. 2001). In our microcosms, the roots explored their entire surroundings and were able to influence them, rising the pH (Table 3). The increased pH (mainly after H. annuus growth) could be attributed to a possible B exclusion mechanism. Even if no universal extractant can predict the available fraction for all plants, the used one (mannitol + CaCl2) was able to distinguish the ability of each plant in reduce B available fraction. The extract obtained can be considered as the leachable portion of B and a tool to assess the potential B available for plant uptake (Chaudhary and Shukla 2004; Rhoades et al. 1970), indicating a rough estimation of the time necessary to reduce B from sediment using specific plants and could be a way to select the best performing plant for subsequent pilot tests.
Based on our results it seems that this extractant was suitable only for B. juncea plants as demonstrated by a mass balance evaluation. The mass balance Eq. 1 was obtained considering that all B found in plants should come from the fraction in sediment that was potentially available and neither the loss of B by leaching nor the addition of B by irrigation water was there (our microcosms were semi closed systems and deionized water was used for plant water needs):
where:
- C p :
-
is the B concentration in the plant (mg kg−1 dry matter)
- Y :
-
is the dry matter yield in each microcosm (kg)
- ∆C s :
-
is the variation of the available B fraction in sediment without plants and after the second growing cycle (mg kg−1 sediment)
- 0.05:
-
is a factor to convert the concentration of available B fraction (mg kg−1 sediment) in mass of B (mg) considering the mass of 50 g of sediment in each microcosm.
Therefore, the total mass of B ions taken up by B. juncea in the two growing cycles explain a significant change in the mass of B ions within the potential available B fraction in sediment, whereas the B recovered by plants corresponds to 86% of the change of B available in sediment. Applying the same equation for the other two plant species, the chemical extraction overestimated the plant available B fraction; thus, the time requested for remediation must be based on the biological test (plants accumulation) rather than the chemical extraction.
5 Conclusions
In this investigation, the aim was to assess in one first phase the feasibility of B phytoextraction from contaminated sediments. The indigenous plants collected colonizing these contaminated areas of the Cecina River basin did not show any ability to hyperaccumulate B. Although some species showed good capacity to accumulate B in the upper parts some inconvenient was posed on its use as a possible diffusion of the weed R. crispus and the relatively low biomass production by, for example, Poa spp. Results of laboratory tests using high biomass crop species showed promising results. All plants used in laboratory tests seem to be efficient in the reducing available B from the most contaminated sediment. B. juncea and Z. mays showed better phytoextraction performance than H. annuus due to their higher B uptake and biomass production. The reduction of 45% of B fraction in only two growing cycles of B. juncea is undoubtedly a promising result. Nonetheless, the feasibility of using annual herbaceous plant species needs to be tested on a larger scale, also assessing the use of specific fertilizers to improve the biomass production and the agronomic manipulation to obtain elevated roots density increasing the phytoextraction efficiency. This phase of study is still in progress; it will complete the feasibility of phytoextraction tests, and will permit establishing a low cost and low impact strategy for the B remediation of sediments from the Cecina basin. Moreover, harvested plant material may provide an additional value to the process as a source of bio energy, stock fodder, or as an amendment for B deficient soils. Thus, obtained results and observations can be helpful for enlarging the data on plants tolerant to B contamination and offer a base to explore specific agronomic manipulation to make the phytoextraction process a valid solution for the remediation of B polluted sediments.
References
Angin, I., & Turan, M. (2008). Humic acid addition enhances B and Pb phytoextraction by Vetiver grass (Vetiveria zizanoides (L.) Nash). Water, Air, and Soil Pollution, 188, 335–343.
Banuelos, G. S. (1996). Managing high levels of Boron and Selenium with trace elements accumulator crops. Journal of Environmental Science and Health. Part A: Toxic/Hazardous Substances & Environmental Engineering, 31(5), 1179–1196.
Banuelos, G. S., Cardon, G., Mackey, B., Ben-Asher, J., Wu, L., Beuselinck, P., et al. (1993). Boron and selenium removal in boron-laden soils by four sprinkler irrigated plant species. Journal of Environmental Quality, 22(4), 786–792.
Branquinho, C., Serrano, H. C., Pinto, M. J., & Martins-Louçao, M. A. (2007). Revisiting the plants hyperaccumulation criteria to rare plants and earth abundant elements. Environmental Pollution, 146(2), 437–443.
Brown, P. H., & Shelp, B. J. (1997). Boron mobility in plants. Plant and Soil, 193(1–2), 85–101.
Cartwright, B., Zarcina, B. A., & Spouncer, L. A. (1986). Boron toxicity in south Australian barley crops. Australian Journal of Agricultural Research, 37(4), 351–359.
Chaudhary, D. R., & Shukla, L. M. (2004). Evaluation of extractants for predicting availability of boron to mustard in arid soils of India. Communications in Soil Science and Plant Analysis, 35(1–2), 267–283.
Del-Campo Marin, C. M., & Oron, G. (2007). Boron removal by the duckweed Lemna gibba: a potential method for the remediation of boron-polluted waters. Water Research, 41(20), 4579–4584.
Di Gregorio, S., Barbafieri, M., Lampis, S., Sanangelantoni, A. M., Tassi, E., & Vallini, G. (2006). Combined application of Triton X-100 and Sinorhizobium sp. P002 inoculum for the improvement of lead phytoextraction by Brassica juncea in EDTA amended soil. Chemosphere, 63(2), 293–299.
Fleming, G. A. (1980). Essential micronutrients. I: Boron and Molibdenum. In B. E. Davies (Ed.), Applied soil trace elements. New York: John Wiley and Sons.
Gobran, G. R., Wenzel, W. W., & Lombi, E. (2001). Trace elements in the rhizosphere. Florida: CRC Press LLC.
Goldbach, H. E., & Wimmer, M. A. (2007). Boron in plants and animals: is there a role beyond cell wall structure? Journal of Plant Nutrition and Soil Science, 170(1), 39–48.
Goldberg, S. (1997). Reactions of boron with soils. Plant and Soil, 193(1–2), 35–48.
Grassi, S., & Squarci, P. (2004). La contaminazione da boro lungo il fiume Cecina. Atti della Società Toscana di Scienze Naturali, Serie A, 109, 21–28.
Keren, R. (1996). Boron. In D. L. Sparks (Ed.), Methods of soil analysis, Part 3-chemical methods (pp. 603–626). USA: SSSA-Soil Science Society of America Book Series, Inc Madison.
Leyshon, J. A., & Jame, W. Y. (1993). Boron toxicity and irrigation management. In U. C. Gupta (Ed.), Boron and its role in crop production (pp. 207–226). Florida: CRC Press Inc.
Litovitz, T. L., Klein-Schwartrtz, W., Oderda, G. M., & Schmitz, B. F. (1988). Clinical manifestation of toxicity in a series of 784 boric acid ingestions. The American Journal of Emergency Medicine, 6, 109–203.
Mazzanti, R. (1966). Geologia della zona di Pomarance-Larderello. Memorie della Società Geologica Italiana, 5, 105–138.
McGrath, S. P., & Zhao, F.-J. (2003). Phytoextraction of metals and metalloids from contaminates soils. Current Opinion in Biotechnology, 14(3), 277–282.
Meers, E., Hopgood, M., Lesage, E., Vervaeke, P., Tack, F. M. G., Verloo, M. G. (2004). Enhanced Phytoextraction: in search of EDTA alternatives. International Journal of Phytoremediation, 6, 95–109.
Murray, F. J. (1995). A human health risk assessment of Boron (boric acid and borax) in drinking water. Regulation of Toxic and Pharmacoogyl, 22, 221–230.
Nable, R. O., Lance, R. C. M., & Cartwrigth, B. (1990). Uptake of boron and silicon by barley genotypes with different susceptibilities to boron toxicity. Annals of Botany, 66(1), 83–90.
Nable, R. O., Banuelos, G. S., & Paul, J. G. (1997). Boron toxicity. Plant and Soil, 193(1–2), 181–198.
O’Neil, M. A., Ishii, T., Albersheim, P., & Darvill, A. G. (2004). Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annual Review of Plant Biology, 55(1), 109–139.
O’Sullivan, K., & Taylor, M. (1983). Chronic boric acid poisoning in infants. Archives of Disease in Childhood, 58(9), 734–749.
Parks, J. L., & Edwards, M. (2005). Boron in the environment. Critical Reviews in Environmental Science and Technology, 35(2), 81–114.
Pedron, F., Petruzzelli, G., Barbafieri, M., & Tassi, E. (2009). Strategies to use phytoextraction in very acidic soil contaminated by heavy metals. Chemosphere, 75(7), 808–814.
Pennisi, M., Gonfiantini, R., Grassi, S., & Squarci, P. (2006). The utilization of boron and strontium isotopes for the assessment of boron contamination of the Cecina River alluvial aquifer (central-western Tuscany, Italy). Applied Geochemistry, 21(4), 643–655.
Rhoades, J. D., Ingvalson, R. D., & Hatcher, J. T. (1970). Laboratory determination of leachable soil boron. Soil Science Society of America Proceedings, 34(6), 871–5.
Robinson, B. H., Green, S. R., Mills, T. M., Clothier, B. E., Van der Velde, M., Leplane, R., et al. (2003). Phytoremediation: using plants as biopumps to improve degraded environments. Australian Journal of Soil Research, 41(3), 599–611.
Robinson, B. H., Green, S. R., Chancerel, B., Mills, T. M., & Clothier, B. E. (2007). Poplar for the phytomanagement of boron contaminated sites. Environmental Pollution, 150(2), 225–233.
Smith, R. A. (2001). Basic geology and geochemistry of borate. Ceramic Engineering and Science Proceedings, 22, 61–75.
Soil Tuscany Maps – Map of the pH of the topsoil - http://sit.lamma.rete.toscana.it/websuoli/. Accessed in June 2010.
SSSA, Soil Science Society of America Book Series. (1996). Methods of soil analysis, Part 3-chemical methods. Inc Madison: USA.
Tassi, E., Pouget, J., Petruzzelli, G., & Barbafieri, M. (2008). The effects of exogenous plant growth regulators in the phytoextraction of heavy metals. Chemosphere, 71(1), 66–73.
USDA-NRCS US Department of Agriculture (2000). Natural resources conservation service, soil quality, urban technical note n.3: heavy metal soil contamination. http://soils.usda.gov/sqi/publications/publications.html. Accessed in January 2010.
Yanqun, Z., Yuan, L., Jianjun, C., Haiyan, C., Li, Q., & Schvartz, C. (2005). Hyperaccumulation of Pb, Zn and Cd in herbaceous grown on lead-zinc mining area in Yunnan, China. Environmental international, 31, 755–762.
Zaller, J. G. (2004). Ecology and non-chemical control of Rumex crispus and R. obtusifolius (Polygonacear): a review. Weed Research, 44, 414–432.
Acknowledgments
Research was funded by “Ministero dell’Ambiente e della Tutela Ambientale”, “Regione Toscana” and “Provincia di Pisa”. Authors are grateful to all subscribers of the Programme Agreement on “Remediation and Environmental Recovery of ex Mining and Industrial Site in the Area of Cecina River Basin” and to Dr. Sergio Grassi (CNR-IGG) for providing site data and assistance in the selection of areas to be investigated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tassi, E.L., Pedron, F. & Barbafieri, M. Evaluating the Absorption of Boron by Plants—A Potential Tool to Remediate Contaminated Sediments from Cecina River Basin in Italy. Water Air Soil Pollut 216, 275–287 (2011). https://doi.org/10.1007/s11270-010-0533-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0533-7