Abstract
This research is focused on evaluating heavy metals (Cd, Cu, Fe, Mn, Pb, and Zn) uptake and removal by Eleocharis ovata, Cyperus manimae, Typha dominguensis, and Pteridium aquilinum in a natural wetland impacted by mining activities. We analyzed heavy metals content and distribution in native plants, soils, and water of a semipermanent natural wetland in Taxco de Alarcón, Guerrero, and we also determined the physicochemical characteristics of the water. Translocation factor (TF) and bioconcentration factor (BCF) were evaluated. Results showed that physical and chemical conditions are favorable for plants development. Correlation analysis showed a good and positive relation (0.95) between Cu and Pb in soils and plants. In the analyzed matrices: Zn (0.62–2.20 mg/L) exceeded the permissible limits in water, high concentrations of Pb and Zn (26.57–525.67 and 266.67–983.33 mg/kg, respectively) were detected in the studied soils, and Pb exceeded the normal range for E. ovata and P. aquilinum in the analyzed plants. Uptake of heavy metals in the tissues of different species was found in the following order: root > leaf. Data of TF and BCF showed that E. ovata is a tolerant plant with respect to heavy metals exposure since TF value was greater than 1. This study showed that E. ovata could be considered as a bioaccumulator of heavy metals in contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are serious contaminants due to their toxicity, environmental persistence, and ability to be incorporated into the food chain and generate risks to the environment and human health (Sasmaz et al. 2008). The most important sources of heavy metals pollution in the environment are those generated by anthropogenic activities such as mining, smelting, mining-metallurgical industry, chemical industry, among others.
Tailings are wastes resulting from the crushing, grinding, and processing (by physical and chemical methods) of rocks until the desired ore is obtained. These residues are usually placed near the processing site, and once exposed to the weather, can be oxidized and release heavy metals by the action of rain and other factors which may reach the soil and water bodies altering water quality (Romero et al. 2008).
The concentration of heavy metals in soil and water increases due to the amount of discharged wastes to the surroundings. Their concentration and availability play an important role in controlling the bioavailability of metals to plants, which depends on soil type, HM solubility in water, plant growth status, and plant species. Heavy metals cannot be degraded in the environment, so they are continuously deposited in water bodies and soil.
The presence of heavy metals in soil and water can affect wildlife, plant growth, and others (Popescu et al. 2009). The collection and accumulation of heavy metals in plants follows two different paths: (1) through the roots and (2) through the leaf surface (Sawidis et al. 2001). This uptake and accumulation are also modified by the plants’ life cycle, biomass, and interaction with microorganisms present in the rhizosphere.
Heavy metals exist in water bodies, sediments, plants, and other organisms (Engin et al. 2015). As they also affect the environment, heavy metals distribution can also change flora and fauna distribution in wetlands, due to different abiotic and biotic factors. It is thus important to investigate pollutants removal processes by different routes as plants, microorganisms, and abiotic factors; they can influence the mobility and translocation of heavy metals.
Contamination by mining activities has generated many environmental problems worldwide. In the study zone of Taxco de Alarcón, Guerrero, several geochemical and biological studies have been published (Romero et al. 2008; Ruiz-Huerta and Armienta-Hernández 2012; Gómez-Bernal et al. 2014). However, important plant species are being defined for the removal of pollutants from anthropogenic activities in the area and the implementation of wetlands around sites close to mineral residues (Gómez-Bernal et al. 2017). Therefore, this study aims to evaluate heavy metals (Cd, Cu, Fe, Mn, Pb, and Zn) uptake and removal by Eleocharis ovata, Cyperus manimae, Typha dominguensis, and Pteridium aquilinum in a natural wetland impacted by mining activities, as a case study possibly to implement at other similar areas and to propose species able to remediate this kind of contamination.
Materials and methods
Geographic delimitation
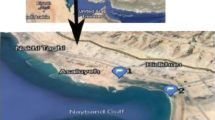
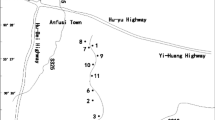
Plants were collected in a semipermanent natural wetland (300 m2) located in Taxco de Alarcon, Mexico (Fig. 1). Taxco is a municipality located in the northern part of the state of Guerrero, at an altitude of 2600 m above sea level (18°21′N, 99°47 W). The annual temperature varies between 12 and 26 °C, and the range of annual precipitation is 800–1500 mm (INEGI 1999). “La Concha” tailing pond is located north of San Antonio mine (Fig. 1), at 18°32′22.27191″N, 99°38′9.0537″. It is a deposit of irregular shape of 140 m long, 50 m wide, and 10 m height (CRM 2003).
Collection of samples
Selection of sampling points was designed according to the following: Soil collection was carried out where the most abundant plant species grew along the natural wetland being eight sampling points at a depth of 30 cm (points 1–8, Fig. 1) and one more point in an artificial wetland receiving wastewater from the mine (point b, Fig. 1). The rhizosphere was also collected at each sampling point. Water samples were taken from the mine water outlet and from the natural wetland during the dry season (Fig. 1).
Physicochemical parameters of water
Water samples were collected in 250 mL polyethylene bottles previously washed with HCl (1 N) and rinsed with distilled water. Temperature, pH, and electrical conductivity were measured with a portable multiparameter (HANNA, HI 9829). Water samples were also collected at each point acidifying with HNO3 (0.1 M), filtered through a 0.45-μm pore membrane and stored under refrigeration until laboratorial analysis. Analyses of main cations and anions were carried out using the techniques described by Armienta et al. (1987) and APHA (2005). Metals concentrations were determined by atomic absorption spectrometry (Perkin Elmer AAnalyst 200).
Determination of heavy metals in soils
Soil samples were dried, homogenized, and sieved through 0.063 mm. Half a gram of each sample was weighed, added with 10 mL aqua regia (HNO3 and HCl 3:1), and digested during 30 min in a microwave oven CEM, MARSXpress. The digestions were taken to 100 mL with deionized water and filtered through Whatman 40. Triplicates of each sample were treated in this way. Concentrations of Cd, Cu, Fe, Mn, Pb, and Zn in soil were determined by flame atomic absorption spectrometry (Perkin Elmer AAnalyst 200). High purity standards (Certified reference materials NIST traceable, Montana soil 2711) were used for calibration.
Determination of heavy metals in plants
We collected randomly nine plant samples. Plants samples were carefully washed in the laboratory with running tap water, followed by three rinses with deionized water (18 MΩ/cm, Milli-Q Millipore), and a rinse of tri-distilled water. All plants were carefully divided into roots and leaves. A dry weight of the collected plants was obtained after heating at 60 °C for 75 h in an oven; the samples were then crushed, sieved (< 325 μm), homogenized, and weighed. The digestions were performed open: 0.5 g of each part was weighed and cut into small pieces and open digested with concentrated HNO3 and HClO4. The accuracy of the procedure was determined by analyzing the certified reference material peach leaves (SRM 1547) for plants for quality control. Concentrations of Cd, Cu, Fe, Mn, Pb, and Zn in plants were determined by flame atomic absorption spectrometry (Perkin Elmer AAnalyst 200).
Translocation and bioconcentration factors
The values of the heavy metals concentrations were used to estimate the translocation factor (TF) and the bioconcentration factor (BCF). TF was defined as the average of the heavy metal concentration in leaves divided by the heavy metal concentration in roots (Ruiz-Huerta and Armienta-Hernández 2012; Gómez-Bernal et al. 2014). The BCF was defined as the average of the heavy metals concentration in plant tissues divided by the heavy metals concentration in soil (Ruiz-Huerta and Armienta-Hernández 2012; Gómez-Bernal et al. 2014).
Results and discussion
Physicochemical parameters of water
The pH in the water ranged from 8.20 to 8.55, complying with ecological criteria for water quality CE-CCA-001/89 (SEDUE 1990), which is 6.0–9.0 for recreational and industrial water, and with USEPA (1986) that establishes mean pH values between 6.5 and 9.0 for aquatic life. The electrical conductivity ranged from 571 to 797 μS/cm (Table 1). The normal range proposed by WHO (1980) is 400–600 μS/cm. Our data exceeded these values for the first four sampling points. The range of carbonates (CO3 2−) was from 13.07 to 22.57 mg/L. The range of bicarbonates (HCO3 −) was 115.96–217.42 mg/L (Table 1). These parameters have their own importance as part of the environment necessary for biological processes. The range of sulfates (SO4 2−) in water was from 165.17 to 212.32 mg/L. Chlorides ranged from 9.21 to 12.05 mg/L. The range of sodium in water was from 5.16 to 6.80 mg/L. Nitrates (NO3 −) varied from 9.32 to 28.20 mg/L (Table 1). Calcium (Ca2+) ranged from 95.03 to 141.06 mg/L. The range of magnesium in water was from 13.50 to 23.63 mg/L.
Table 2 reports concentrations of heavy metals in water. Magnesium ranged from 13.50 to 23.63 mg/L. Manganese was not detected in any sample. The range of Zn was from 0.62 to 2.20 mg/L. The limit for this last metal proposed by WHO (1980) is 5 and 2 mg/L by SEDUE (1990). The limit proposed by WHO (1980) was not exceeded. However, for SEDUE (1990), concentrations were higher at two sampling points (1 with 2.20 mg/L and 2 with 2.15 mg/L) decreasing toward the sampling point 4 (0.62 mg/L) and increasing to 1.60 mg/L at sampling point 5. This could be explained for sampling point 4 because there is in this site abundant Eleocharis ovata, a plant species that has been reported to colonize mining shafts and impacted areas with high concentrations of heavy metals (Lottermoser and Ashley 2011).There is also a difference in altitude with respect to sampling points 3 and 4. Cadmium was not detected in the water at any sampling point. This could be explained by the pH values since the solubility of Cd, Pb, Cu, and Zn is low (Sukreeyapongse et al. 2002) at the measured pH (Table 1). Concentrations of heavy metals are below the maximum allowable limits established by Mexican regulation (NOM-001-SEMARNAT-1996) for natural wetlands (Table 2).
Soils
Cd occurred only in inorganic form and in one oxidation state (2+) in natural environments, and its bioavailability depends greatly on soil conditions (Clemens and Ma 2016). Alloway (1995) proposed a limit of 60–125 mg/kg of Cu for soils which was not exceeded at any sampling point. The NOM (2003) indicates that the permissible limit is 20 mg/kg, which was not exceeded at the sampling points. The US EPA (1992) contemplates a range of 3–10 ppm of Cd in surface soil, considering the excessively phytotoxic sites exceeding this range. Concentrations of the heavy metals in the studied soils were within the permissible limits (Table 3). The range of Cd in wetland soils was 1.88–9.25 mg/kg. Sampling points with the highest concentrations of Cd were 6.33, 7.77 and 9.25 mg/kg, for points 6, 7, and 8, respectively; the latter concentrated more Cd in the soil (Table 3). This shows that Cd in the wetland soils did not exceed any proposed limit by US EPA (1992), Alloway (1995) and NOM (2003). Cd toxicity in soil is well known as a heavy metal that causes toxicity even at low concentrations (Khan et al. 2015).
European Union’s regulatory commission (EU) (2006) sets a limit of 100 mg/kg of Cu in soils, while the limit in India is 135–270 mg/kg (Awashthi 2000). However, in any case, this limit was not exceeded at the studied area. Cu ranged from 0.56 to 9.83 mg/kg in soils. The highest concentrations of Cu were measured at sampling points 6, 7, 8, and 9 (9.73, 9.83, 9.13, and 10.83 mg/kg, respectively) (Table 3).
Iron concentration ranged from 205.00 to 1200.03 mg/kg in the soil. The highest concentrations were at the sampling points 6, 7, and 9 (1039.63, 750.00, and 1200.03 mg/kg, respectively). The highest concentration was measured at sampling point 9 (Table 3).
Alloway (1995) proposed a limit for Mn of 5000 mg/kg in soils, which was not exceeded at any of the sampling points. The range at the sampling points of soils was from 31.17 to 94.66 mg/kg. The highest sampling point concentrations were 82.00, 68.33, 66.33, and 94.66 mg/kg, for points 6, 7, 8, and 9, respectively. The highest concentration was measured at sampling point 9 (Table 3).
Pb is one of the first anthropogenic pollutants, being highly persistent in soils and hardly bioavailable under most conditions due to its low solubility at pH greater than 5; it has a strong interaction with organic matter (Clemens and Ma 2016). Pb concentration ranged from 26.57 to 525.67 mg/kg; sampling points 6, 7, and 8 exceeded the limit of the Mexican regulation (NOM-147-SEMARNAT/SAA1-2004) of 400 ppm, the limit proposed by Alloway (1995) of 100–400 mg/kg of Pb and the limit of 420 mg/kg established by the USEPA (2005). Sampling points with concentrations higher than those proposed by NOM-147-SEMARNAT/SAA1-2004 Alloway (1995) and USEPA (2005) were 6, 7, and 8 (457.33, 480.33 and 525.67 mg/kg, respectively) (Table 3).
Alloway (1995) proposed a limit of 70–400 mg/kg of Zn in soils, which was exceeded at sampling points 1, 2, 6, 7, 8, and 9 (891.67, 983.33, 583.33, 658.33, 816.67, and 608.33 mg/kg, respectively) (Table 3). The sampling site with the highest concentration of Zn was point 2 (983.33 mg/kg), exceeding the limit of Alloway (1995). Gómez-Bernal et al. (2014) found the following concentrations of heavy metals in “La Concha” tailings: Pb (19125.05 mg/kg), Zn (37994.35 mg/kg), Cu (1039.51 mg/kg), Mn (15.84 mg/kg), and Fe (19.15 mg/kg). In the soils surrounding “La Concha,” the concentrations were as follows: Pb (3.87–49.91 mg/kg), Zn (70.48–109 mg/kg), and Cu (50.90–52 mg/kg). The values measured in this study were higher for Pb and Zn (27–525.67 and 266.67–983.33 mg/kg, respectively) and lower for Cu (0.56–10.83 mg/kg). This difference in concentrations in the wetland may be due to the proximity of points 7–9 to “La Concha” tailings.
Correlation analysis is widely used in environmental studies and provides an efficient method of revealing relationships between multiple variables (Zhuang and Gao 2014; Xie et al. 2016). Table 4 shows the correlation of heavy metals in the soil, indicating the high correlation between Fe and Mn (0.99), Pb and Cu (0.96), as well as Pb and Cd (0.96), Cu and Fe (0.91), Cu and Mn (0.90). Principal component analysis (PCA) (Fig. 2) shows that Zn has no significant relation with the other metals. Likewise, the relationship between sampling points 1 and 2, as well as, 3–5 is evidenced by PCA.
The distribution of heavy metals in the soil was variable at sampling points 1 and 2, decreasing at sampling points 3–5 in almost all heavy metals with the exception of Cd, Pb, and Zn at sampling point 5. On the other hand, heavy metals concentrations varied at sampling points 6–9. At sampling point 9, the highest values for Cu, Fe, and Mn were observed (Table 3). Sampling point 8 presented the highest values for Cd and Pb. Clearly, there is retention at sampling points 3–5. The mobility and bioavailability of heavy metals in contaminated soils are affected by a number of biological processes and physicochemical properties such as soil characteristics, pH, organic matter content (Ahmad and Goni 2010), cation exchange capacity, and microbiota of the soil (Khan et al. 2015) (Fig. 3).
Plants
Plants growing on contaminated soils with high levels of cadmium show visible signs of leaf damage reflecting chlorosis, growth inhibition, oxidative stress, genotoxicity, inhibition of the photosynthetic apparatus, brown margins, wavy leaves, inhibition of the roots metabolism, and finally death (Sanita di Toppi and Gabbrielli 1999; Benavides et al. 2005; Mohanpuria et al. 2007; Guo et al. 2008; Mishra and Tripathi 2008; Andersen and Küpper 2013). Cd has a tendency to show interference with Fe uptake and transport and to the use of several elements such as Ca, Mg, P, and K in water by plants (Das et al. 1997). According to Allen (1989), plants in non-contaminated environments contain 0.01–0.30 mg/kg of Cd. In this study, the highest concentration for leaf was measured in E. ovata at sampling point 2 and in the roots of P. aquilinum at sampling point 8 (Table 5). A high translocation of Cd from the roots (0.48 mg/kg) to the leaves (0.96 mg/kg) in E. ovata was observed at sampling point 2; this sampling point is the only case. For the case of P. aquilinum at sampling point 9, leaf accumulation (0.48 mg/kg) was higher than in roots (0.42 mg/kg). It has been reported that both roots and leaves absorb considerable amounts of Cd (Kabata-Pendias and Pendias 2001; Sasmaz et al. 2008). Carranza-Alvarez et al. (2008) reported that roots of T. latifolia accumulated 25 mg/kg of Cd, which means that they exceed 50 times the phytotoxic concentrations. Sasmaz et al. (2008) reported average concentrations of Cd in soils of 0.23 mg/kg, as well as in plants of 0.44 mg/kg in roots and 0.21 mg/kg in leaves of T. latifolia, which show a great absorption of cadmium from sediments. Chandanshive et al. (2017) found lower concentrations of Cd in a textile effluent (0.07 mg/kg) for T. angustifolia (0.05 mg/kg), Paspalum scrobiculatum (0.05 mg/kg), and in the association of both plants (0.02 mg/kg). This shows that concentrations of Cd vary for each species of Typha and also present a different translocation; however, the lower concentrations may also be due to the aqueous speciation of Cd in the effluent.
Copper (Cu) is also an essential element for the growth of plants as an important constituent of oxidation–reduction reactions (Aksoy et al. 2005). However, it could cause toxic effects when it accumulates in plants’ roots and leaves exceeding health limits producing effects such as decreased plant growth and leaf chlorosis (Lewis et al. 2001). For Cu, the established limit by Borkert et al. (1998) is 20 mg/kg in plants; it was not exceeded at any sampling point. Sampling point 9 corresponding to P. aquilinum was the one that accumulated the highest Cu in leaves (0.41 mg/kg) and in roots (2.46 mg/kg) (Table 5). At sampling points 2 and 3, the roots concentration (0.29 and 0.15 mg/kg, respectively) with respect to leaves (0.32 and 0.15 mg/kg, respectively) indicated a high translocation being the only present case for E. ovata. Kabata-Pendias and Pendias (2001) reported Cu levels of several plants of uncontaminated zones in different countries in the range of 2.1 and 8.4 mg/kg. Ganjali et al. (2014) found that Cu tends to be accumulated in roots and is scarcely translocated to other organs of the plant. This was observed in the analyzed samples in this work (Table 5). Ha et al. (2011) found that E. acicularis accumulated a range of 14.4–20.5 mg/kg compared to the samples from this study in which E. ovata accumulated in the range of 0.15–0.77 mg/kg in root and 0.10–0.32 mg/kg in leaf, showing much less accumulation. It has been reported that Cu and Cd in combination strongly affect germination, seedling length, and number of lateral roots in Solanum melongena (Neelima and Reddy 2002).
Iron (Fe) is one of the essential elements for plants growth (Wintz et al. 2002) and plays an important role in energy transformation processes for synthesis and other life processes in cells (Klink et al. 2013). On the other hand, excessive accumulation damages tissues by the formation of free radicals (Engin et al. 2015). The damages caused by iron in the plants are related to the high uptake of Fe2+ by the roots and their transportation to the leaves and stems. The excess of Fe2+ causes the production of free radicals that alters irreversibly the cellular structure and damages plants membranes, DNA, and proteins. (Dorlodot et al. 2005). In the study, the highest concentration in the root was from T. dominguensis (75.83 mg/kg) and in leaves from E. ovata at sampling point 2 (29.20 mg/kg) (Table 5). At sampling point 2, concentrations in the root (16.30 mg/kg) and leaves (29.20 mg/kg) of E. ovata indicate a higher translocation; it is the only present case.
Mn is an essential element for plants and is used in many redox enzymatic processes and photosynthesis (Carranza-Álvarez et al. 2008). Mn is rapidly transported from the roots to the aerial parts through the stem, but is not rapidly mobilized through the phloem to other organs after the leaves (Loneragan 1988). However, the Mn limit proposed by Kabata-Pendias and Pendias (2001) is 300 mg/kg for phytotoxic concentrations, and this was not exceeded at the sampling points. The highest concentrations in roots were observed in P. aquilinum (11.83 mg/kg) and in leaves from E. ovata at sampling point 2 (22.01 mg/kg) (Table 3). E. ovata showed a high translocation from roots to leaves only in site 2. Wu (1994) found that the visible symptoms of Mn toxicity are necrotic scores in leaves, petioles, and stems, which were not observed in the species of studied plants. Pais and Jones (2000) found that some plants can grow at concentrations as high as 1500 mg/kg without damage. Baldantoni et al. (2004) indicate that a high accumulation of Mn in the roots implies a high availability in sediments. Engin et al. (2015) found for T. latifolia concentrations of Mn in the range of 177.09–906.35 mg/kg; the concentration of T. dominguensis at sampling point 1 was 4.63 mg/kg indicating the great variability of accumulation of heavy metals in the genus Typha.
Lead (Pb) is not an essential element for the growth of plants but it is absorbed by them with other elements. Kabata-Pendias and Pendias (2001) reported that Pb contents of plants growing in non-contaminated areas ranged from 0.05 to 3.0 mg/kg. Reeves and Baker (2000) present a normal range of Pb in plants from 0.1 to 5 mg/kg, which is exceeded in E. ovata and P. aquilinum at sampling points 4, 5, 8, and 9 (5.30, 7.43, 62.38, and 92.50 mg/kg, respectively). The highest values were in P. aquilinum at sampling point 9 in leaves (2.68 mg/kg) and roots (92.50 mg/kg) (Table 5). Beckett and Davis (1977) proposed a toxic concentration for plants of 27 mg/kg, which was exceeded by samples 8 and 9 in roots, indicating a high accumulation of Pb in plants. Outridge and Noller (1991) found ranges of 6.3 and 9.9 mg/kg in freshwater plants without contamination. Chandanshive et al. (2017) found a decrease in Pb concentration in a textile effluent (0.42 ppm) for T. angustifolia (0.23 ppm), Paspalum scrobiculatum (0.21 ppm), and the association of both plants (0.13 ppm). In the present study, T. dominguensis contained high concentration in roots.
Zn is an essential element which is related to physiological and metabolic processes in all plants. However, in high concentrations, it can be toxic. Reeves and Baker (2000) present a normal range in plants of 20–400 mg/kg, which was not exceeded at any sampling point. The highest concentrations of Zn were observed in E. ovata leaves at sampling point 2 (311.66 mg/kg) and in E. ovata roots at sampling point 4 (254.16 mg/kg). E. ovata showed a high translocation of Zn from roots to leaves at sampling point 2 (89.16 and 311.66 mg/kg, respectively) (Table 5). Engin et al. (2015) found for T. latifolia concentrations of Zn in the range of 9.96–292.04 mg/kg, while for sampling point 1 corresponding to T. dominguensis was 163.33 mg/kg, which demonstrates the great variability of accumulation of heavy metals that can be altered depending on the size of the plant, as well as on the characteristics of the environment. Ha et al. (2011) found that E. acicularis accumulated Zn in the range from 44 to 73.5 mg/kg compared to the samples from this study in which E. ovata accumulated in the range of 35–254.16 mg/kg in root and 9.33–311.66 mg/kg in leaf. These values are much higher than those found by Ha et al. (2011).
The accumulation of heavy metals in the studied plants followed the order Zn > Fe > Pb > Mn > Cu > Cd. Data demonstrated that Zn is the most available element, which is in agreement with the studies by Morton-Bermea et al. (2014), who reported that in “La Concha” tailings 32.2–39.8% of a total concentration of 23,625–40,250 mg/kg is in the available fraction, indicating a high availability of Zn to the environment. However, different factors may modify this accumulation behavior since at other sampling points a decrease in the accumulation of heavy metals was observed such as E. ovata at point 4 for Zn, and P. aquilinum at sampling points 8 and 9 for all heavy metals. This may be due to the fact that these sampling points are located on the edges of the wetland. It is also notable that different plant species show different toxicities to the same pollutant and in the same environmental condition, being due to the mechanism of capture of heavy metal by plants, which is not the same for all plant species (Clemens 2006).
Table 6 shows the correlations of heavy metals in plants, indicating the high correlation between Pb and Cu (0.95) and Zn and Mn (0.89). Principal component analysis (Fig. 4) showed that Zn and Mn, as well as Pb and Cu have a good correlation.
Translocation factor
In our study, high translocation factor values were calculated for E. ovata at sampling point 2 for Cd (1.97), Cu (1.10), Fe (1.79), Mn (2.01), Pb (1.52), and Zn (3.49) (Table 7). High values were also present in E. ovata at sampling point 3 for Cu (1.02) and P. aquilinum at sampling point 9 for Cd (1.13). Also, the decrease in heavy metals from sampling points 2 to 5 was observed, demonstrating the accumulation of heavy metals, which E. ovata develops. The translocation factor in T. dominguensis is smaller due to its location in the artificial wetland where the flow of the water is very fast affecting the translocation of heavy metals.
Cadmium is a highly toxic non-essential element, which influences the growth, metabolism, and water status of plants (Bonanno and Giudice 2010). Sasmaz et al. (2008) reported that both roots and leaves absorb Cd considerably. Copper is an element for the nutrition of plants and necessary for various oxide-reduction enzymatic activities. Thus, Cu tends to accumulate in roots and is scarcely translocated to the upper organs (Siedlecka et al. 2001). This can be seen in most of the sampling points (Table 7).
Yanqun et al. (2005) reported that higher TF values correspond to accumulating species and lower values to species that exclude heavy metals. These values greater than 1 indicate an ability to transport heavy metals from the root to the leaf and probably to the metal sequestration in vacuoles of the leaf and apoplast (Lasat et al. 2000). It is also important to mention that many factors can influence TF values in plants, such as plant species, physiological factors, and metals characteristics. Table 7 shows that at sampling point 2 corresponding to E. ovata, TF values are higher than 1 for all the analyzed heavy metals, the highest one was for Mn (2.01) and Zn (3.49). At the other studied points (sampling point 3 with 1.02 for Cu), TF was also generally higher with respect to the other plant species with the exception of sampling point 9 in which Cd TF was 1.13 corresponding to P. aquilinum.
Figure 5 shows the decrease in the concentration of Zn in both soil and water at sampling points 3–5, so in E. ovata, at sampling point 2, a high transfer of all heavy metals from the roots to the leaves was observed demonstrating that the first plants in the natural wetland receiving wastewater from the mine are those that have a greatest impact on the accumulation of heavy metals. The high population density of E. ovata may also be a factor of the heavy metals concentration at sampling points 3–5.
The morphological and physiological characteristics of E. ovata have developed a unique strategy to reduce the damages caused by heavy metals, and this may be partially responsible for the differences of the values of TF in this study. It should be noted that E. ovata may be used as an important filter for the removal of heavy metals from the following considerations: (1) they did not show visible damage and there were no differences in height between the different samples of E. ovata; (2) TF and BCF had values greater than 1; (3) it has a fast growth when being herbaceous; (4) wide geographic distribution; (5) ease of cultivation by seeds or stolons; and 5) facility to be collected. This is based on their form and life cycle, as well as on the data obtained during this research.
Bioconcentration factors
Table 8 shows the bioconcentration factors of all the studied plants. The highest values of the BCF were for E. ovata at sampling point 2 for Cd (0.42), Cu (1.08), Mn (0.59), and Pb (0.42), sampling point 4 for Zn (1.05) and for sampling point 5 in Cu (1.14), and for T. dominguensis at sampling point 1 for Fe (0.21). It should be noted that the species E. ovata presented the highest values of the BCF in general for heavy metals. It is observed, at sampling point 2, that it acts as a barrier for heavy metals dispersion (Fig. 3).
Conclusion
Results of this study indicate that E. ovata might capture Cd, Cu, Mn, Pb, and Zn of contaminated soils with heavy metals showing a good efficiency since the physicochemical factors of water are in the normal range for the development of the plants. The soils were mostly within the allowable ranges (Cd, Cu, and Mn), and only Fe, Pb, and Zn exceeded the limits at sampling points 6, 7 and 8, and also at points 1 and 2. The analyzed species, Eleocharis ovata, Cyperus manimae, Typha dominguensis, and Pteridium aquilinum, grow in a wetland near the heavy metals contaminated site and are tolerant to these metals. Plants accumulate Cd, Fe, Pb, and Zn over the toxic limits at several sampling points without any visible changes. From the analyzed species, E. ovata showed the highest values for TF (1.10–3.49) for most analyzed heavy metals and BCF for Cd, Cu and Zn (0.17–0.42, 0.20–1.14, and 0.17–1.05, respectively). Therefore, E. ovata might be considered as a good candidate to be a phytostabilizing species of heavy metals in natural and artificial wetlands, thus preventing their dispersion to the environment. On the other hand, it is necessary to investigate the phytoremediation potential of E. ovata in future greenhouse studies with different concentrations of heavy metals to confirm the potential of this plant as well as to establish the requirements of cultivation and its possible implementation in artificial wetlands.
References
Ahmad JU, Goni MA (2010) Heavy metal contamination in water, soil, and vegetables of the industrial areas in Dhaka, Bangladesh. Environ Monit Asses 166:347–357
Aksoy A, Demirezen D, Duman F (2005) Bioaccumulation, detection and analyses of heavy metal pollution in Sultan Marsh and its environment. Water Air Soil Pollut 164:241–255
Allen SE (1989) Chemical analysis of ecological materials. Blackwell Scientific, Oxford
Alloway BJ (1995) Heavy metals in soils. Chapman and Hall, Cambridge
American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF) (2005) Standard methods for the examination of water and wastewater, vol 1, 21st edn. American Public Health Association, Washington
Andersen E, Küpper H (2013) Cadmium toxicity in plants. In: Sigel A, Sigel H, Sigel RKO (eds) Cadmium: From toxicity to essentiality. Springer, pp 395–413
Anteproyecto de Norma Oficial Mexicana (2003) Que establece criterios para determinar niveles de limpieza para la remediación de suelos contaminados por metales y metaloides. Secretaría del Medio Ambiente y Recursos Naturales
Armienta MA, Zamora V, Juárez F (1987) Manual para el Análisis Químico de Aguas Naturales, en el Campo y en el Laboratorio, instituto de Geofísica. Universidad Nacional autónoma de México, Comunicaciones técnicas, Serie Docencia y Divulgación No. 4
Awashthi SK (2000) Prevention of Food Adulteration Act No 37 of 1954. Central and state rules as amended for 1999. Ashoka Law House, New Delhi
Baldantoni D, Alfani AD, Tommasi P et al (2004) Assessment of macro and microelement accumulation capability of two aquatic plants. Environ Pollut 130:149–156
Beckett HT, Davis RD (1977) Upper critical levels of toxic elements in plants. New Phytol 79:95–106
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34
Bonanno G, Giudice RL (2010) Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol Indic 10:639–645
Borkert CM, Cox FR, Tucker Mr (1998) Zinc and copper toxicity in peanut, soybean, rice, and corn in soil mixtures. Commun Soil Sci Plant Anal 29:2991–3005
Carranza-Alvarez C, Alonso-Castro AJ, Alfaro-De La Torre MC, García-De La Cruz RF (2008) Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial lagoon in San Luis Potosí, México. Water Air Soil Pollut 188:297–309
Chandanshive VV, Rane NR, Tamboli AS, Gholave AR, Khandare RV, Govindwar SP (2017) Co-plantation of aquatic macrophytes Typha angustifolia and Paspalum scrobiculatum for effective treatment of textile industry effluent. J Hazard Mater 338:47–56
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Clemens S, Ma JF (2016) Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol 67:489–512
Consejo de Recursos Minerales (CRM) (2003) Carta Geológico-Minera Taxco E14-A68 Secretaria de Economía –CRM
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98:29–36
Dorlodot S, Lutts S, Bertin P (2005) Effects of ferrous iron toxicity on the growth and mineral composition of an inter specific rice. J Plant Nutr 28:1–20
Engin MS, Uyanik A, Kutbay HG (2015) Accumulation of heavy metals in water, sediment and wetland plants of Kizilirmak delta (Samsun, Turkey). Int J Phytoremediation 17:66–75
European Union (2006) Commission Regulatory (EC)No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union 364:5–24
Ganjali S, Tayebi L, Atabati H et al (2014) Phragmites australis as a heavy metal bioindicator in the Anzali wetland of Iran. Toxicol Environ Chem 96:1428–1434
Gómez-Bernal JM, Morton-Bermea O, Ruiz-Huerta EA et al (2014) Microscopic evidences of heavy metals distribution and anatomic alterations in breaching-leaves of Cupressus lindleyi growing around mining wastes. Microsc Res Tech 77:714–726
Gómez-Bernal JM, Ruiz-Huerta EA, Armienta-Hernandez Ma et al (2017) Phytoavailability index of heavy metals and arsenic in Pioneer plants of a semi-permanent natural wetlands. Environ Prog Sustain Energy. https://doi.org/10.1002/ep.12759. Version of Record online: 21 Sept 2017
Guo J, Dai X, Xu W et al (2008) Over expressing GSHI and AsPCSI simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere 72:1020–1026
Ha NTH, Sakakibara M, Sano S (2011) Accumulation of Indium and other heavy metals by Eleocharis acicularis: an option for phytoremediation and phytomining. Bioresour Technol 102:2228–2234
INEGI (Instituto Nacional de Estadística, Geografía e Informática) (1999) Síntesis Geográfica del Estado de Guerrero. Instituto Nacional de estadística, Geografía e Informática, Aguascalientes, México
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants. CRC Press, Washington
Khan S, Waqas M, Ding F, Shamshad I, Arpd HPH, Li G (2015) The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J Hazard Mater 300:1–11
Klink A, Maciol A, Wislocka M, Krawczyk J (2013) Metal accumulation and distribution in the organs of Typha latifolia L. (cattail) and their potential use in bioindication. Limnol Ecol Manag Inland Waters 43:164–168
Lasat MM, Pence NS, Garvin DF et al (2000) Molecular physiology of zinc transport in the Zn hyperaccumulator Thlaspi caerulescens. J Exp Bot 51:71–79
Lewis S, Donkin ME, Depledge MH (2001) Hsp 70 expression in Enteromorpha intestinalis (Chlorophyta) exposed to environmental stressors. Aqua Toxicol 51:277–291
Loneragan JF (1988) Distribution and movement of manganese in plants. In: Graham RD, Hannam RJ, Uren NC (eds) Manganese in soils and plants. Springer, Berlin
Lottermoser BG, Ashley PM (2011) Trace element uptake by Eleocharis equisetina (spike rush) in an abandoned acid mine tailings pond, northeastern Australia: implications for land and water reclamation in tropical regions. Environ Pollut 159:3028–3035
Mishra VK, Tripathi BD (2008) Concurrent removal and accumulation of heavy metals by the three aquatic macrophytes. Bioresour Technol 99:7091–7097
Mohanpuria P, Rana NK, Yadav SK (2007) Cadmium induced oxidative stress influence on glutathione metabolic genes of Camella sinensis (L.) O Kuntze. Environ Toxicol 22:368–374
Morton-Bermea O, Gómez-Bernal JM, Armienta MA et al (2014) Metal accumulation by plant species growing on a mine contaminated site in Mexico. Environ Earth Sci 71:5207–5213
Neelima P, Reddy KJ (2002) Interaction of copper and cadmium with seedlings growth and biochemical responses in Solanum melongena. Environ Pollut Technol 1:285–290
Norma Oficial Mexicana NOM-147-SEMARNAT/SAA1-2004 (2007) Que establece criterios para determinar las concentraciones de remediación de suelos contaminados por Arsénico, Bario, Berilio, Cadmio, Cromo hexavalente, Mercurio, Níquel, Plata, Plomo, Selenio, Talio y Vanadio. Publicado en el Diario Oficial de la Federación el viernes 2 de marzo de
Outridge PM, Noller BN (1991) Accumulation of toxic trace elements by freshwater vascular plants. In: Ware GW (ed) Reviews of environmental contamination and toxicology. Springer, Berlin, pp 1–63
Pais I, Jones JB (2000) The handbook of trace elements. St. Luice Press, Florida
Popescu IV, Stihi C, Cimpoca GhV et al (2009) Environmental samples analysis by atomic absorption spectrometry (AAS) and inductively coupled plasma-optical emission spectroscopy (ICP_AES). Rom J Phys 54:7–8
Reeves RD, Baker AJM (2000) Metal-accumulating plants. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York, pp 193–229
Romero FM, Armienta MA, Gutierrez ME et al (2008) Factores geológicos y climáticos que determinan la peligrosidad y el impacto ambiental de jales mineros. Rev Int Contam Ambient 24(2):43–54
Ruiz-Huerta EA, Armienta-Hernández MA (2012) Acumulación de arsénico y metales pesados en maíz en suelos cercanos a jales o residuos mineros. Rev Int Contam Ambient 28:103–117
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Sasmaz A, Obek E, Hasar H (2008) The accumulation of heavy metals in Typha latifolia L. grown in a stream carrying secondary effluent. Ecol Eng 33:278–284
Sawidis T, Chettri MK, Papaioannou A et al (2001) A study of metal distribution from lignite fuels using trees as biological monitors. Ecotoxicol Environ Saf 48:27–35
Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT) (1997) Norma Oficial Mexicana NOM-001-SEMARNAT-1996, Que establece los límites máximos permisibles de contaminantes en las descargas de agua residuales en aguas y bienes nacionales. Diario Oficial de la Federación. 6 de enero de 1997
SEDUE (Secretaría de Desarrollo Urbano y Ecología) (1990) Acuerdo por el que se establecen los Criterios Ecológicos de Calidad del Agua. CE-CCA-001/89. Gaceta Ecológica
Siedlecka A, Tukendorf A, Skórzyńska-Polit E et al (2001) Angiosperms (Asteraceae, Convolvulaceae, Fabaceae and Poaceae; other than Brassicaceae). In: Prasad MNV (ed) Metals in the environment. Analysis by biodiversity. Marcel Dekker Inc, New York, pp 171–217
Sukreeyapongse O, Holm PE, Strobel BW et al (2002) pH-dependent release of cadmium, copper, and lead from natural and sludge-amended soils. J Environ Qual 31:1901–1909
U.S. Environmental Protection Agency (EPA) (1986) Quality criteria for water. Office of Water Regulations and Standards, Washington
U.S. Environmental Protection Agency (EPA) (2005) Guidance for developing ecological soil screening levels. Office of Solid Waste and Emergency Response, Washington
US EPA (1992) Technical support document for land application of sewage sludge, Document No. EPA-822/R0-93-001a
Wintz H, Fox T, Vulpe C (2002) Responses of plants to iron, zinc and copper deficiencies. Portland Press Limited, London
World Health Organization (1980) Recommended health-based limits in occupational exposure to heavy metals: report of a WHO study group [meeting held in Geneva from 5 to 11 June 1979]
Wu S (1994) Effect of manganese excess on the soybean plant cultivated under various growth conditions. J Plant Nutr 17:993–1003
Xie Z, Jiang Y, Zhang H et al (2016) Assessing heavy metal contamination and ecological risk in Poyang Lake area, China. Environ Earth Sci 75:549
Yanqun Z, Yuan L, Jianjun C et al (2005) Hyperaccumulation of Pb, Zn and Cd in herbaceous grown on lead–zinc mining area in Yunnan, China. Environ Int 31:755–762
Zhuang W, Gao XL (2014) Integrated assessment of heavy metal pollution in the surface sediments of the Laizhou Bay and the coastal waters of the Zhangzi Island, China: comparison among typical marine sediment quality indices. PLoS ONE 9(4):e94145
Acknowledgments
Dr. Gomez-Bernal appreciates the postdoctoral fellowship at the Facultad de Química, UNAM, granted by the Directorate General of the Academic Staff (DGAPA) of the UNAM for the development of this research, which was funded by PAIP 50009111 granted to VMLP by the Facultad de Química, UNAM, Luciano Hernández Gómez, MSc, for his technical support in processing plants samples, and Nora Ceniceros, Alejandra Aguayo, and Olivia Cruz for their technical support in processing soils and water samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez-Bernal, J.M., Ruiz-Huerta, E.A., Armienta-Hernández, M.A. et al. Evaluation of the removal of heavy metals in a natural wetland impacted by mining activities in Mexico. Environ Earth Sci 76, 801 (2017). https://doi.org/10.1007/s12665-017-7144-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-7144-1