Abstract
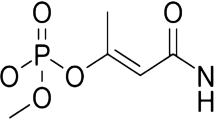
The present study focused on the degradation of mixed pesticides using UV-induced photocatalytic degradation of lindane (1α,2α,3β,4α,5α,6β-hexachlorocyclohexane), methyl parathion (O,O-dimethyl-O-4-nitrophenyl phosphorothioate), and dichlorvos (2,2-dichlorovinyl-O-O-dimethyl phosphate). Different grades of TiO2 were prepared through the acid route (AR), alcohol route (AlR), and surfactant route (SR) and their photocatalytic activity were compared with commercially available Degussa P-25 TiO2. The rate of degradation of pesticides was high for TiO2 prepared through the SR compared to the other three catalysts. The crystalline structure and morphology of SR TiO2 was identified with scanning electron microscope, energy dispersive X-ray analyzer, UV, and transmission electron microscope analyses and was compared with that of Degussa P-25 TiO2. Degradation studies of individual as well as mixed pesticides were carried out. The intermediate formed during the photodegradation of methyl parathion, lindane, and dichlorvos were identified by gas chromatography–mass spectrometry analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pesticides of different chemical nature are being used extensively for agricultural purposes all over the world. Migration of pesticides to groundwater and surface water has become an issue of great concern as numerous incidents of contamination have been documented in developed countries (Mohammadi et al. 2006). Even when present in small quantities, their variety, toxicity, and persistence have an adverse effect on ecological systems such as birds, fish, and plants with which human welfare is inseparably bound (Gupta 2004). New methods for water treatment as well as improvements of the existing processes are essential to meet the stringent regulations that resulted from the increasing awareness about the adverse effects of these pollutants.
Methyl parathion (O,O-dimethyl-O-4-nitrophenyl phosphorothioate), dichlorvos (2,2-dichlorovinyl-O-O-dimethyl phosphate), and lindane (1α,2α,3β,4α,5α,6β-hexachlorocyclohexane) are the most commonly used pesticides in India (Ma et al. 2003; Hoyer et al. 1998; Atiqur Rahman and Muneer 2005). Many researchers have reported the photocatalytic degradation of methyl parathion, lindane, and dichlorvos using TiO2 in suspension form (Moctezuma et al. 2007; Evgenidou et al. 2007; Wang et al. 2007; Kim et al. 2006; Prakash et al. 2004; Zaleska et al. 2004; Evgenidou et al. 2005; Atiqur Rahman and Muneer 2005). Most of these studies focused on the degradation of a single pesticide in distilled water system using aqueous TiO2 suspension. However, in real-life conditions, a mixture of pesticides along with other organic compounds might be present. Moreover, there are several problems associated with using TiO2 in suspension form such as requirement of an extensive solid–liquid separation process to separate the nano-TiO2 particles from the drinking water system (Gelover et al. 2004). Hence, immobilizing TiO2 on suitable support and employing this coated surface as the photocatalyst may be a promising solution for the above-mentioned problems (Gelover et al. 2004). Dionysiou et al. (2000) reported the degradation of lindane using TiO2 immobilized on a continuous-flow rotating disk and achieved 63% of lindane degradation. The sol–gel method is considered as an effective approach for the preparation of immobilized TiO2 films on various substrates (Shang et al. 2003; Chen and Dionysiou 2006).
TiO2 in the anatase form appears to be the most photocatalytically active crystal phase compared to rutile (Adachi et al. 2004). Moreover, control of the morphology, particle size, particle size distribution, phase composition, and porosity of TiO2 is vital to achieve optimum photocatalytic activity (Khanna et al. 2007). A variety of methods have been proposed for the preparation of nano-TiO2 like flame synthesis, sol–gel routes, thermal hydrolysis, and reverse microemulsion method (Li-yuan et al. 2007). Among these techniques, a sol–gel thin film deposition offers several advantages over other techniques. The main disadvantage of sol–gel process is agglomeration of hydrolyzed TiO2 particles (Mohammadi et al. 2006). Many methods have been developed to disperse the aggregated TiO2 particles such as washing with organic solvent, freeze drying followed by heating, and adding surfactants (Li-yuan et al. 2007). The most economical and efficient way to prevent the aggregation of TiO2 is the addition of surfactants. Surfactants are well known as stabilizers, template agents, and shape directors. Therefore, they have been adopted to realize controllable synthesis of nanoparticles. Highly photocatalytically active mesoporous TiO2 with the particle sizes ranging from submicrometer to micrometer were synthesized using nonionic surfactants (Wang et al. 2007; Choi et al. 2006). The preparation of surfactant-assisted nano-TiO2 microsphere showed better catalytic efficiency than Degussa P-25 TiO2 (Zhang et al. 2009).
Very little work has been carried out on the degradation of mixed pesticides with immobilized TiO2. Most of the studies dealing with photocatalytic degradation of a single pesticide were carried out using Degussa P-25 TiO2. The present work is focused on the preparation of nano-TiO2 by sol–gel process using acid, alcohol, and surfactant routes (AR, AlR, and SR, respectively). Nano-TiO2 immobilized on Pyrex glass tube was employed to conduct photocatalytic degradation studies of methyl parathion, lindane, and dichlorvos. The photocatalytic activities of TiO2 prepared by AR, AlR, and SR were compared with that of commercially available photocatalyst Degussa P-25 TiO2. Rates of degradation of lindane, dichlorvos, and methyl parathion were evaluated for individual and mixed pesticides using surfactant-assisted TiO2.
2 Materials and Methods
2.1 Chemicals
Analytical grade isopropanol (purity 99.7%) and ethyl alcohol (purity 99.9%) purchased from Ranbaxy Chemicals, India were used for the coating of titanium oxide nanoparticles. Methyl parathion, dichlorvos, and lindane of high-performance liquid chromatography (HPLC)-grade were also procured from Ranbaxy Chemicals, India, whereas methyl parathion, dichlorvos, and lindane of commercial grade (purity 80%) were purchased from the local market.
2.2 Preparation of Nano-TiO2
Preparation of TiO2 was carried out by three different methods using alcohol, acid, and surfactant.
2.2.1 Acid Route
For the preparation of acid-assisted (AR) TiO2, the colloidal solution was prepared according to the method suggested by Torimoto et al. (1997). Titanium isopropoxide (7.4 mL, 99% purity) was added dropwise to 30 mL of 1 M HNO3 solution and agitated to give a transparent TiO2 solution. The transparent solution was diluted with 100 mL of distilled water and the pH of the colloidal solution was adjusted to 3 using 1 M NaOH solution. The suspension was agitated for 2 h at room temperature, centrifuged, and then washed with distilled water. The isolated TiO2 was dried for 1 h at 100°C in a hot air oven. The resulting solid was calcined in a muffle furnace at 500°C.
2.2.2 Alcohol Route
For the preparation of alcohol-assisted (AlR) TiO2, a 0.1-M titanium isopropoxide solution in isopropyl alcohol was prepared and added to water in 1:1 ratio. The suspension was agitated for 1 h and centrifuged. The isolated TiO2 was dried for 1 h at 100°C in a hot air oven. The resulting solid was calcined in a muffle furnace at 500°C.
2.2.3 Surfactant Route
The preparation of surfactant-assisted (SR) TiO2 was carried out with hydrolysis of titanium isopropoxide in the presence of cationic surfactant (dodecyl amine). Titanium isopropoxide (0.4 M) was prepared in isopropanol and equimolar quantity of metal complexing agent acetyl acetone was added to it. The acetyl acetone forms complex with titanium and becomes golden yellow in color (Adachi et al. 2004). Dodecyl amine hydrochloride (0.4 M) in water was prepared by adjusting the pH to 4.5 with HCl. Both the solutions were mixed together and warmed up to 40°C until the mixture became transparent. The above solution was autoclaved up to 80ºC for 72 h. The suspension was centrifuged at 8,000 rpm and residue was washed with alcohol for three times to remove the surfactant (Adachi et al. 2004; Sakulkhaemaruethai et al. 2004). The dried sample was calcined at 500°C.
2.3 Immobilization of TiO2
A Pyrex glass tube with an inner surface area of 169.56 cm2 (with a height of 90 mm and a diameter of 60 mm) was used for coating the photocatalyst. Before coating, the inner surface of the Pyrex glass tube was treated with 5% (v/v) hydrofluoric acid for 30 min to get a rough surface and washed with distilled water. The Pyrex glass tube coating was carried out using different concentrations of TiO2 and isopropanol suspension. Before coating, the suspension was sonicated for 15 min and the Pyrex tube was inserted slowly into the suspension and allowed to stay in the suspension for 5 min, then taken out and dried in an oven for 30 min at 150°C. The weight of the coated materials was measured each time. The film thickness and surface morphology was also monitored.
2.4 Photoreactor
The schematic diagram of the photoreactor employed in the present study is given in Fig. 1. Photocatalytic degradation studies of methyl parathion, dichlorvos, and lindane were performed in an immobilized batch reactor. The cylindrical photochemical reactor with a volume of 400 mL was provided with water circulation arrangement to maintain the temperature in the range of 25–30°C. Irradiation was carried out with 125 W medium pressure mercury lamps supplied by Haber Scientific, India, which emitted predominantly UV radiation at a wavelength of 365 nm. For immobilized batch reactor, a coated Pyrex tube (inner surface area of 169 cm2) was inserted into the reactor. Before adding the pesticide, the UV lamp was warmed up for 15 min to attain sufficient energy. Oxygen flow rate of 300 mL/min and a stirring rate of 150 rpm were maintained in all the experiments. Samples were collected at regular intervals and extracted with HPLC-grade hexane (purity 99%) and analyzed using a gas chromatograph (GC) as mentioned earlier. The change in pH during the degradation of the pesticide was monitored with digital pH meter supplied by Digisun Electronics, India (model DI-707).
2.5 Analytical Methods
2.5.1 GC and GC-MS Analysis
Methyl parathion, dichlorvos, and lindane were analyzed using a Perkin Elmer Clarus 500 gas chromatograph with electron capture detector (GC/ECD) equipped with an autosampler, an on-column, split/splitless capillary injection system, and a Perkin Elmer (PE)-5 capillary column (30 m × 0.53 mm × 0.5 µm film thickness). The operating conditions were as follows: the column was initially held at a temperature of 80°C for 5 min and then the temperature was increased at a rate of 10°C/min to 180°C, followed by 8°C/min to 250°C and finally held at 250°C for 1 min. The temperatures of the injector and detector were maintained at 180°C and 350°C, respectively. Nitrogen was used as a carrier gas at a flow rate of 8.0 mL/min and the injections were made in the split mode with a split ratio of 1:10. The metabolites formed during the degradation were monitored using a gas chromatograph–mass spectrometer (GC-MS) supplied by Agilent, USA, and a temperature program similar to the one used for GC analysis was employed for the GC-MS analyses also. Concentrations of NO −3 , SO 2−4 , PO 3−4 , and Cl− ions formed during the degradation of methyl parathion, dichlorvos, and lindane were analyzed using an ion chromatograph supplied by Dionex, USA with electrochemical detector (ED50).
2.5.2 X-Ray Diffraction and UV Analysis
The crystalline structure of prepared TiO2 was examined by X-ray diffraction (XRD) analyses, using Copper K alpha radiation with Lynx detector at an operating voltage of 35 kV and current of 25 mA supplied by Bruker Axs, USA (model D8 Discover). The Cary-5E UV–VIS-NIR, high-resolution spectrophotometer was used to find the absorption pattern of TiO2 samples.
2.5.3 SEM, TEM, and EDAX Analysis
The surface morphology of the TiO2 and particle size were determined using a scanning electron microscope (SEM) equipped with a field emission gun (JEOL, JSM-6380, Japan). The spot elemental analysis of synthetically prepared nano-TiO2 and Degussa P-25 TiO2 was carried out using SEM equipped with an energy dispersive X-ray analyzer (EDAX; FEI, Quanta 200, Czechoslovakia). High-resolution transmission electron microscope (TEM), JEOL 3010 with a UHR pole piece operating at an accelerating voltage of 300 kV, was used to measure the particle size of SR TiO2.
2.6 Kinetic Studies
The kinetic study using SR TiO2 was carried out with two different concentrations (1.0 and 2.5 mg/L) of methyl parathion, lindane, and dichlorvos. Coated TiO2 was prepared separately for each study. Pseudo-first-order kinetic expression was used to analyze the photocatalytic reaction rate using the kinetic study data, and the kinetic expression is given by Eq. 1:
where k is the pseudo-first-order rate constant, C and C 0 are the concentrations at time t and t = 0, respectively.
3 Results and Discussion
3.1 Effect of Coating Solution Concentration
Coating thickness is one of the important parameters in the performance of immobilized catalysts (Guillard et al. 2002). For effective degradation, TiO2 should cover the entire area of the exposed reaction surface (Pyrex glass tube). TiO2 suspensions with TiO2 concentrations of 0.1%, 0.25%, 0.5%, 0.75%, and 1.0%, respectively, were prepared in isopropanol and used for coating the Pyrex glass tube. Preliminary degradation studies were carried out using 10 mg/L of methyl parathion with a reaction time of 60 min. The results are presented in Fig. 2. Methyl parathion was completely degraded in reactors which employed immobilized glass plates prepared using 0.5%, 0.75%, and 1.0% TiO2 in isopropanol suspension solution. However, only 40% and 70% removals were attained in other reactors which employed 0.1% and 0.25% TiO2 in isopropanol suspension for immobilization, respectively. Hence, 0.5% TiO2 in isopropanol suspension solution was employed for immobilization in further studies. The weight of coated TiO2 on Pyrex tube was monitored in each batch and it was around ∼59 ± 6 mg. The surface morphology of the coated Pyrex tube using 0.5% TiO2 suspension is given in Fig. 3.
3.2 Screening of TiO2 Prepared in Various Routes
The degradation of methyl parathion was carried out using three grades of TiO2 immobilized on Pyrex glass tubes prepared through three different routes and the rates of degradation were compared with that of Degussa P-25 TiO2. The results of methyl parathion degradation with different TiO2 catalysts are presented in Fig. 4. Methyl parathion degradation efficiency was high for TiO2 prepared through SR compared to the other three catalysts (Degussa P-25 TiO2, AlR, and AR). Complete mineralization was achieved within 45, 55, 120, and 165 min for SR TiO2, Degussa P-25 TiO2, AR TiO2, and AlR TiO2, respectively. The rate of degradation was dependent on particle size, crystalline structure and purity of the TiO2. As SR TiO2 performed better than other grades of TiO2 during the screening study, this grade was selected for further studies.
3.3 Characterization of SR TiO2
3.3.1 X-Ray Diffraction and UV–Vis Spectra
The SR TiO2 was dried in an oven at 500°C before it was used in the XRD analysis. The XRD patterns of TiO2 were compared with those for Degussa P-25 TiO2 and the results are given in Fig. 5. The strong XRD anatase peaks at 2θ = 25.4° (101) and 48.0° (101) were observed in SR TiO2 as well as in Degussa P-25 TiO2. A strong rutile peak was observed at 2θ = 27.50° in Degussa P-25 TiO2, whereas a weak rutile peak was observed in SR TiO2 at 54.5° (110). The XRD analysis showed a well-crystallized anatase peak and a weak rutile peak in SR TiO2.
The UV–Vis spectra of SR TiO2 (500°C) and Degussa P-25 TiO2 were analyzed using 0.0025 M concentration of each catalyst and are given in Fig. 6. The absorption pattern of SR TiO2 and Degussa P-25 TiO2 indicated that the absorption was in the far UV region around 325 nm (3.85 eV). A similar UV absorption range, i.e., ∼325 nm, for Degussa P-25 TiO2 was reported earlier (Jwo et al. 2005).
3.3.2 EDAX, SEM, and TEM Analyses
EDAX analysis was used to characterize the elemental composition of SR TiO2. A typical EDAX pattern of SR TiO2 is shown in Fig. 7. The elemental composition of oxygen and Ti were 38.21% and 60.79%, respectively. As per stoichiometric equation, titanium and oxygen in TiO2 should be 59.9% and 40.06%, respectively. There was no other element found in the EDAX analyses. The particle size and morphology of TiO2 catalyst were studied using SEM. It is clear from Fig. 8 that the size of the TiO2 particles is approximately 30–50 nm. The exact sizes of the nanoparticles are very difficult to determine using SEM analysis; therefore, TEM analysis was carried out to find the exact size of the TiO2 prepared through the SR (Gallezot and Leclercq 1994). From the TEM analysis, it was found that the particle size of SR TiO2 is ∼12 nm. Figures 8 and 9 show the SEM and TEM analysis of SR TiO2.
3.4 Effect of Calcination Temperature on Photocatalytic Activity of SR TiO2
Calcination improves the crystallization of TiO2 powders and accelerates the transformation from the amorphous phase to the anatase or rutile phase (Zhang et al. 2000). Usually, beyond a calcination temperature of 500°C, strong rutile peak appears. The particle agglomeration is inversely proportional to its activation energy. It is reported that, when the calcination temperature is high, the activation energy is very low and the particle growth rate is large (Li et al. 2002). When the particle growth of TiO2 increases, the catalytic activity decreases. Up to 500°C, the reduction in activation energy is very small (Li et al. 2002). Hence, in the present study, calcination temperature of TiO2 was limited to 500°C. To find the optimum calcination temperatures, SR TiO2 calcined at different temperatures (varying between 100°C and 500°C) and used for degradation studies. The XRD of SR TiO2 prepared at different calcination temperatures are presented in Fig. 10. No anatase or rutile peak was observed for SR TiO2 calcined at 100°C. As the calcination temperature increased beyond 200°C, sharp peaks of anatase and weak rutile peak started appearing. The degradation of methyl parathion (10 mg/L) with SR TiO2 prepared at different calcination temperatures is given in Fig. 11. The photocatalytic activity of SR TiO2 increased with increasing calcination temperature up to 500°C. A similar trend was observed for lindane and dichlorvos degradation also.
3.5 Effect of Pesticide Concentrations on Photodegradation by SR TiO2
Degradation studies were carried out with two different concentrations (1.0 and 2.5 mg/L) of methyl parathion, dichlorvos, and lindane. The rate of degradation of methyl parathion, dichlorvos, and lindane was inversely affected by the initial pesticide concentration. A lower photodegradation rate was observed for lindane compared to methyl parathion and dichlorvos. This may be due to its high resistance to oxidation via hydroxyl radical attack. It is reported that lindane is less prone to oxidation due to its non-aromatic and saturated structure and the absence of the double bond (Dionysiou et al. 2000).
The rate of degradation of the three pesticides was compared with Degussa P-25 TiO2. The reaction rates, k (per minute), were determined by plots of ln(C/C 0) versus time for all the three pesticides and the results are given in Table 1. The rate of degradation of surfactant-assisted nano-TiO2 was higher compared to Degussa P-25.
3.6 Plausible Degradation Pathways of Methyl Parathion, Dichlorvos, and Lindane
During the photocatalytic degradation of lindane, dichlorvos, and methyl parathion using SR TiO2 as the photocatalyst, various organic intermediates were produced. Using the GC and GC-MS techniques, three by-products were identified during methyl parathion oxidation and five by-products were identified in the case of lindane oxidation. The degradation pathways of lindane and methyl parathion are given in Figs. 12 and 13. Only three intermediate by-products such as methyl paraoxon (m/z 246), O,O,O-trimethyl phosphonic thionate (m/z 156), and p-nitrophenol (m/z 139) were observed during the methyl parathion degradation. At the end of the reaction, methyl parathion and its by-products were completely degraded. Similarly, in the case of lindane degradation, hexachlorocyclohexane (m/z 289), pentachlorocyclohexane (m/z 256), hexachlorobenzene (m/z 284), 1-hydroxy-2,3,4,5,6-pentachlorobenzene (m/z 265),1-hydroxy-2,3,4,5,6-pentachloro cyclohexane, and penta-hydroxyl cyclopentadiene (m/z 146) were identified. Most of the by-products appeared during the middle of the reaction and, as time progressed, the by-products gradually disappeared. Almost similar types of by-products were observed during the degradation of dichlorvos, lindane, and methyl parathion with Degussa P-25 TiO2. At the end of the experiment, none of the by-products were observed. In the case of dichlorvos degradation, no intermediate by-product was identified in GC-MS analysis.
To confirm the complete mineralization of pesticides, the end products SO 2−4 , NO −3 , PO 3−4 , and Cl− were monitored during the degradation of all the three pesticides at regular time intervals. Methyl parathion degradation studies were conducted with an initial concentration of 40 mg/L and this amount of methyl parathion contains 14.59, 9.43, and 14.44 mg/L of SO 2−4 , NO −3 , and PO 3−4 , respectively. During methyl parathion degradation, removal of the sulfur group occurred first. A maximum concentration of 14.17 mg/L of free SO 2−4 , 9.11 mg/L of free NO −3 , and 14.11 mg/L of free PO 3−4 were identified. A slight difference between theoretical and experimental values of SO 2−4 , NO −3 , and PO 3−4 was observed. This may be due to the experimental and analytical errors. The concentration of chloride ions and change in pH during the degradation of lindane (initial concentration of 10 mg/L) and dichlorvos (initial concentration of 7.5 mg/L) were also monitored and the results are presented in Table 2. Chloride concentrations in the range of 2.79 and 5.36 mg/L were obtained for dichlorvos and lindane, respectively. The observed values were well in agreement with the stoichiometric concentrations of chlorides that can be generated from the given concentrations of pesticides.
3.7 Photodegradation of Mixed Pesticides
Photodegradation studies of mixed pesticides were carried out using identical mass concentrations of dichlorvos, lindane, and methyl parathion. Already, the attempt was made to photodegrade a high concentration of mixed pesticides (alachlor, atrazine, chlorfenvinphos, diuron, and isoproturon), but complete mineralization was hard to accomplish (Maldonado et al. 2006). Therefore, a low concentration of (1.0 mg/L) mixed pesticides were employed for the photodegradation studies and all other experimental conditions were kept similar to the previous set of experiments. The results of the mixed pesticides degradation studies are presented in Fig. 14. Though degradation of all the three pesticide took place simultaneously, dichlorvos degradation was much faster compared to methyl parathion and lindane. Dichlorvos was completely degraded within 40 min, whereas 70 and 220 min were needed for the complete degradation of methyl parathion and lindane, respectively. Similar experiments were carried out with Degussa P-25 TiO2; dichlorvos was completely degraded within 45 min, whereas 80 and 260 min were needed for the complete degradation of methyl parathion and lindane, respectively. The surfactant-assisted TiO2 showed better catalytic efficiency compared to Degussa P-25 TiO2 even in mixed pesticide systems. The GC and GC-MS analysis confirmed that there was no accumulation of intermediates in the system.
4 Conclusion
The photocatalytic degradation of lindane, methyl parathion, and dichlorvos was studied using surfactant-assisted nano-TiO2. The rate of degradation was high for TiO2 prepared through the SR compared to that for TiO2 prepared through AR and A1R and Degussa P-25 TiO2. SEM and TEM studies showed that the particle size of SR TiO2 was in the range of ∼12 nm. The XRD studies showed the presence of strong anatase crystalline structure in SR TiO2. EDAX analysis confirmed that the SR TiO2 contains no impurities. GC-MS analysis showed that similar intermediates were identified for methyl parathion and lindane in single as well as mixed conditions. No reaction intermediate was identified during the degradation of dichlorvos. The rates of degradation of mixed pesticides were completely different from single pesticide.
References
Adachi, M., Murata, Y., Takao, J., Jiu, J., Sakamoto, M., & Wang Highly, F. (2004). Efficient dye-sensitized solar cells with a titania thin-film electrode composed of a network structure of single-crystal-like TiO2 nanowires made by the “Oriented Attachment” mechanism. Journal of American Chemical Society, 126, 14943–14949.
Atiqur Rahman, M., & Muneer, M. (2005). Photocatalysed degradation of two selected pesticide derivatives, dichlorvos and phosphamidon, in aqueous suspensions of titanium dioxide. Desalination, 181, 161–172.
Chen, Y., & Dionysiou, D. D. (2006). TiO2 photocatalytic films on stainless steel: The role of Degussa P-25 in modified sol–gel methods. Applied Catalysis B: Environmental, 62, 255–264.
Choi, H., Stathatos, E., & Dionysiou, D. D. (2006). Synthesis of nanocrystalline photocatalytic TiO2 thin films and particles using sol–gel method modified with nonionic surfactants. Thin Solid Films, 510, 107–114.
Dionysiou, D. D., Khodadoust, A. P., Kern, A. M., Suidan, M. T., Baudin, I., & Michel Laîné, J. (2000). Continuous-mode photocatalytic degradation of chlorinated phenols and pesticides in water using a bench-scale TiO2 rotating disk reactor. Applied Catalysis B: Environmental, 24, 139–155.
Evgenidou, E., Fytianos, K., & Poulios, L. (2005). Semiconductor-sensitized photodegradation of dichlorvos in water using TiO2 and ZnO as catalysts. Applied Catalysis B: Environmental, 59, 81–89.
Evgenidou, E., Konstantinou, I., Fytianos, K., Poulios, I., & Albanis, T. (2007). Photocatalytic oxidation of methyl parathion over TiO2 and ZnO suspensions. Catalysis Today, 124, 156–162.
Gallezot, P., & Leclercq, C. (1994). Catalyst characterization: Physical techniques for solid materials. New York: Plenum. Quoted by B. Imelik & J.C. Vedrine.
Gelover, S., Mondragónb, P., & Jiménez, A. (2004). Titanium dioxide sol–gel deposited over glass and its application as a photocatalyst for water decontamination. Journal of Photochemistry and Photobiology A: Chemistry, 165, 241–246.
Guillard, C., Beaugiraud, C. D., Herrmann, J. M., Jaffrezic, H., Renault, N. J., & Lacroix, M. (2002). Physiochemical properties and photocatalytic activities of TiO2 films prepared by sol gel methods. Applied Catalyst B: Environment, 39, 331–342.
Gupta, P. K. (2004). Pesticide exposure—Indian scene. Toxicology, 198, 83–90.
Hoyer, A., Grandjean, P., Jorgensen, T., Brock, J. W., & Hartvig, H. B. (1998). Organochlorine exposure and risk of breast cancer. Lancet, 352, 1816–1820.
Jwo, C. S., Tien, D. C., Teng, T. P., Chang, H., Tsung, T. T., Liao, C. Y., et al. (2005). Preparation and UV characterization of TiO2 nanoparticles synthesized by SANSS. Review on Advanced Material Science, 10, 283–288.
Khanna, P. K., Singha, N., & Charan, S. (2007). Synthesis of nano-particles of anatase-TiO2 and preparation of its optically transparent film in PVA. Materials Letters, 61, 4725–4730.
Kim, T., Kim, J., Choi, K., Stenstrom, M. K., & Zoh, K. (2006). Degradation mechanism and the toxicity assessment in TiO2 photocatalysis and photolysis of parathion. Chemosphere, 62, 926–933.
Li, B., Wang, X., Yan, M., & Li, L. (2002). Preparation and characterization of nano-TiO2 powder. Materials Chemistry and Physics, 78, 184–188.
Li-yuan, C., Yan-fen, Y., Gang, Z., Bing, P., & Shun-wen, W. (2007). Effect of surfactants on preparation of nanometer TiO2 by pyrohydrolysis. Transactions of Nonferrous Metals Society of China, 17, 176–180.
Ma, T., Kramer, R. E., Baker, R. C., Fan, L. W., & Ho, I. K. (2003). Effects of chronic dermal exposure to nonlethal doses of methyl parathion on brain regional acetyl cholinesterase and muscarinic cholinergic receptors in female rats. Journal of Neuroscience Research, 71, 138–145.
Maldonado, M. I., Malato, S., Pérez-Estrada, L. A., Gernjak, W., Oller, I., Domenech, X., et al. (2006). Partial degradation of five pesticides and an industrial pollutant by ozonation in a pilot-plant scale reactor. Journal of Hazardous Materials, 138, 363–369.
Moctezuma, E., Leyva, E., Palestino, G., & de Lasa, H. (2007). Photocatalytic degradation of methyl parathion: reaction pathways and intermediate reaction products. Journal of Photochemistry and Photobiology A: Chemistry, 186, 71–84.
Mohammadi, M. R., Cordero-Cabrera, M. C., Fray, D. J., & Ghorbani, M. (2006). Preparation of high surface area titania (TiO2) films and powders using particulate sol–gel route aided by polymeric fugitive agents. Sensors and Actuators B: Chemical, 120, 86–95.
Prakash, O., Suar, M., Raina, V., Dogra, C., Pal, R., & Lal, R. (2004). Residues of hexachlorocyclohexane isomers in soil and water samples from Delhi and adjoining areas. Current Science, 87, 73–77.
Sakulkhaemaruethai, S., Suzuki, Y., & Yoshikawa, S. (2004). Surfactant-assisted preparation and characterization of mesoporous titania nanocrystals. Journal of the Ceramic Society of Japan, 112, 547–552.
Shang, J., Li, W., & Zhu, Y. (2003). Structure and photocatalytic characteristics of TiO2 film photocatalyst coated on stainless steel webnet. Journal of Molecular Catalysis A: Chemical, 202, 187–195.
Torimoto, T., Okawa, Y., Takeda, N., & Yoneyama, H. (1997). Effect of activated carbon content in TiO2-loaded activated carbon on photodegradation behaviors of dichloromethane. Journal of Photochemistry and Photobiology A: Chemistry, 103, 153–157.
Wang, J., Suna, W., Zhang, Z., Zhang, X., Li, R., Maa, T., et al. (2007). Sonocatalytic degradation of methyl parathion in the presence of micron-sized and nano-sized rutile titanium dioxide catalysts and comparison of their sonocatalytic abilities. Journal of Molecular Catalysis A: Chemical, 272, 84–90.
Zaleska, A., Hupka, J., Wiergowski, M., & Biziuk, M. (2004). Photocatalytic degradation of lindane, p, p DDT and methoxychlor in an aqueous environment. Journal of Photochemistry and. Photobiology A: Chemistry, 135, 213–220.
Zhang, Q., Gao, L., & Guo, J. (2000). Effects of calcination on the photocatalytic properties of nanosized TiO2 powders prepared by TiCl4 hydrolysis. Applied Catalysis B: Environmental, 26, 207–215.
Zhang, X., Hong Pan, J., Du Jianhong, A., Ng, J., Darren, D., Sun, J., et al. (2009). Fabrication and photocatalytic activity of porous TiO2 nanowire microspheres by surfactant-mediated spray drying process. Materials Research Bulletin, 44, 1070–1076.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Senthilnathan, J., Philip, L. Removal of Mixed Pesticides from Drinking Water System Using Surfactant-Assisted Nano-TiO2 . Water Air Soil Pollut 210, 143–154 (2010). https://doi.org/10.1007/s11270-009-0230-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0230-6