Abstract
This study focused on the synthesis and evaluation of mixed metal oxides ZnO/Al2O3 and ZnO/Al2O3/TiO2 as heterogeneous photocatalysts for the degradation of 2,4-dichlorophenoxyacetic acid (2,4-D). Hydrotalcites were synthesized using three different methods (conventional, microwave, and ultrasonic) and then treated at 700 ºC to obtain the corresponding Zn/Al and Zn/Al/Ti mixed metal oxides. The layered double hydroxide (LDH) and the mixed metal oxides were characterized by various techniques including X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), X-ray photoelectron spectrometry (XPS), N2 adsorption–desorption, Fourier transform infrared spectroscopy (FTIR), pHPZC analysis and UV–Vis techniques. A 95.6% photodegradation of 2,4-D was achieved after 240 min of UV exposure radiation (λ = 254 nm). The degradation of the products was confirmed by mass spectrometry analysis. The activity of the samples under UV irradiation followed this order: OZnTU > OZnTC > OZnTM, with ZnO/Al2O3/TiO2 showing the highest activity due to its chemical composition and the interaction between ZnO and TiO2. The degradation process was described by a Langmuir–Hinshelwood type kinetic model. Mass spectrometry was used to analyze the photodegradation results, suggesting the potential of these photocatalysts for the oxidation of 2,4-D in industrial wastewater under UV irradiation, particularly for the degradation and mineralization of 2,4-D herbicides from an aqueous solution.
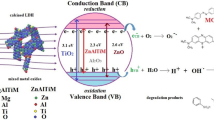
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2,4-Dichlorophenoxyacetic acid (2,4-D) is a synthetic herbicide that was developed in the United States in 1945. It is one of the most important and widely used herbicides [1]. In Mexico, it is mainly used to control weeds in crops such as rice, sugar cane, and corn and for managing aquatic weeds in water treatment plants [2]. Its popularity is attributed to its efficacy and affordability [3].
2,4-D works as a systemic hormonal herbicide, meaning that when applied to a weed, it is absorbed through the leaves and roots, disrupting the plant’s hormonal balance, and ultimately leading to its death [1, 4, 5]. It is important to note that phenoxy-type herbicides like 2,4-D are often contaminated with highly toxic and environmentally stable substances such as dioxins. These contaminants can lead to soil, sediment, and food contamination upon release into the environment [4,5,6,7,8].
Exposure to 2,4-D can result in respiratory, cardiovascular, and cancer-related health issues [4, 9]. Some of the physicochemical properties of 2,4-D include its water solubility (677 ppm at 25 °C), n-octanol-water partition coefficient (log Kow = 2.81), and pKa of 2.64–3.31 [10, 11]. Additionally, 2,4-D is stable within a pH range of 5–9, exhibits high mobility in soil, degrades slowly in water, and undergoes moderate photolysis [4, 12, 13].
Heterogeneous photocatalysis is an advanced oxidation process (AOP) known for its ability to deactivate microbes and break down organic matter. In this process, a solid catalyst is activated by visible or ultraviolet light in the presence of oxygen, producing hydroxyl radicals (OH·). These OH· radicals can then oxidize organic compounds and form peroxy radicals by abstracting hydrogen or adding electrophilically to double bonds [5, 13]. As a result, these reactions can cause oxidative stress in microorganisms and break down organic molecules, sometimes leading to the complete mineralization of contaminants. Photocatalysis is effective in breaking down dioxins, drugs, dyes, and pesticides [12, 13].
Layered double hydroxides (LDH), which is a two-dimensional (2D) clay-like material, also known as anionic clays or hydrotalcites, are nanocomposites that may contain one or more metals in their structure, allowing them to combine and create a catalyst with enhanced properties. Their structure is similar to brucite [Mg(OH)2], with Mg2+ ions forming sheets with octahedral coordination and being stacked on top of each other through hydrogen bonds [14, 15]. The great advantage of these materials lies in their versatility. They require a minimum of two metals, which function as divalent and trivalent cations. The material can acquire different properties by exchanging these ions with anions such as CO32−, NO3−, Cl−, SO42−, PO43−, among others, in the interlayer region [14,15,16,17,18,19].
The LDHs used in this study were synthesized using conventional, microwave, and ultrasound methods. The conventional method involves preparing a solution containing the precursor salts of the desired metals and slowly mixing it with an alkaline solution to precipitate the cations. The mixture is then subjected to hydrothermal treatment for at least 8 h with continuous stirring. The synthesis methods proposed in this study are influenced by factors such as pH, temperature, and aging time, which affect the precipitation of hydrotalcites and the size of their particles [20]. Microwave radiation reduces the size of the crystals [20], while ultrasonic radiation disperses metals more effectively, increases the specific surface area, generates more uniform pore sizes, and decreases the synthesis time [21, 22].
Hydrotalcites can withstand high temperatures, but calcination above 500 °C causes the laminar structure to collapse, resulting in a mixture of metal oxides. This process imparts new properties to the materials, including increased surface area, particle size reduction, improved stability, synergistic effects between metals, and memory effect (regeneration of hydrotalcite) [16, 19, 22].
This study used conventional, microwave, and ultrasonic synthesis methods to incorporate Ti (IV) as a dopant into LDH at a concentration of 2.8–5.3 wt%. The oxide mixtures (ZnAlTi) and (ZnAl) obtained from the calcination of LDH at 700 °C exhibited degradation efficiencies of 95.6% and 91.8%, respectively. At least three types of oxides responsible for the photodegradation of 2,4-D were identified. The mixed oxides doped with Ti showed higher catalytic activity than the TiO2 used as a reference [23]. The effectiveness of the catalyst was evaluated by 2,4-D degradation and analyzed by UV–Vis spectra and mass spectrometry. The kinetics of 2,4-D degradation was studied as a first-order reaction. Therefore, this work aims to characterize these materials and analyze the influence of synergy in the synthesis of LDH with titanium and their catalytic behavior.
Materials and methods
Chemical substances
Aluminum nitrate nonahydrate [Al(NO3)3⋅9H2O, 98%, Sigma-Aldrich], zinc nitrate hexahydrate [Zn(NO3)2⋅6H2O, 98%, Sigma-Aldrich], titanium (IV) chloride [TiCl4, reagent Plus 99.9%, Sigma-Aldrich] and sodium hydroxide [NaOH, analytical grade, ≥ 98% Sigma-Aldrich] were used for the preparation of the catalysts. 2,4-D amine (isopropyl ester of 2,4-dichlorophenoxyacetic acid) [C8H6Cl2O3, commercial grade, Herbipol®] in a water solution was used in the photocatalytic experiments. Solutions of NaCl (0.01 M), HCl (0.01 M), and NaOH (0.01 M) were used for pH control. Deionized water was used as the solvent to prepare solutions in all experiments. All the reagents were used without further purification.
Catalysts preparation
ZnO–Al2O3–TiO2
The catalysts were prepared using three different methods: conventional method, ultrasound synthesis, and microwave synthesis.
Conventional method
The corresponding nitrate salts of Al (0.0262 mol) and Zn (0.0786 mol), along with TiCl4 (2 mL) were mixed in 300 mL of distilled water with a metallic molar ratio [M3+ / (M3+ + M2+)] of X = 0.25. Sodium hydroxide (1.86 N) was slowly added to the mixture until a pH of 12 was reached, while the mixture was vigorously stirred. The resulting gel was heated for 8 h at 80 °C and atmospheric pressure, then filtered and rinsed with hot distilled water until a pH of 8 was reached to remove alkaline metals and unreacted nitrate ions (5 times). The product was dried at 80 °C for 24 h [19, 20]. The resulting hydrotalcite sample was identified as ZnTC. When the LDH was calcined at 700 °C for 4 h under airflow, the resulting mixed oxide was identified as OZnTC.
Ultrasound synthesis
The preparation of LDH and the subsequent procedures were carried out in the same manner as the conventional synthesis. The hydrotreatment was carried out using VEVOR ultrasonic cleaning equipment -model JPS-10A, for 10 min at 80 °C and atmospheric pressure [21]. The hydrotalcite sample was identified as ZnTU. The calcined sample was identified as OZnTU.
Microwave synthesis
The preparation of hydrotalcite, as well as the washing and drying procedures, were carried out similarly to the conventional synthesis. The hydrotreatment was carried out in a General Electric (GE) microwave oven (model JES735WJ) at 80% power for 10 intervals of 1 min each at atmospheric pressure. The glass was removed from the microwave between each minute, and its temperature was recorded. The resulting hydrotalcite sample was identified as ZnTM, and the calcined sample was identified as OZnTM [22].
ZnO–Al2O3
The catalysts were prepared using three different methods: conventional method, ultrasound synthesis, and microwave synthesis.
Conventional synthesis
The nitrate salts of Al (0.0262 mol) and Zn (0.0786 mol) were mixed in 300 mL of distilled water. While vigorously stirring, sodium hydroxide (1.86 N) was slowly added to the mixture until a pH of 12 was reached. The preparation of LDH, washing, and drying procedures were carried out in the same manner as the conventional synthesis of the ZnTC sample. The resulting hydrotalcite sample was identified as ZnC. When the LDH was calcined at 700 °C for 4 h under airflow, the resulting mixed oxide was identified as OZnC.
Ultrasound synthesis
The preparation of LDH was carried out in ultrasonic cleaning equipment, model JPS-10A, for 10 min at 80 °C and atmospheric pressure. The resulting hydrotalcite sample was identified as ZnU. The calcined sample was identified as OZnU.
Microwave synthesis
The preparation of hydrotalcite was carried out in a microwave oven, model JES735WJ at 80% power for 10 min. The resulting hydrotalcite sample was identified as ZnM. The calcined sample was identified as OZnM.
Table 1 describes the identifications of all the synthesized hydrotalcite samples based on the type of treatment applied.
Characterization of LDH and mixed metal oxides
The LDH and mixed metal oxides underwent various physicochemical characterization techniques. X-ray diffraction patterns were obtained in the 2θ = 5–70° range using a Rigaku Miniflex 600 diffractometer with Cu-Kα radiation at 40 kV and 15 mA. The microstructural characterization involved obtaining N2 adsorption–desorption isotherms at 77 K using a Bel-Japan Minisorp II instrument via the multipoint technique (Praxair N2); the BET model was used to calculate the specific surface area. Before analysis, the sample (100–250 mg) was pretreated overnight at 120 °C in a nitrogen stream (40 mL/min). The surface area, average pore diameter, and pore volume were determined from the N2 adsorption–desorption isotherms. The materials’ diffuse reflectance spectra (UV–Vis DRS) were measured using a Cintra 4040 spectrophotometer (GBC) equipped with an integration sphere. The band gap energy (Eg) was estimated from Tauc’s plot, assuming a direct transition. Scanning electron micrographs were taken using a JSM-7800F electronic microscope, equipped with a microanalysis system and an EDS detector. The elemental composition and valence band of the samples were determined using a THERMO Scientific K-Alpha spectrometer, which is equipped with an Al X-ray source Kα (1486.6 eV) coupled to a hemispherical electron analyzer.
pH drift method
The pH drift method is used to determine the pHPZC (Point of Zero Charge) of catalysts [18, 23]. The pHPZC is the pH at which the net charge on the surface of the material is zero. The procedure involves preparing 25 mL of 0.01 N NaCl and adding it to a series of flasks. Then, using 0.01 N HCl solution and 0.01 N NaOH, their pH values are adjusted between 2 and 12 (pH0). Next, 75 mg of catalysts are added to each flask and stirred for 14 h. After this time, the pH of the solutions is measured with a pH meter and recorded as pH1. The pHPZC is the point where pH0 equals pH1.
Degradation of 2,4-D
The degradation of 2,4-D is carried out in a batch mode. A solution of 2,4-D is prepared using deionized water. The reaction takes place in a glass reactor at 40 ± 1 °C (using a Polyscience water bath). For each batch test, 200 mL of aqueous 2,4-D solution at 86.4 ppm and 0.1 g of catalyst are used at natural pH (pH 6.67), and the reactor is subjected to magnetic stirring at 300 rpm. The suspension is stirred for 60 min for adsorption and then exposed to UV light (UV Pen Ray, λ = 254 nm, 4.4 mw/cm2) for 240 min while immersed in the reaction solution. The concentration of 2,4-D in the solution is determined using a spectrophotometer in the UV–Visible range at a wavelength of 284 nm. Aliquots of the degraded solution (2.5 mL) are collected at different time intervals and filtered through a Millipore filter (0.45 mm pore size).
Identification of degradation products
Following the degradation test, a portion of the solution was directly introduced into a micrOTOF-Q II ™ ESI-TOF time-of-flight mass spectrometer (Bruker Daltonics) to analyze the degraded compounds. Peaks corresponding to the samples were detected in negative and positive ion modes (ESI ±). Operating conditions included a capillary potential of 3500 V, with ion polarity switching between negative and positive. The drying temperature was set at 180 °C, with a dry gas flow rate of 4 L/min. Charge/mass (m/z) ion chromatograms ranged from 50 to 3000 [24].
Results and discussion
Characterization of LDH
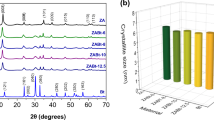
The JCPDS (Joint Committee on Powder Diffraction Standards) database facilitated the identification of crystalline phases present in the synthesized samples. Fig. 1a illustrates characteristic diffraction signals of hydrotalcite-type compounds (ZnTU-LDH) at angles 2θ = 11.5°, 23.3°, 34.3°, 39°, 46.6°, 60.1° and 61.4°. Additionally, Fig. 1a displays reflection lines corresponding to the crystalline phase of ZnO at angles 2θ = 31.6°, 34.3°, 36.1°, 47.4°, 56.4°, 62.7°, 66.2°, 67.8° and 68.9°. These signals correspond to the planes (100), (002), (101), (102), (110), (103), (200), (112) and (201) [25,26,27,28]. Similarly, Fig. 1b identifies hydrotalcite-type compounds at angles 2θ = 11.6°, 23.4°, 34.5°, 39.2°, 46.8°, 60.2° and 61.5° of the ZnU-LDH [25, 26], thus identifying the synthesized materials. These signals correspond to planes (003), (006), (009), (015), (018), (110) and (113) identified with the ICDD card 01–089-5434 of a typical hydrotalcite [27,28,29]. Additionally, reflection lines indicating the presence of ZnO crystalline phase are observed at angles 2θ = 31.7°, 34.5°, 36.2°, 47.5°, 56.5°, 62.8°, 67.9° and 69°. It can be concluded that hydrotalcite-type compounds and, to a lesser extent, the zinc oxide phase are identified in both samples.
All the hydrotalcites synthesized were calcined at 700 °C. At this temperature, the hydrotalcite-type structure collapses, so that the diffraction spectrum observed in Fig. 2a corresponds only to the mixed oxides formed, with ZnO being the only phase present, observed at angles 2θ = 31.9°, 34.6°, 36.4°, 47.7°, 56.8°, 63°, 66.6°, 68.1° and 69.3°. In Fig. 2b, ZnO is also the only phase present where the angles 2θ = 32.1°, 34.7°, 36.5°, 47.8°, 56.9°, 63.1°, 66.7°, 68.2° and 69.3° are observed. For both samples, these signals correspond to planes (100), (002), (101), (102), (110), (103), (200), (112) and (201) [27, 28].
Fig. 3 presents the infrared spectra of ZnAlTi-LDH samples synthesized via three distinct methods. Given the similarity of absorption spectra among hydrotalcites, the primary absorption bands of LDH exhibit signals at nearly identical wavenumbers. At 3382.46 cm–1, a characteristic stretching of OH- groups within the brucite-type layers is observed. At 2974.41 cm–1, corresponding to the stretching frequency of water molecules, a signal is evident in samples synthesized via the conventional method, ZnTC. The broad band at 1639.53 cm–1 signifies the torsion mode of OH– groups in interstitial water molecules of hydrotalcite. Stretching of NO3−1 groups in the interlaminar region is found at 1353.85 cm–1 [22, 25, 27]. Vibrations of M(II)-OM(III) network stretching and bending, along with M–O stretching, manifest in the range from 832 cm–1 to 607 cm–1. Notably, the most intense signal within this range potentially corresponds to Ti–O [8, 18].
Significant changes in sample composition occur upon heating hydrotalcite at 700 °C for 4 h. The characteristic signal of OH- groups in the brucite-type layer at 3379.41 cm−1 (Fig. 3d) diminishes markedly post-calcination. The band at 1633.31 cm−1, associated with angular deformation of water’s H-OH group, shifts rightward compared to the fresh sample (Fig. 3c). While the band at 1359.35 cm−1 corresponding to the nitrate ion decreases noticeably, it persists. Bands indicative of metal oxides (ZnO, Al2O3, TiO2) may emerge from 1000 cm−1 [18]. Not much difference can be seen from the FTIR spectrum of materials prepared using conventional, ultrasonic, or microwave-assisted methods. Both spectrums exhibited the same absorption band and functional groups in the materials.
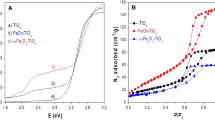
Nitrogen physisorption analyses were conducted to identify the isotherm characterizing calcined samples and to compute the specific area, diameter, and pore volume of the catalysts. Table 1 summarizes nitrogen physisorption determinations of synthesized samples. Samples synthesized with ultrasound, containing two cations (Zn and Al) and three cations (Zn, Al, and Ti), exhibit higher surface area values as hydrotalcites than mixed oxides. Incorporating a third cation (Ti) in the hydrotalcites and subsequent calcination leads to a doubling or tripling of area values compared to samples with only two cations (ZnAl). Microwave-synthesized samples report the lowest surface area and pore volume values but the highest pore diameter values [22, 25, 29]. It was clear that the average pore size consistently decreased with ultrasonic treatment. Zhao et al. mentioned that the effect of ultrasonic on the formation of pore structure can be conceptually considered the cavitation effect, which can not only improve the crystal quality of the LDHs, but also bring a smaller gain size and higher degree of dispersion [21].
Fig. S1 exhibits Type IV isotherms for all catalysts, indicative of mesoporous materials. Across all isotherms, capillary condensation accompanied by hysteresis is observed. According to the IUPAC classification, the hysteresis loop corresponding to type H3 is evident in all graphs, which is characteristic of clay-type materials or aggregate materials with plate-like or lamellar particles [30].
Intending to illustrate the morphology of the hydrotalcites and the mixed oxides obtained by calcining the sample at 700 °C, the textural characterization using SEM (Fig. 4a and b) is presented. It can be seen in Fig. 4a that the LDHs have a structure made up of flake-shaped agglomerates, or the nanostructures shows a cauliflower-like shape [31]. Once the sample was calcined, the characteristic flakes of LDH faded, and many agglomerates of dispersed material were formed. The EDS mapping (of the sample shows that O, Zn, Al, and Ti are highly homogeneously distributed on the surface of the OZnTU catalyst (see Fig. S2) [21].
XPS spectra were employed to identify and determine the oxidation states of metals and oxygen atoms in the samples (Al 2p, Zn 2p, Ti 2p, and O 1 s). Fig. S3 displays deconvoluted peaks from XPS spectra. The binding energies of Al 2p components were identified and deconvoluted (Fig. S3a), revealing peaks attributed to Al–OH (Al 2p—OH) and Al-O (Al 2p–O) at 74.15 and 74.80 eV, respectively. The peak at 74.25 eV corresponds to the Al-M state. Peaks indicative of Al2O3 in oxidized and hydroxyl forms are observed in all catalysts containing Al2O3, possibly representing surface Al–OH groups [32, 33]. Fig. S3b illustrates the deconvoluted peak of TiO2 species from the OZnTU catalyst. A change in the oxidation state of TiO2 is discerned from the spin–orbit splitting of 2p1/2 and 2p3/2 at 464 and 458.7 eV, respectively. The shape of the Ti 2p1/2 excludes the presence of traceable amounts of Ti3+ at 464 eV. Species of Ti 2p 3/2 are visible at 458.7 eV and correspond to TiO2 species (Ti4+). When irradiated with UV light, these species produce photogenerated electron–hole pairs. In this process, the electrons tend to reduce the Ti4+ cations to a Ti3+ state, while the holes can oxidize the O2− anions to form O− ions. Also, Ti3+ ions can enhance photoactivity by trapping photogenerated electrons and promoting free charge formation, possibly adjacent to oxygen vacancies. Since Ti3+ ions are strongly polarized, they can improve photoactivity [34, 35]. At 459.0 eV, the observed influence of Zn, suggests potential substitution of some Ti ions with Zn ions in the lattice structure.
Additionally, the catalyst exhibits characteristic peaks at 1044.70 eV and 1021 eV, corresponding to Zn 2p 1/2 and Zn 2p 3/2, respectively (Fig. S3c), indicative of Zn2+ [36] with a slight shoulder observed at 1040 eV, potentially corresponding to Zn 2p 1/2 of ZnO. The asymmetric O 1 s peak on the surface is fitted coherently by three nearly Gaussian components centered at 530.2 eV, 531.4 eV, and 532.6 eV. The 530.2 eV peak is attributed to the O2− ions, surrounded by the OZnTU catalyst [37]. The higher binding energy at 532.6 eV is typically associated with chemisorbed or dissociated oxygen or OH species on the catalyst’s surface. The medium binding energy component at 531.4 eV of the O 1 s peak is linked with O2− ions present in oxygen-deficient regions, such as oxygen vacancies, which are crucial for electron transfer when irradiated with ultraviolet light. The composition of the OZnTU catalyst, measured by XPS, is detailed in Table S1, showing a metallic ratio (M2+/M3+) of 5.2, corroborating the presence of hydrotalcites, ZnO, Al2O3, and TiO2. Additionally, probable surface contamination with carbonate or CO2 species from atmospheric air and impurities of Cl and Na, likely derived from sample washing during synthesis, was observed.
The curves associated with the variation of (F(R)hν)2 vs. energy for the samples are shown in Fig. S4. Band gap energy (Eg) estimation derived from Tauc’s plot, assuming a direct transition [39], yields values of 3.22 eV in both catalysts. When exposed to ultraviolet light, this band gap energy could induce degradation effects on 2,4-D [39, 40]. Notably, this material may serve as a photocatalyst akin to TiO2, with bandgap values closely aligned with the reported value of 3.2 eV for Titania [40]. Ekennia et al. reported a band gap range of 2.72 to 4.72 eV for ZnO nanoparticles [27], while other studies have reported biosynthesized ZnO nanoparticles with a band gap energy of 3.28 eV [39, 41]. From the results in Fig. S4, the band gap energy for both catalysts, OZnU and OZnTU, falls within the reported range.
Photocatalytic degradation of 2,4-D
Fig. 5a and b depict the UV–Vis spectra of the degradation reaction of 2,4-D over time using the OZnU and OZnTU catalysts. The spectra show characteristic peaks at 284 and 229 nm. The peak at 284 nm is assigned to n → π* transitions of C–Cl bonds, and the peak at 229 nm is assigned to π → π* transitions of aromatic rings [38]. A significant decrease in these peaks suggests the susceptibility of C–Cl bonds and aromatic rings to degradation by hydroxyl radicals. Additionally, a slight increase in the signal at 250 nm, observed from 50 to 180 min, is attributed to intermediate formation, possibly benzoquinones generating maleic and fumaric acid, formic acid, and oxalic acid [42, 43]. Notably, after 210 min, a substantial decrease in signals at 284 and 229 nm is observed, with degradation more pronounced with the OZnTU catalyst, suggesting effective mineralization of 2,4-D to simpler molecules like CO2 and H2O.
Determining the point of zero charges (pHPZC) is essential for predicting catalysts’surface charge across varying pH levels, which is crucial during the photodegradation process. Photocatalysis primarily occurs on catalyst surfaces, hence, its pH sensitivity significantly impacts its performance during pollutant degradation [41, 42]. The pHPZC values for the OZnU and OZnTU catalysts, tested across a pH range from 2 to 12, are illustrated in Fig. S5. The experimentally determined pHPZC of the OZnU catalyst was 9.64, while for OZnTU, it was 11.0. Comparable values by Yashni et al. reported pHpzc values of 8.5 and 9.01 for ZnO NPs [40], while Castro et al. reported a pHPZC value of 9.26 for mixed oxides containing ZnO and TiO2 [18]. At pH values below pHPZC, the catalysts carry a positive charge, while higher pH values promote the formation of a negative charge on the catalyst’s surface.
Being a negatively charged anionic molecule, 2,4-D can adsorb onto catalyst surfaces via electrostatic attraction and interactions, particularly when catalysts exhibit a highly positive charge below their respective pHpzc (9.64). Such interactions between 2,4-D and catalysts enhance adsorption properties, thereby improving degradation efficiency. Degradation efficiency (DE%) was calculated using the formula DE% = (C0−C)/ C0 × 100, where C0 and C are 2,4-D solution concentrations at time t = 0 and any subsequent time t.
The pH of the solution is a critical parameter in the photocatalytic system. The degradation effect on 2,4-D was evaluated at an initial solution pH of 6.67, with initial 2,4-D concentration, contact time, catalyst amounts, and shaking speed set at 86.4 mg/L, 240 min, 0.1 g/200 mL, and 300 rpm. Degradation was monitored by observing the decrease in the 2,4-D band at 284 nm in UV–Vis absorption spectra. Before each photocatalysis assay, 2,4-D solution and each catalyst were stirred in darkness for 60 min until equilibrium was attained to mitigate any adsorption effects. Photolysis experiments in the absence of photocatalysts confirmed that light alone is insufficient for 2,4-D degradation. All photodegradation experiments were conducted in triplicate to ensure repeatability of the results.
Fig. 6a illustrates the degradation progress of 2,4-D in aqueous solution over time using Zn/Al catalysts synthesized via conventional (OZnC), ultrasound (OZnU), and microwave (OZnM) methods. Additionally, the photolysis process, where 2,4-D undergoes degradation by 21.65% in 240 min under ultraviolet light without a catalyst, is depicted. The degradation process reveals that OZnU degraded 2,4-D to 34.4% of its original concentration in 60 min (Fig. 6a), while OZnM degraded 17.14%. OZnC did not exhibit significant adsorption, maintaining the initial concentration, potentially due to its lower surface area and porosity. Upon UV light irradiation, photodegradation commences. OZnU displayed the highest degradation and adsorption, reaching 91.8% of the original concentration, attributed to its higher surface area and lowest pore diameter (Table 1). OZnC degraded 85.9%, while OZnM degraded 30.8% of the initial concentration.
In Fig. 6b, photocatalytic evaluations with Zn/Al/Ti oxides are presented. OZnTU degraded 2,4-D to 23.3% of its original concentration at 60 min, while OZnTC degrading 13.0%. OZnTM exhibited minimal changes. The most effective catalyst was OZnTU, degrading 95.6% of the original concentration, followed by OZnTC (75.6%), and OZnTM (31.34%). The substantial increase in degradation percentages may be attributed to the addition of titanium (Ti), enhancing surface area up to 3 times. This increased surface area facilitates more significant adsorption of 2,4-D, enhancing degradation efficiency. However, the presence of Ti may hinder adsorption due to increased material hardness, potentially reducing porosity. Considering the memory effect of hydrotalcite, mixed oxides may revert to their original structure in the photocatalysis process, impacting adsorption and photodegradation efficiency. These processes are influenced by catalyst crystalline structure, synthesis methods, and chemical composition.
Kinetic studies of degradation were conducted using various experimental runs. Following previously published studies [24], the photodegradation reaction of 2,4-D follows a pseudo-first-order reaction. Rate constants were determined from the exponential equation [44]:
Here Abs represents the instrumental signal, A is the process amplitude, k1 is the pseudo-first-order rate constant, and E is the endpoint. The correlation coefficient (R2) validates the linear behavior of Eq. 1. Kinetic parameters results are summarized in Table 2, and the relationship between absorbance and reaction time for 2,4-D is depicted in Fig. 5.
The recyclability of the OZnTU material was assessed by subjecting it to three consecutive cycles of photodegradation of 2,4-D, aiming to evaluate its potential for regeneration. Sequential runs were conducted after recovering the photocatalyst through filtration. Post-treatments such as washing or thermal treatments at 50 °C were carried out during the recycling experiments to reactivate the active sites on the material’s surface after each cycle. The photodegradation of 2,4-D using OZnTU achieved degradation efficiencies of 89–95% in the first four cycles under conditions of 0.1 g/200 mL solution [2,4-D]0 = 86.4 mg/L, pH = 6.67, 40 °C and 300 rpm.
By-products identification
In the subsequent analysis, by-products of the photodegradation process were identified. Fig. S6a displays the ESI–MS spectra of fragmentation of the [M-H]- ion obtained through direct injection of an aqueous solution of 2,4-D at the experiment’s onset. The principal ions observed were at m/z 160.95 and m/z 218.96, with intensities exceeding 1 × 105, consistent with previous reports for 2,4-D [42, 45]. Fig. S6b shows the ESI-MS spectra of the [M-H]− ion fragmentation of 2,4-D after 240 min of photodegradation using OZnU. In addition, the main ions observed were at m/z 160.95, m/z 170.83, m/z 218.96, and m/z 354.82 with intensities above 3 and 1 × 103 corresponding to the main signals reported for the degradation of 2,4-D, and a decrease in intensity occurs with some new compounds signals such as at m/z 170.83, m/z 310.75, m/z 354.82, m/z 414.75 and m/z 490.68 indicating photodegradation of the 2,4-D compound [46]. Jaafarzadeh et al. had reported that 2,4-D had a half-life of 50 min in water irradiated at 254 nm with the reaction products 2,4-dichlorophenol, 4-chlorocatechol, 2-hydroxy-4-chlorophenoxyacetic acid, 1,2,4-benzenetriol, and polymeric humic acids [42].
Similarly, in Fig. S6c, the ESI–MS spectra of the [M-H]− ion fragmentation of 2,4-D after 240 min of photo-degradation using OZnTU are depicted. New signals corresponding to potential degradation products were observed, at m/z 170.83, m/z 194.87, m/z 265.14, m/z 297.15, m/z 354.82, m/z 490.68 indicating photodegradation of 2,4-D compound [47]. This spectrum reflects a signal of all the subproducts with an intensity less than 1 × 103, and the reduction in intensity of the rest of the signals is indicative of possible photocatalytic degradation and/or change in the structure of the rest of the components in the formulation of the commercial herbicide solution (Herbipol ®). From these results, it can be concluded that there is a significant degradation of the active ingredient 2,4-D and the other compounds present in the formulation of the herbicide used.
Conclusions
Hydrotalcite-type compounds were obtained by incorporating three and two different cations and their corresponding mixed oxides derived from hydrotalcites. It is possible to observe the differences in the textural properties obtained depending on the heat treatment method used in the synthesis of hydrotalcites. All the synthesized catalysts were extensively characterized, with particular emphasis on two key samples: OZnU and OZnTU. Both catalysts exhibit semiconductor properties with a band gap of 3.22 eV. Characterization studies and UV radiation-induced photo-degradation experiments using OZnU and OZnTU have demonstrated their efficacy as photocatalysts for the degradation of 2,4-D. Optimal conditions for initial degradation, involving natural pH and minimal catalyst dosage, yielded the most promising results in degrading 86.4 ppm of 2,4-D (0.1 g). Analysis of the reported ESI ± spectra allowed for the examination of potential degradation pathways of the herbicide, complemented by UV–Vis spectra analysis of the various samples studied. In addition, the determination of first-order kinetic constants for the degradation process of 2,4-D provided valuable insights. These results highlight the practical application of mixed oxides as photocatalysts, particularly in wastewater treatment, potentially reducing the costs associated with photodegradation processes of organic compounds.
References
Garabrant D, Philbert M (2002) Review of 2,4-Dichlorophenoxyacetic acid (2,4-D) epidemiology and toxicology. Crit Rev Toxicol 32:233–257
Ávila V, Meraz L, Padilla JL (1997) Metodología en línea para la determinación de trazas en los herbicidas 2,4-D y 2,4-DB en agua. Revista Internacional de Contaminación Ambiental 13:63–71
Dolatabadi M, Taghi-Ghaneian M, Wang C, Ahmadzadeh S (2021) Electro-Fenton approach for highly efficient degradation of the herbicide 2,4-dichlorophenoxyacetic acid from agricultural wastewater: process optimization, kinetic and mechanism. J Molecular Liquids 334:116116. https://doi.org/10.1016/j.molliq.2021.116116
Peterson MA, McMaster SA, Riechers DE, Skelton J, Stahlman PW (2017) 2,4-D past, present, and future: a review. Weed Technol 30:303–345. https://doi.org/10.1614/WT-D-15-00131.1
Girón-Navarro R, Linares-Hernández I, Teutli-Sequeira EA, Martínez-Miranda V, Santoyo-Tepole F (2021) Evaluation and comparison of advanced oxidation processes for the degradation of 2,4-dichlorophenoxyacetic acid (2,4-D): a review. Environ Sci Pollut Res Int 28:26325–26358
Kundu S, Pal A, Dikshit AK (2005) UV induced degradation of herbicide 2,4-D: kinetics, mechanism and effect of various conditions on the degradation. Sep Purif Technol 44:121–129
Cai J, Zhou M, Yang W, Pan Y, Lu X, Serrano KG (2018) Degradation and mechanism of 2,4-dichlorophenoxyacetic acid (2,4-D) by thermally activated persulfate oxidation. Chemosphere. https://doi.org/10.1016/j.chemosphere.2018.08.127
Araújo ES, Pereira MFG, da Silva GMG, Tavares GF, Oliveira CYB, Faia PM (2023) A Review on the use of metal oxide-based nanocomposites for the remediation of organics-contaminated water via photocatalysis: fundamentals bibliometric study and recent advances. Toxics 11(8):658. https://doi.org/10.3390/toxics11080658
Organización Mundial de la Salud. (29 de noviembre de 2023). Las dioxinas y sus efectos en la salud humana. Organización Mundial de la Salud. https://www.who.int/es/news-room/fact-sheets/detail/dioxins-and-their-effects-on-human-health#:~:text=Efectos%20de%20las%20dioxinas%20en,as%C3%AD%20como%20alteraciones%20funcionales%20hep%C3%A1ticas
Henkel M (2015) 21st Century Homestead: Sustainable Agriculture III: Agricultural Practices. Lulu.com. https://books.google.com.mx/
Environmental health criteria for 2,4-dichlorophenoxyacetic acid, https://www.inchem.org/documents/ehc/ehc/ehc29.htm#PartNumber:1
Pozdnyakov I, Sherin P, Grivin V, Plyusnin V (2016) Degradation of herbicide 2,4-dichlorophenoxybutanoic acid in the photolysis of [FeOH]2+ and [Fe(Ox)3]3− complexes: a mechanistic study. Chemosphere 146:280–288. https://doi.org/10.1016/j.chemosphere.2015.12.041
Gaya UI, Abdullah AH (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem Photobiol, C 9(1):1–12
Farhan A, Khalid A, Maqsood N, Iftekhar S, Muhammad H et al (2024) Progress in layered double hydroxides (LDHs): synthesis and application in adsorption, catalysis and photoreduction. Sci Total Environ 912:169160. https://doi.org/10.1016/j.scitotenv.2023.169160
Mohanty UA, Sahoo DP, Paramanik L, Parida K (2023) A critical review on layered double hydroxide (LDH)-derived functional nanomaterials as potential and sustainable photocatalysts. Sustain Energy Fuels 7:1145–1186. https://doi.org/10.1039/D2SE01510B
Tamboli AH, Jadhav AR, Chung W, Kim H (2015) Structurally modified cerium doped hydrotalcite-like precursor as efficient catalysts for hydrogen production from sodium borohydride hydrolysis. Energy 93:955–962. https://doi.org/10.1016/j.energy.2015.09.059
Osorio G, Duque A et al (2015) Propiedades dieléctricas de hidróxidos dobles laminares de Mg:Al. Revista de La Sociedad Química Del Perú 81:224–231
Castro LV, Manríquez ME, Ortiz-Islas E et al (2023) Kinetic study of the photodegradation of ibuprofen using tertiary oxide ZnO–Al2O3–TiO2. Reac Kinet Mech Cat 136:1705–1721. https://doi.org/10.1007/s11144-023-02430-y
Sikander U, Sufian S, Salam MA (2017) A review of hydrotalcite based catalysts for hydrogen production systems. Int J Hydrogen Energy 42:19851–19868. https://doi.org/10.1016/j.ijhydene.2017.06.089
Bukhtiyarova MV (2018) A review on effect of synthesis conditions on the formation of layered double hydroxides. J Solid State Chem 269:494–506. https://doi.org/10.1016/j.jssc.2018.10.018
Zhao S, Yi H, Tang X, Gao F, Yu Q, Zhou Y, Wang J, Huang Y, Yang Z (2016) Enhancement effects of ultrasound assisted in the synthesis of NiAl hydrotalcite for carbonyl sulfide removal. Ultrason Sonochem 32:336–342. https://doi.org/10.1016/j.ultsonch.2016.04.001
Jubri Z, Hussein MZ, Yahaya A, Zainal Z (2012) The effect of microwave assisted synthesis on the physico-chemical properties of pamoate-intercalated layered double hydroxide. Nanosci Methods 1:152–163. https://doi.org/10.1080/17458080.2011.630036
Mahadeva M, Nagabhushana BM, Hari Krishna R, Nagaraju K, Raveendra RS, Prashanth R (2017) Fast adsorptive removal of methylene dye from aqueous solution onto a wild carrot fower activated carbon: isotherms and kinetics studies. Desalin Water Treat 71:399–405
Castro LV, Alcántar-Vázquez B, Ortiz-Islas E, Manriquez ME (2023) Photocatalytic performance of copper slag in the degradation of 2,4-dichlorophenoxyacetic acid herbicide. Reac Kinet Mech Cat 136:3211–3226
Zhang G, Hu L, Zhao R, Su R, Wang Q, Wang P (2018) Microwave-assisted synthesis of ZnNiAl-layered double hydroxides with calcination treatment for enhanced PNP photo-degradation under visible-light irradiation. J Photochem Photobiol, A 356:633–641. https://doi.org/10.1016/j.jphotochem.2018.02.010
Zidi Z, Ltifi M, Ayadi B, Mir EL, Nóvoa L (2020) Effect of nano-ZnO on mechanical and thermal properties of geopolymer. Journal of Asian Ceramic Societies 8:1–9. https://doi.org/10.1080/21870764.2019.1693682
Ekennia AC, Uduagwu DN, Nwaji NN, Oje O, Emma-Uba CO, Mgbii SI, Nwanji OL (2020) Green synthesis of biogenic zinc oxide nanoflower as dual agent for photodegradation of an organic dye and tyrosinase inhibitor. J Inorg Organomet Polym Mater. https://doi.org/10.1007/s10904-020-01729-w
Negrón G, Soto L, Guerra A, Lomas L, Méndez J (2000) Preparación de hidrotalcitas mediante radiación de microondas. Evaluación de la actividad catalítica. Revista de la Sociedad Química de México 44:251–256
Thommes M, Kaneko K, Neimark A, Olivier J, Rodriguez R, Rouquerol J, Sing K (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87(9–10):1051–1069. https://doi.org/10.1515/pac-2014-1117
Scarpellini D, Falconi C, Gaudio P, Mattoccia A, Medaglia PG, Orsini A, Pizzoferrato R, Richetta M (2014) Morphology of Zn/Al layered double hydroxide nanosheets grown onto aluminum thin films. Microelectron Eng 126:129–133. https://doi.org/10.1016/j.mee.2014.07.007
Chubar N (2018) The influence of sulfate on selenate sorption on Mg–Al–CO3 layered double hydroxides prepared by fine inorganic sol–gel synthesis studied by X-ray photoelectron spectroscopy. Appl Surf Sci 459:281–291
Wu G, Dash K, Galano ML, O’Reilly KAQ (2019) Oxidation studies of Al alloys: part II Al-Mg alloy. Corros Sci 155:97–108
Biesinger MC, Lau LWM, Gerson AR, Smart RSC (2010) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc Ti, V, Cu and Zn. Appl Surf Sci 257:887–898
Biesinger M, Hart B, Grosvenor B, Mcintyre A, Lau N, Smart R (2008) Quantitative chemical state XPS analysis of first row transition metals, oxides and hydroxides. J Phys: Conf Ser 100:012025. https://doi.org/10.1088/1742-6596/100/1/012025
Ilyas U, Rawat RS, Tan TL, Lee P, Chen R, Sun HD, Fengji L, Zhang S (2011) Oxygen rich p-type ZnO thin films using wet chemical route with enhanced carrier concentration by temperature-dependent tuning of acceptor defects. J Appl Phys 110(9):093522
Ren C, Wang H, Ji J, Li W (2022) Molecular scale assessment of defluoridation of coal-mining wastewater by calcined Mg/Al layered double hydroxide using 19F solid-state NMR, XPS, and HRTEM. Chemosphere 303:135072. https://doi.org/10.1016/j.chemosphere.2022.135072
López R, Gomez R (2012) Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: a comparative study. J Sol-Gel Sci Technol 61:1–7
Estrella-González A, Asomoza M, Solís S, García-Sanchez MA, Cipagauta-díaz S (2020) Enhanced photocatalytic degradation of the herbicide 2,4-dichlorophenoxyacetic acid by Pt/TiO2–SiO2 nanocomposites. Reac Kinet Mech Cat 131:489–503
Angel RD, Durán-Álvarez JC, Zanella R (2018) TiO2-low band gap semiconductor heterostructures for water treatment using sunlight-driven photocatalysis. Titan Dioxide: Mater Sustain Environ. https://doi.org/10.5772/intechopen.76501
Gopalakrishnan Y, Al-Gheethi E, Mohamed R, Al-Sahari M (2020) Reusability performance of green zinc oxide nanoparticles for photocatalysis of bathroom greywater. Water Pract Technol 16:364–376. https://doi.org/10.2166/wpt.2020.118
Belhamdi B, Merzougui Z, Trari M, Addoun A (2016) A kinetic, equilibrium and thermodynamic study of 1-phenylalanine adsorption using activated carbon based on agricultural waste (date stones). J Appl Res Technolgy 14:354–366
Jafarzadeh N, Ghanbari F, Ahmadi M (2017) Efficient degradation of 2,4- dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: a novel combination of advanced oxidation processes. Chem Eng J 320:436–447
Girón-Navarro R, Linares-Hernández I, Teutli-Sequeira EA, Martínez-Miranda V, Santoyo-Tepole F (2021) Evaluation and comparison of advanced oxidation processes for the degradation of 2,4-dichlorophenoxyacetic acid (2,4-D): a review. Environ Sci Pollut Res 28:26325–26358
Lente G (2018) Facts and alternative facts in chemical kinetics: remarks about the kinetic use of activities, termolecular processes, and linearization techniques. Curr Opin Chem Eng 21:76–83
NIST Mass Spectrometry Data Center, https://webbook.nist.gov/cgi/inchi?ID=C94757&Mask=200#Mass-Spec. Accessed 18 March, 2024
Liu M, Huang D, Quan S, Zheng J, Zhang W, Liu L (2014) Degradation of 2,4-dichlorophenoxyacetic acid in an internal circulation three-phase fluidized photoreactor using N-TiO2=γ-Al2O3 granule as adsorbent and photocatalyst. J Environ Eng 140:04014026
Golshan M, Kakavandi B, Ahmadi M, Azizi M (2018) Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. J Hazard Mater 359:325–337
Acknowledgements
The authors thank the IPN for its financial support (SIP-20231201) and the Centro de Nano, Micro y Nanotecnología of the IPN for its technical support. Finally, we thank the Laboratorio Universitario of the UNAM for its technical support.
Author information
Authors and Affiliations
Contributions
LVC and GMB conceived and designed the experiments; LVC, GMB, BAV, MEM, EA, and RCS performed the experiments; LVC, BAV, EA, and MEM analyzed the data and wrote the manuscript; LVC, EA, RCS, and BAV contributed reagents/materials/synthesis tools; and all the authors revised and discussed the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bahena, G.M., Castro, L.V., Alcántar-Vázquez, B. et al. Photocatalytic performance of ZnAl vs ZnAlTi in the degradation of 2,4-dichlorophenoxyacetic acid herbicide. Reac Kinet Mech Cat (2024). https://doi.org/10.1007/s11144-024-02717-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11144-024-02717-8