Abstract
In this study, the degradation of trichloroethylene (TCE) with the existence of tween-80 (TW-80) or sodium dodecyl sulfate (SDS) using peroxymonosulfate (PMS) activated by nano-zero-valent iron (nZVI) was investigated. Over 87.6% TCE (with 1.3 g L−1 TW-80 presence) was degraded by 0.9 mM PMS and 0.12 g L−1 nZVI, while 89.7% TCE (with 2.3 g L−1 SDS presence) was degraded by 1.2 mM PMS and 0.20 g L−1 nZVI, in which more than 71.9% TCE with TW-80 existence and 87.5% TCE with SDS existence were dechlorinated. Besides, the effects of some factors (i.e., PMS and nZVI dosages, initial solution pH, and inorganic anions) on TCE removal were evaluated. The degradation of TCE was restrained continuously with increasing surfactant concentration, and TW-80 was more easily decomposed than SDS in PMS/nZVI system. Furthermore, sulfate radical (SO4–•) and hydroxyl radical (HO•) were demonstrated the main reactive oxygen species (ROS) contributing to TCE degradation and SO4–• played a dominant role through EPR tests and ROS scavenging experiments. Finally, the results of TCE degradation in actual groundwater confirmed that PMS/nZVI process has great advantages and potential in remediation of actual TCE-contaminated groundwater with TW-80 or SDS existence.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the widespread use in industrial production and personal daily life, chlorinated solvents have attracted increasing attention as growing contaminants in soil and groundwater (Jiao et al. 2009; Moran et al. 2007). Among the chlorinated solvents, trichloroethylene (TCE) is one of the most frequently detected contaminants in soil and groundwater. TCE can easily migrate and diffuse to the depth of groundwater and exist for a long time due to its properties of low solubility and biodegradability (Alonso-de-Linaje et al. 2019; Wu et al. 2016). Hence, it is necessary to establish high-efficient technology to remove TCE in deep groundwater.
Surfactant-enhanced aquifer remediation technology (SEAR), as an improved groundwater extraction and treatment technology, is increasingly employed to treat deep groundwater for its low cost, high efficiency, and short-time remediation (Fountain et al. 2010). Surfactants are injected into the contaminated sites; thus, organic contaminants could be desorbed from soil medium and enter the aqueous phase under solubilization by surfactants (Maturi et al. 2009). In addition, once surfactant concentration is equal to or more than its critical micelle concentration (CMC), surfactant molecules will aggregate together to form micelles, and the formation of micelles can enhance the solubilization of contaminants (West and Harwell 1992). Surfactants can be classified as anionic, nonionic, and cationic surfactants, in which nonionic and anionic surfactants are more widely used such as tween-80 (TW-80) (representative of nonionic surfactants) and sodium dodecyl sulfate (SDS) (representative of anionic surfactants) (Mao et al. 2015). The features of some commonly used surfactants are shown in Table S1. When TW-80 or SDS is used to enhance the solubility of organic contaminants, how to deal with TCE solutions containing surfactants is the next crucial issue.
Recent years, in situ chemical oxidation (ISCO) has become an effective choice, especially for the remediation of polluted groundwater (Huguenot et al. 2015). Many oxidants, such as peroxymonosulfate (PMS), peroxydisulfate (PDS), and hydrogen peroxide (H2O2), are applied in ISCO technology to produce reactive oxygen species (ROS) for degrading contaminants (Yu et al. 2018). Particularly, compared with other oxidants, PMS shows better performance in degrading organic contaminants because of its asymmetric structure and short O-O bond length (Rastogi et al. 2009). Generally, PMS can be activated by alkali, thermal, transition metals, and ultrasound to release sulfate radicals (SO4–•) and hydroxyl radicals (HO•) through a series of chain reactions (Paritosh et al. 2020; Li et al. 2018; Yin et al. 2018; Huang et al. 2009). However, alkali, thermal, and ultrasound suffer the limitations of high cost and complicated operation. Hence, much attention has been paid on transition metals for activating PMS. Among these transition metals, nano-zero-valent iron (nZVI), as an efficient catalyst, shows great potential in activating PMS. Compared with zero-valent iron (ZVI) or micron zero-valent iron (mZVI), nZVI displays greater reactivity because of its large surface area and low diffusion resistance (Zhou et al. 2015; Fu et al. 2014). As a long-lasting activator, nZVI can activate PMS gradually and reach the better utilization of the generated ROS. In a word, considering PMS a strong oxidant and the outstanding catalytic performance of nZVI, PMS/nZVI process can become highly-efficient and appliable technology for TCE removal containing surfactants.
Although nZVI has been successfully employed for the degradation of TCE, as far as we know, TCE removal with the existence of TW-80 (or SDS) in PMS/nZVI system has not been clearly reported yet (Zhou et al. 2015). Thus, the targets of this research are as follows: (1) to assess the effectiveness of PMS/nZVI process for removing TCE in the presence of TW-80 or SDS; (2) to elucidate the effect of different surfactant concentrations and the decomposition of surfactants; (3) to determine the dominant reactive oxygen species (ROS) for TCE removal and dechlorination of TCE; (4) to illuminate PMS/nZVI process performance to the remediation of the actual TCE-contaminated groundwater in the existence of TW-80 or SDS.
Materials and methods
Materials
All materials and related reagents used were listed in Supporting Information (Text S1).
Experimental processes
The 250-mL transparent reactors were applied to carry out a series of tests. In order to ensure the uniformity of reaction solution, a magnetic stirrer was placed in reactor and constantly stirred with speed of 600 rpm. A water bath was used to control constant temperature (20 ± 0.5°C) of reactors. Besides, according to the investigation of most contaminated groundwater in practice, TCE concentration was set to 0.15 mM (Sun et al. 2019). Considering the high concentration of surfactants used in the actual remediation of contaminated sites, the TW-80 concentration was set as 1.3 g L−1 and the SDS concentration was fixed as 2.3 g L−1, unless conducting experiments of different surfactants concentration (Besha et al. 2017).
Firstly, 0.15 mM TCE and surfactants in 250 mL were prepared in the reactor and stirred constantly. Secondly, the pre-calculated amount of nZVI was added into the reactor and the reaction started after adding PMS. 0.2-mL samples were taken from the reactor as designed intervals and rapidly added into a vial containing 2.8 mL n-hexane. Finally, samples were shaken for 5 min and the supernatants were taken for the gas chromatograph (GC) analysis.
In scavenging experiments, tert-butyl alcohol (TBA) and isopropanol (IPA) were employed to identify the main ROS for TCE removal (Buxton et al. 1988; Liang and Su 2009). 5,5-2 Dimethyl-1-pyrroline N-oxide (DMPO) was employed to trap radical species and ROS contained in PMS/nZVI system was detected by electron paramagnetic resonance (EPR). For chloride ion (Cl–) analysis, 6-mL samples were taken from solutions at preset intervals and filtered by 0.22-μm water phase filter head, then 5-mL filtered solution was added by 1 mL of methanol to terminate reaction. Finally, samples were placed standby for several hours to evaporate remaining TCE. Ion chromatograph (IC) was applied to quantify Cl–.
In actual groundwater experiments, tests were carried out in 250-mL reactors using the collected actual groundwater instead of ultrapure water. Firstly, TCE solution and surfactants were added to the reactors containing actual groundwater in which TCE was set to 0.15 mM, while TW-80 or SDS concentration was set to 1.3 g L−1 or 2.3 g L−1, respectively. Secondly, nZVI was added into reactors and the reaction began after adding PMS. 0.2-mL samples were taken from reactors at set time points and added into a vial including 2.8 mL n-hexane. Thirdly, samples were shaken for 5 min and the supernatants were taken for analysis by GC.
Analytical methods
The GC was employed to analyze TCE extras and further details about instrument are provided in Text S2. For the analysis of PMS concentration, the KI colorimetric method at the wavelength of 352 nm was employed (Liang et al. 2008). The 1,10-phenanthroline spectrophotometry at 512 nm was used to quantify the dissolved iron and total Fe (Harvey et al. 1955). The concentration of TW-80 was analyzed by cobalt ammonium thiocyanate spectrophotometric method at 623 nm and the concentration of SDS was determined with the methylene blue spectrophotometric method at 650 nm (Lin et al. 1986; Hayashi 1975). Besides, some information about EPR was provided in Text S2.
Results and discussion
The degradation performance of TCE
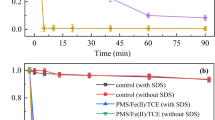
For evaluating the degradation performance of TCE in PMS/nZVI system, four experimental systems, namely PMS, PMS/Fe (II), PMS/ZVI, and PMS/nZVI systems, were investigated. As shown in Fig. 1a, no significant loss of TCE (less than 7.0%) was observed with or without the presence of TW-80 in control group. Furthermore, only 14.8% TCE was removed in PMS alone system with the presence of TW-80, which suggested that without activator PMS could not degrade TCE effectively. Under the existence of TW-80, about 30.5%, 75.6%, and 87.6% TCE were degraded in PMS/Fe (II), PMS/ZVI, and PMS/nZVI systems within 180 min, respectively. In addition, the kinetic constant (K1) and the correlation index of kinetic formulas (R2) at equilibrium of the pseudo-first-order kinetics were 0.0016 min−1 (R2 > 0.77), 0.0147 min−1 (R2 > 0.92), and 0.0693 min−1 (R2 > 0.91) respectively, which corresponded to the performance of TCE removal in PMS/Fe (II), PMS/ZVI, and PMS/nZVI systems. The above results indicated that the addition of nZVI could remarkably enhance the efficiency of TCE removal, showing the excellent catalytic performance of nZVI. Especially, the degradation of TCE quickly reached 81.6% within 15 min in PMS/nZVI system without TW-80 and eventually raised to 97.8%. Similar results could also be achieved with the presence of SDS. As shown in Fig. 1b, the result of control group with SDS presence (less than 8.5%) was similar to that with TW-80, which could be ascribed to the natural volatilization of TCE. Corresponding to TCE removal in PMS/Fe(II), PMS/ZVI, and PMS/nZVI systems, the K1 were calculated as 0.0026 min−1 (R2 > 0.83), 0.0072 min−1 (R2 > 0.99), and 0.0321 min−1 (R2 > 0.99), respectively. In addition, TCE removal in PMS/nZVI system increased from 89.7% (with SDS) to 95.5% (without SDS). The above results strongly proved that the catalytic performance of nZVI on PMS was much better than Fe(II) or ZVI. Some researches reported that PDS/Fe(II)/nZVI process also exhibited excellent TCE removal performance when containing surfactants (Xu et al. 2021). However, the higher chemical dosages and lower surfactant concentration used in their study limited practical applications. Compared with PDS, PMS is more easily catalyzed due to its asymmetric structure; therefore, using less oxidants and catalysts could achieve better degradation to pollutants. As long-lasting catalysts, nZVI or ZVI can gradually activate PMS, while Fe(II) may rapidly react with ROS duo to its short lifespan (Xue et al. 2018). Moreover, TCE removal was inhibited with TW-80 or SDS addition from experimental results in PMS/nZVI system (with or without surfactants).
The investigation of key factors in PMS/nZVI system
Effect of PMS dose
To investigate the effect of oxidant dosage on TCE removal, various concentrations of PMS were set to conduct experiments (fixed nZVI dose as 0.12 g L−1 with TW-80 existence and 0.20 g L−1 with SDS existence). From Fig. 2a and b, it was obviously noted that PMS alone could not degrade TCE (both less than 6%). The degradation of TCE with TW-80 existence (with SDS existence) rapidly improved from 64.8% (52.5%) to 87.6% (88.8%) with the raise of PMS dosage from 0.3 mM (0.3 mM) to 0.9 mM (1.2 mM), and the K1 increased from 0.0658 min−1 (0.0250 min−1) to 0.1094 min−1 (0.0321 min−1), respectively. Unexpectedly, TCE removal including TW-80 (or SDS) began to decrease from 80.3% (86.8%) to 73.0% (70.2%) when PMS dosage continuously increased from 1.2 mM (1.5 mM) to 1.5 mM (1.8 mM). These results suggested that TCE removal was restricted by PMS concentration and more ROS would produce with the increase of PMS concentration. Similar to the mechanisms of persulfate activated by zero-valent iron (Xiong et al. 2014), PMS could also be activated by nZVI via the transfer of electrons on the nZVI surface during the reaction. However, the generated ROS could be consumed by PMS to form SO5–• (Eqs. 1–2), which is a less reactive free radical and cannot degrade contaminants effectively. Furthermore, the extra SO4−• and the generated SO5–• might be consumed by self-scavenging reactions (Eqs. 3–4). Hence, the quenching processes may hinder or inhibit the degradation of TCE when the dosage of PMS surpassed 0.9 mM (with TW-80) or 1.2 mM (with SDS) in PMS/nZVI system. In order to keep highly efficient TCE degradation, the PMS dosages were selected as 0.9 mM (with TW-80) and 1.2 mM (with SDS) in the following experiments except in actual groundwater tests. PMS decomposition was also monitored in PMS/nZVI system with surfactants presence. As illustrated in Fig. S1, 99.5% (with TW-80 existence) and 67.0% (with SDS existence) PMS were consumed in 180 min, which indicated the utilization ratio of PMS in PMS/nZVI with TW-80 was much sufficient but it was relatively insufficient with SDS existence.
Effect of nZVI dose
Figure 3 shows the effect of different nZVI doses on TCE removal. Evidently, the degradation of TCE with TW-80 existence (with SDS existence) was raised from 9.1% (16.0%) to 87.5% (91.7%) with the addition of nZVI raised from 0 g L−1 (0 g L−1) to 0.12 g L−1 (0.20 g L−1). However, the catalytic effect of nZVI was not as efficient as expected when nZVI dosage was increased continuously. TCE removal with TW-80 existence (with SDS existence) was decreased slightly from 87.4% (90.7%) to 84.9% (72.5%) when the addition of nZVI was increased from 0.15 g L−1 (0.28 g L−1) to 0.18 g L−1 (0.36 g L−1). In addition, nZVI could react with PMS or hydrogen ions to form Fe(II) (Eqs. 5–6), and PMS also would produce SO4−• and HO• under the activation of generated Fe(II) (Eqs. 7–8). But excess nZVI will be applied to compensate for electron wastage instead of activating PMS (Liu et al. 2013). When PMS was insufficient whereas Fe(II) was excessive, the extra Fe(II), as a radical scavenger, could react with SO4−•, thus leading to the decrease of TCE degradation (Eq. 9). In order to make full utilization of Fe(II) and reduce the self-scavenging effect of Fe(II) on ROS, the nZVI dosages were chosen as 0.12 g L−1 (with TW-80 presence) and 0.20 g L−1 (with SDS presence) in the following experiments apart from the actual groundwater tests. As shown in Fig. S2, the changes of total Fe and Fe(II) concentration were also monitored. In the presence of TW-80 (or SDS), more than 1.45 mM (2.75 mM) total Fe and 0.59 mM (1.65 mM) Fe(II) were produced in 180 min.
Effect of initial solution pH
According to literature, initial pH value of solution has a strong influence on ROS generation in Fenton-like system (Xue et al. 2018). Therefore, initial pH value (3.0, 5.0, 7.0, 9.0, and 11.0) in solution was adjusted by H2SO4 or NaOH to investigate its effect on TCE removal. As shown in Fig. 4a, approximately 89.1%, 86.5%, 90.1%, 90.3%, and 22.7% TCE in the presence of TW-80 were removed when initial pH value of solution was set to 3.0, 5.0, 7.0, 9.0, and 11.0, respectively. In Fig. 4b, about 90.3%, 90.4%, 91.3%, 86.8%, and 72.5% TCE (with SDS) were removed at initial solution pH values of 3.0, 5.0, 7.0, 9.0, and 11.0, respectively. Some relevant data about TCE degradation and pH value are listed in Table 1. These findings indicated that pH below 9.0 had few negative influence on TCE removal with TW-80 existence (with SDS existence) whereas alkaline conditions (pH = 11.0) were not favorable for TCE removal. Ling et al. (2017) reported that in most cases, PMS/Fe(II) system was required to operate under acidic conditions to prevent the formation of Fe(III) precipitates. Although the initial solution pH in the range of 7.0 and 9.0 is not favorable to the catalysis of nZVI, PMS is acidic after dissolution in aqueous solution; thus, the pH value can be decreased effectively by adding PMS. Under pH = 11.0, the addition of PMS was not sufficient to lower the pH and more iron precipitated out of the solution, thus inhibited TCE degradation.
Effect of inorganic anions
To our knowledge, the existence of inorganic anions may have impacts on TCE removal by scavenging ROS, generating new ROS, or absorbing on the catalyst surface (Qi et al. 2014). Thus, four kinds of common anions (Cl–, NO3–, PO43–, and CO32–) on TCE degradation in PMS/nZVI system were investigated. Figure S3 shows that TCE removal with TW-80 existence (with SDS existence) decreased from 83.8% (90.0%) to 58.1% (70.6%) when 1 mM and 10 mM Cl– were added into the solution. There are several reasons accounting for above results. Firstly, PMS can react with Cl– to produce Cl2 (Eq. 10) (Ao and Liu 2017). Secondly, HO• and SO4–• could be depleted by Cl– to generate ClOH– or Cl• (Eqs. 11–12) (Wang et al. 2017). So TCE degradation containing TW-80 or SDS were decreased mainly because of the consumption of oxidants, HO•, and SO4–•. Figure S4 describes that TCE removal with TW-80 presence (with SDS presence) was slightly declined from 85.6% (83.4%) to 83.0% (77.3%) with the raise of NO3– from 1 to 10 mM, and TCE removal was slightly inhibited due to reactions between NO3– and HO• or SO4–• (Eqs. 13–14) (Neta et al. 1988). Compared with Cl– and NO3–, PO43– possessed an obviously inhibitive effect on TCE degradation. Figure S5 shows that the removal efficiency of TCE with TW-80 presence (with SDS presence) decreased from 34.4% (28.0%) to 6.5% (12.4%) with the addition of PO43– from 1 to 10 mM, which was attributed to the occupation of reaction sites and consumption of ROS. PO43– could restrain the corrosion of nZVI by absorbing the catalyst surface and react with ROS or iron ions (Cao et al. 2019). As illustrated in Fig. S6, TCE removal efficiency with TW-80 presence (with SDS presence) was reduced from 34.7% (49.4%) to 9.5% (9.9%) with the increase of CO32– from 1 to 10 mM, which was due to the quenching effect of CO32– on ROS (Eqs. 15–16) (Neta et al. 1988). In addition, the formed HCO3– could also scavenge ROS due to the conversion of CO32– under acidic condition.
The influence of surfactants
As discussed in the “The degradation performance of TCE” section, the presence of surfactants would inhibit TCE removal. Depending on the CMC of surfactants, experiments were carried out by setting different surfactant concentrations (TW-80: 0, 1.0, 100, 200, and 400 CMC; SDS: 0, 0.5, 1, 2, and 4 CMC). As shown in Fig. 5, without surfactant or with low concentration of surfactants, there was little effect on TCE removal. Clearly, TCE degradation with TW-80 existence (with SDS existence) was decreased from 97.8% (95.4%) to 87.5% (85.1%) when surfactant concentration was increased from 0 CMC (0 CMC) to 200 CMC (2 CMC). However, only 23.0% and 54.9% TCE removal were obtained with 400 CMC TW-80 or 4 CMC SDS addition. For high concentration of surfactants, ROS would be consumed during the decomposition of surfactants, which resulted in insufficient ROS to degrade TCE (Li 2004). Additionally, TCE molecules were easy to be wrapped by micelles; thus, ROS firstly attacked micelles before attacking TCE molecule, leading to the reduced utility of ROS (Tao et al. 2019).
Furthermore, for the purpose of better exploring the influence of surfactants on TCE removal, the changes of surfactant concentration were monitored during reaction. As illustrated in Fig. S7, the concentration of TW-80 was decreased from 1.3 to 0.6 g L−1 in 180 min, while SDS concentration was reduced from 2.3 to 1.8 g L−1 during the reaction. Compared to 21.7% SDS decomposition, the decomposition of TW-80 reached to 53.1%. These results demonstrated that TW-80 was more easily decomposed than SDS in PMS/nZVI system. The main reason is that the CMC of SDS is 2.3 g L−1, while the CMC of TW-80 is 0.013 g L−1, and the difference of concentration between SDS and TW-80 in experiments was large. Therefore, SDS was less decomposed in the reaction due to the high concentration and the large consumption of free radicals.
It can be seen from the previous discussion that the decomposition of surfactants was mainly attacked by ROS. To further confirm that surfactants could consume HO• and SO4–•, scavenging tests were conducted in which TBA was selected as the scavenger for HO• and IPA was selected as the scavenger for both HO• and SO4–•. As shown in Fig. 6a, when there was only TCE or TW-80 or SDS in the system, TCE removal and the decomposition of TW-80 or SDS were all below 5% in 180 min, which were hardly affected with 100 mM TBA or 100 mM IPA addition. As illustrated in Fig. 6b, when no scavenger was added, TW-80 and SDS were decomposed to 53.1% and 21.7% in PMS/nZVI system, respectively. However, the decomposition of TW-80 was decreased from 53.1 to 41.3% with 100 mM TBA addition and from 53.1 to 11.5% with 100 mM IPA addition. Similar results were also found in tests with SDS. The decomposition of SDS was reduced from 21.7% (without scavenger) to 11.3% (with 100 mM TBA). When 100 mM IPA was added, only 6.5% SDS was decomposed during the reaction. These results verified that HO• and SO4–• attacked TW-80 and SDS, causing the consumption of HO• and SO4–•. Furthermore, compared with HO•, SO4–• played the dominant role in TW-80 decomposition.
Effect of free radical scavengers on TCE removal and the decomposition of TW-80 or SDS in PMS/nZVI system. a Blank groups ([TW-80]0 = 1.3 g L−1, [SDS]0 = 2.3 g L−1, [TCE]0 = 0.15 mM). b Experimental groups ([PMS]0 = 0.9 mM, [nZVI]0 = 0.12 g L−1, [TW-80]0 = 1.3 g L−1, [TCE]0 = 0.15 mM; [PMS]0 = 1.2 mM, [nZVI]0 = 0.20 g L−1, [SDS]0 = 2.3 g L−1, [TCE]0 = 0.15 mM)
Identification of ROS and dechlorination of TCE
Identification of ROS
According to literature, much ROS would generate in PMS-based Fenton-like reaction; thus, DMPO was used to trap ROS and EPR was employed for analysis (Madden and Taniguchi 2001). Yang et al. (2018) and Wang et al. (2015) reported that typical characteristic peaks of DMPO-HO• and DMPO-SO4–• adducts were found in EPR spectrum. As shown in Fig. 7, the results of EPR experiments were in agreement with their studies, which proved the existence of HO• and SO4–• in PMS/nZVI system. Based on published articles, O2–• was also found in bisphenol A removal in PMS/Fe system; however, no typical signs could be discovered for DMPO-OOH adducts formed by the reaction between DMPO and O2–•, owing to a less O2–• generated, causing the lower amount of DMPO-OOH adducts, which was covered by characteristic peaks of HO• and SO4–• (Liu et al. 2020; Tan et al. 2012). Furthermore, DMPO-OOH adducts was unstable and was transformed easily to DMPO-OH in acidic conditions (Zhou et al. 2006).
To further determine the dominant ROS in PMS/nZVI system during TCE degradation, IPA (kSO4–• = 8.2 × 107 M−1 s−1, kHO• = 1.9 × 109 M−1 s−1) and TBA (kHO• = 5.3 × 108 M−1 s−1) were selected as scavengers to carry out reactive radicals scavenging tests (Buxton et al. 1988; Liang et al. 2008). As seen in Fig. 8a, about 87.6% TCE in the existence of TW-80 was degraded without scavengers. Unexpectedly, TCE removal was declined to 48.3% and 17.8% when adding 10 mM and 100 mM IPA, separately. The K1 was dropped from 0.0592 min−1 (without scavenger) to 0.0167 min−1 (with 10 mM IPA) and 0.0009 min−1 (with 100 mM IPA), demonstrating that SO4–• was predominant ROS in PMS/nZVI system in the existence of TW-80. Besides, 83.8% and 85.2% TCE were removed when 10 mM and 100 mM TBA were added into solution separately. The degradation of TCE was slightly inhibited with the addition of TBA, indicating that HO• hardly promoted the removal of TCE with TW-80 presence. As illustrated in Fig. 8b, in the existence of SDS, about 91.7% TCE was removed without scavenger addition. When 10 mM and 100 mM TBA were added into the solution, over 89.7% and 85.9% TCE were degraded, respectively. Similar to the experimental groups containing TW-80, about 54.5% and 25.1% TCE with SDS existence were removed when 10 mM and 100 mM IPA were added. In addition, K1 was reduced from 0.0433 min−1 (without scavenger) to 0.0252 min−1 (with 10 mM IPA) and 0.0004 min−1 (with 100 mM IPA). Although scavengers functioned on tests with TW-80 presence and tests with SDS presence to a different degree, results of reactive radicals scavenging tests in two experimental groups strongly proved that PMS/nZVI process primarily involved SO4–• and HO• generation, and SO4–• played a leading role.
Dechlorination of TCE
To investigate dechlorination of TCE including surfactants, experiments of Cl– analysis were conducted. As shown in Fig. 9 and Table 2, about 58.4% and 72.6% Cl– were detected with TW-80 and SDS presence, separately. Considering the natural volatilization of 6.3% and 5.8% TCE with TW-80 and SDS during the whole reaction, the actual released Cl– were 71.9% (with TW-80) and 87.5% (with SDS). Furthermore, based on the theoretical released Cl– (removing volatilized Cl–), the maximum released Cl– were 86.7% (with TW-80) and 90.0% (with SDS) in theory. From the experimental results, it could be seen that the dechlorination of TCE containing SDS was slightly better than that containing TW-80.
Effectiveness of TCE degradation in actual groundwater
To evaluate the applicability of PMS/nZVI process for removing TCE in actual groundwater under TW-80 or SDS existence, various experiments were conducted. Detailed parameters of groundwater and pH values before and after reaction were listed in Table 1 and Table S2, respectively. Figure 10 shows that TCE removal with TW-80 existence (with SDS existence) merely reached to 14.5% (20.8%) under the initial dosages of 0.9 mM PMS and 0.12 g L−1 nZVI (1.2 mM PMS and 0.20 g L−1 nZVI). Regrettably, only 25.5% (23.1%) TCE was removed when the dosages of PMS and nZVI were increased to 2 folds of the initial dosage, separately. These experimental results in actual groundwater were worse than that in ultrapure water mainly due to the strong buffering capacity of actual groundwater (pH = 7.7) and the presence of high concentration of inorganic ions. It could be seen from the above results that increasing chemicals doses could not only fail to achieve better degradation performance, but also lead to the high remediation cost. Hence, in order to improve efficiency of TCE removal and save remediation cost, H2SO4 is applied to regulate pH of actual groundwater to 5.0 firstly. Encouragingly, compared to the groups without pH adjustment, TCE removal with TW-80 presence (with SDS presence) dramatically increased to 73.8% (71.3%), 81.0% (87.4%) at one and two folds of initial chemicals dose, separately. Although the pH of the solution was around 3.0 after reaction, it could be cheaply adjusted with NaOH. Apparently, this is a better way that can be employed to deal with the negative effects caused by the complicated actual groundwater matrix, thus showing a great potential in application.
Conclusion
This study mainly explored TCE degradation performance in PMS/nZVI system in the existence of TW-80 or SDS. The synergistic catalytic interaction between PMS and nZVI has been confirmed by various controlled experiments. As a long-lasting activator, nZVI could activate PMS efficiently and improve the utility of PMS. Compared with ZVI or Fe(II), the application of nZVI not only increased TCE removal, but also reduced the oxidant dosage and saved the remediation cost. In investigation of surfactant influence, TW-80 or SDS could consume HO• and SO4–• during the whole reaction, and SO4–• played the main role in the decomposition of TW-80. Therefore, more free radicals would be consumed with increasing surfactant concentration and hence resulting in a decrease in TCE removal. Additionally, the effect of different inorganic anions (Cl–, NO3–, PO43–, and CO32–) and initial solution pH (3.0, 5.0, 7.0, 9.0, and 11.0) on TCE removal was investigated. Except for pH = 11, TCE with TW-80 (or SDS) was well degraded at other pH conditions, which reflected a wide adaptability of PMS/nZVI process. The EPR detection proved the generation of HO• and SO4–•, in which SO4–• played an important role in TCE removal through the ROS scavenging tests. Although increasing dosages of chemicals could slightly enhance the degradation of TCE with surfactants in actual groundwater, the cost of remediation was significantly increased. Encouragingly, about 81.0% (87.4%) of TCE with TW-80 (with SDS) was degraded under the dosages of 1.8 mM PMS and 0.24 g L−1 nZVI (2.4 mM PMS and 0.40 g L−1 nZVI) by previously adjusting the pH of actual groundwater to 5, which strongly demonstrated that PMS/nZVI process has great potential for dealing with TCE pollutant containing surfactants.
Data availability
All relevant data and material have been provided in the manuscript and supplementary information.
References
Alonso-de-Linaje V, Mangayayam MC, Tobler DJ, Dietmann KM, Espinosa R, Rives V, Dalby KN (2019) Sorption of chlorinated hydrocarbons from synthetic and natural groundwater by organo-hydrotalcites: towards their applications as remediation nanoparticles. Chemosphere 236:124369. https://doi.org/10.1016/j.chemosphere.2019.124369
Ao X, Liu W (2017) Degradation of sulfamethoxazole by medium pressure UV and oxidants: peroxymonosulfate, persulfate, and hydrogen peroxide. Chem Eng J 313:629–637. https://doi.org/10.1016/j.cej.2016.12.089
Besha AT, Bekele DN, Naidu R, Chadalavada S (2017) Recent advances in surfactant-enhanced in-situ chemical oxidation for the remediation of non-aqueous phase liquid contaminated soils and aquifers. Environ Technol Innov 9:303–322. https://doi.org/10.1016/j.eti.2017.08.004
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms, and hydroxyl radicals OH/O− in aqueous solution. J Phys Chem Ref Data 17:513–886. https://doi.org/10.1063/1.555805
Cao J, Lai L, Lai B, Yao G, Chen X, Song L (2019) Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: performance, intermediates, toxicity and mechanism. Chem Eng J 364:45–56. https://doi.org/10.1016/j.cej.2019.01.113
Fountain JC, Starr RC, Middleton T, Beikirch M, Taylor C, Hodge D (2010) A controlled field test of surfactant-enhanced aquifer remediation. Groundwater 34:910–916. https://doi.org/10.1111/j.1745-6584.1996.tb02085.x
Fu F, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205. https://doi.org/10.1016/j.jhazmat.2013.12.062
Harvey AE, Smart JA, Amis ES (1955) Simultaneous spectrophotometric determination of Fe(II) and total iron with 1,10-phenanthroline. Anal Chem 27:26–29. https://doi.org/10.1021/ac60097a009
Hayashi K (1975) A rapid determination of sodium dodecyl sulfate with methylene blue. Anal Biochem 67:503–506. https://doi.org/10.1016/0003-2697(75)90324-3
Huang YH, Huang YF, Huang C, Chen C (2009) Efficient decolorization of azo dye Reactive Black B involving aromatic fragment degradation in buffered Co2+/PMS oxidative processes with a ppb level dosage of Co2+-catalyst. J Hazard Mater 170:1110–1118. https://doi.org/10.1016/j.jhazmat.2009.05.091
Huguenot D, Mousset E, Hullebusch ED, Oturan MA (2015) Combination of surfactant enhanced soil washing and electro-Fenton process for the treatment of soils contaminated by petroleum hydrocarbons. J Environ Manage 153:40–47. https://doi.org/10.1016/j.jenvman.2015.01.037
Jiao Y, Qiu C, Huang L, Wu K, Ma H, Chen S, Ma L, Wu D (2009) Reductive dechlorination of carbon tetrachloride by zero-valent iron and related iron corrosion. Appl Catal B-Environ 91:434–440. https://doi.org/10.1016/j.apcatb.2009.06.01
Li Z (2004) Surfactant-enhanced oxidation of trichloroethylene by permanganate-proof of concept. Chemosphere 54:419–423. https://doi.org/10.1016/S0045-6535(03)00752-5
Li X, Yuan X, Wang D, Wang H, Wu Z, Jiang L, Mo D, Yang G, Guan R, Zeng G (2018) Recyclable zero-valent iron activating peroxymonosulfate synchronously combined with thermal treatment enhances sludge dewaterability by altering physicochemical and biological properties. Bioresour Technol 262:294–301. https://doi.org/10.1016/j.biortech.2018.04.050
Liang C, Su HW (2009) Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind Eng Chem Res 48:5558–5562. https://doi.org/10.1021/ie9002848
Liang CJ, Huang CF, Mohanty N, Kurakalva RM (2008) A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 73:1540–1543. https://doi.org/10.1016/j.chemosphere.2008.08.043
Lin JT, Cornell DG, Micich TJ (1986) Cobaltothiocyanate colorimetric analysis for homologous polyoxyethylated alkyl amides. J Am Oil Chem Soc 63:1575–1579. https://doi.org/10.1007/BF02553089
Ling L, Zhang D, Fan C, Shang C (2017) A Fe(II)/citrate/UV/PMS process for carbamazepine degradation at a very low Fe(II)/PMS ratio and neutral pH: the mechanisms. Water Res 124:446–453. https://doi.org/10.1016/j.watres.2017.07.066
Liu H, Wang Q, Wang C, Li X (2013) Electron efficiency of zero-valent iron for groundwater remediation and wastewater treatment. Chem Eng J 215:90–95. https://doi.org/10.1016/j.cej.2012.11.010
Liu L, Li Y, Li W, Zhong R, Lan Y, Guo J (2020) The efficient degradation of sulfisoxazole by singlet oxygen (1O2) derived from activated peroxymonosulfate (PMS) with Co3O4–SnO2/RSBC. Environ Res 187:109665. https://doi.org/10.1016/j.envres.2020.109665
Madden KP, Taniguchi H (2001) The role of the DMPO-hydrated electron spin adduct in DMPO-(OH)-O-center dot spin trapping. Free Radic Biol Med 30:1374–1380. https://doi.org/10.1016/S0891-5849(01)00540-8
Mao X, Jiang R, Xiao W, Yu J (2015) Use of surfactants for the remediation of contaminated soils: a review. J Hazard Mater 285:419–435. https://doi.org/10.1016/j.jhazmat.2014.12.009
Maturi K, Reddy KR, Cameselle C (2009) Surfactant-enhanced electrokinetic remediation of mixed contamination in low permeability soil. Sep Sci Technol 44:2385–2409. https://doi.org/10.1080/01496390902983745
Moran MJ, Zogorski JS, Squillace PJ (2007) Chlorinated solvents in groundwater of the United States. Environ Sci Technol 41:74–81. https://doi.org/10.1021/es061553y
Neta P, Huie RE, Ross AB (1988) Rate constants for reactions of inorganic radicals in aqueous solution. J Phys Chem Ref Data 17:1027–1284. https://doi.org/10.1063/1.555808
Paritosh K, Balan V, Vijay VK, Vivekanand V (2020) Simultaneous alkaline treatment of pearl millet straw for enhanced solid state anaerobic digestion: experimental investigation and energy analysis. J Clean Prod 252:119798. https://doi.org/10.1016/j.jclepro.2019.119798
Qi F, Chu W, Xu B (2014) Modeling the heterogeneous peroxymonosulfate/Co-MCM41 process for the degradation of caffeine and the study of influence of cobalt sources. Chem Eng J 235:10–18. https://doi.org/10.1016/j.cej.2013.08.113
Rastogi A, Al-Abed SR, Dionysiou DD (2009) Sulfate radical-based ferrous-peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl Catal B-Environ 85:171–179. https://doi.org/10.1016/j.apcatb.2008.07.010
Sun Y, Lyu S, Brusseau ML, Tang P, Jiang W, Gu M, Li M, Lyu Y, Qiu Z, Sui Q (2019) Degradation of trichloroethylene in aqueous solution by nanoscale calcium peroxide in the Fe(II)-based catalytic environments. Sep Purif Technol 226:13–21. https://doi.org/10.1016/j.seppur.2019.05.075
Tan C, Gao N, Chu W, Li C, Michael RT (2012) Degradation of diuron by persulfate activated with ferrous ion. Sep Purif Technol 95:44–48. https://doi.org/10.1016/j.seppur.2012.04.012
Tao Y, Brigante M, Zhang H, Mailhot G (2019) Phenanthrene degradation using Fe(III)-EDDS photoactivation under simulated solar light: a model for soil washing effluent treatment. Chemosphere 236:124366. https://doi.org/10.1016/j.chemosphere.2019.124366
Wang Y, Indrawirawan S, Duan X, Sun H, Ang HM, Tade MO, Wang S (2015) New insights into heterogeneous generation and evolution processes of sulfate radicals for phenol degradation over one-dimensional α-MnO2 nanostructures. Chem Eng J 266:12–20. https://doi.org/10.1016/j.cej.2014.12.066
Wang Z, Ai L, Huang Y, Zhang J, Li S, Chen J, Yang F (2017) Degradation of azo dye with activated peroxygens: when zero-valent iron meets chloride. RSC Adv 7:30941–30948. https://doi.org/10.1039/C7RA03872K
West CC, Harwell JH (1992) Surfactants and subsurface remediation. Environ Sci Technol 26:2324–2330. https://doi.org/10.1021/es00036a002
Wu X, Gu X, Lu S, Qiu Z, Sui Q, Zang X, Miao Z, Xu M, Danish M (2016) Accelerated degradation of tetrachloroethylene by Fe(II) activated persulfate process with hydroxylamine for enhancing Fe(II) regeneration. J Chem Technol Biotechnol 91:1280–1289. https://doi.org/10.1002/jctb.4718
Xiong X, Sun B, Zhang J, Gao N, Shen J, Li J, Guan X (2014) Activating persulfate by Fe0 coupling with weak magnetic field: performance and mechanism. Water Res 62:53–62. https://doi.org/10.1016/j.watres.2014.05.042
Xu Z, Huang J, Fu R, Zhou Z, Ali M, Ali S, Yang R, Zeng G, Zhou Z, Ayesha I, Lyu S (2021) Enhanced trichloroethylene degradation in the presence of surfactant: pivotal role of Fe(II)/nZVI catalytic synergy in persulfate system. Sep Purif Technol 272:118885. https://doi.org/10.1016/j.seppur.2021.118885
Xue Y, Sui Q, Brusseau ML, Zhang X, Qiu Z, Lyu S (2018) Insight on the generation of reactive oxygen species in the CaO2/Fe(II) Fenton system and the hydroxyl radical advancing strategy. Chem Eng J 353:657–665. https://doi.org/10.1016/j.cej.2018.07.124
Yang Q, Yang X, Yan Y, Sun C, Wu H, He J, Wang D (2018) Heterogeneous activation of peroxymonosulfate by different ferromanganese oxides for tetracycline degradation: structure dependence and catalytic mechanism. Chem Eng J 348:263–270. https://doi.org/10.1016/j.cej.2018.04.206
Yin R, Guo W, Wang H, Du J, Zhou X, Wu Q, Zheng H, Chang J, Ren N (2018) Enhanced peroxymonosulfate activation for sulfamethazine degradation by ultrasound irradiation: performances and mechanisms. Chem Eng J 335:145–153. https://doi.org/10.1016/j.cej.2017.10.063
Yu S, Gu X, Lu S, Xue Y, Zhang X, Xu M, Qiu Z, Sui Q (2018) Degradation of phenanthrene in aqueous solution by a persulfate/percarbonate system activated with CA chelated-Fe(II). Chem Eng J 333:122–131. https://doi.org/10.1016/j.cej.2017.09.158
Zhou N, Qiu T, Liu Y (2006) Superoxide anion radical generation in the NaOH/H2O2/Fe(III) system: a spin trapping ESR study. Magn. Reson. Chem. 44:38–44. https://doi.org/10.1002/mrc.1730
Zhou H, Shen Y, Lv P, Wang J, Li P (2015) Degradation pathway and kinetics of 1-alkyl-3-methylimidazolium bromides oxidation in an ultrasonic nanoscale zero-valent iron/hydrogen peroxide system. J Hazard Mater 284:241–252. https://doi.org/10.1016/j.jhazmat.2014.10.050
Funding
The study was financially supported by the National Key R&D Program of China (No. 2018YFC1802500) and “One Belt and One Road” International Academic Cooperation and Exchange Program of Shanghai Science and Technology Committee (No. 19230742200).
Author information
Authors and Affiliations
Contributions
Peng Wang: conceptualization, methodology, software, investigation, data curation, writing—original draft. Zhiqiang Xu: formal analysis, validation, writing—review and editing. Guilu Zeng: formal analysis, validation, writing—review and editing, funding acquisition. Shuguang Lyu: validation, writing—review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
The authors confirm that this manuscript has not been previously published as a whole or part and it is not under consideration by any other journal.
Consent to participate
All authors have approved the content and consent to submit it.
Consent to publish
All authors have approved the content and consent to publish it.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
•nZVI is an efficient and long-lasting activator of PMS on TCE removal.
•Surfactants can be decomposed by consuming ROS and TCE removal is affected by surfactants.
•SO4−• was the primary ROS in PMS/nZVI system in the presence of TW-80 or SDS.
•Significant TCE degradation in actual groundwater containing TW-80 or SDS was demonstrated.
Supplementary information
Supplementary materials 1:
Table S1. Features of some surfactants commonly used in groundwater remediation. Table S2. The main characteristics of the actual groundwater. Fig. S1. The decomposition of PMS and TCE removal in PMS/nZVI system with the presence of (a) TW-80 ([PMS]0 = 0.9 mM, [nZVI]0 = 0.12 g L-1, [TW-80]0 = 1.3 g L−1, [TCE]0 = 0.15 mM) and (b) SDS ([PMS]0 = 1.2 mM, [nZVI]0 = 0.20 g L-1, [SDS]0 = 2.3 g L−1, [TCE]0 = 0.15 mM). Fig. S2. The concentration of total Fe and Fe(II) in PMS/nZVI system with the presence of (a) TW-80 ([PMS]0 = 0.9 mM, [nZVI]0 = 0.12 g L-1, [TW-80]0 = 1.3 g L−1, [TCE]0 = 0.15 mM) and (b) SDS ([PMS]0 = 1.2 mM, [nZVI]0 = 0.20 g L-1, [SDS]0 = 2.3 g L−1, [TCE]0 = 0.15 mM). Fig. S3. Effect of Cl– on TCE removal in PMS/nZVI system with (a) TW-80 ([PMS]0 = 0.9 mM, [nZVI]0 = 0.12 g L-1, [TW-80]0 = 1.3 g L-1, [TCE]0 = 0.15 mM) and (b) SDS ([PMS]0 = 1.2 mM, [nZVI]0 = 0.20 g L-1, [SDS]0 = 2.3 g L-1, [TCE]0 = 0.15 mM). Fig. S4. Effect of NO3– on TCE removal in PMS/nZVI system with (a) TW-80 ([PMS]0 = 0.9 mM, [nZVI]0 = 0.12 g L-1, [TW-80]0 = 1.3 g L-1, [TCE]0 = 0.15 mM) and (b) SDS ([PMS]0 = 1.2 mM, [nZVI]0 = 0.20 g L-1, [SDS]0 = 2.3 g L-1, [TCE]0 = 0.15 mM). Fig. S5. Effect of PO43– on TCE removal in PMS/nZVI system with (a) TW-80 ([PMS]0 = 0.9 mM, [nZVI]0 = 0.12 g L-1, [TW-80]0 = 1.3 g L-1, [TCE]0 = 0.15 mM) and (b) SDS ([PMS]0 = 1.2 mM, [nZVI]0 = 0.20 g L-1, [SDS]0 = 2.3 g L-1, [TCE]0 = 0.15 mM). Fig. S6. Effect of CO32– on TCE removal in PMS/nZVI system with (a) TW-80 ([PMS]0 = 0.9 mM, [nZVI]0 = 0.12 g L-1, [TW-80]0 = 1.3 g L-1, [TCE]0 = 0.15 mM) and (b) SDS ([PMS]0 = 1.2 mM, [nZVI]0 = 0.20 g L-1, [SDS]0 = 2.3 g L-1, [TCE]0 = 0.15 mM). Fig. S7. The changes of TW-80 and SDS concentrations in PMS/nZVI system. ([PMS]0 = 0.9 mM, [nZVI]0 = 0.12 g L-1, [TW-80]0 = 1.3 g L-1 [TCE]0 = 0.15 mM; [PMS]0 = 1.2 mM, [nZVI]0 = 0.20 g L-1, [SDS]0 = 2.3 g L-1 [TCE]0 = 0.15 mM).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, P., Xu, Z., Zeng, G. et al. Efficient degradation of trichloroethene with the existence of surfactants by peroxymonosulfate activated by nano-zero-valent iron: performance and mechanism investigation. Environ Sci Pollut Res 30, 48351–48362 (2023). https://doi.org/10.1007/s11356-023-25725-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25725-y