Abstract
Selected trace metals were determined in stormwater runoff and sediments of the highly urbanized Brunette watershed in Metro-Vancouver. Surface sediment samples from three tributaries and a lake between 1974 and 1998 were analyzed for total and acid-extractable trace metals. Metal bioavailability was also investigated using Chelex-100 resin. Sediment geochemistry was determined by sequential extraction. Total trace metal concentrations decreased as stormwater moved through the hydrologic gradient of stormwater runoff, headwater stream to outflow river. The percentage of dissolved metals increased downstream largely due to disposition. Higher concentrations of particle-associated trace metals were flushed in stormwater runoff as the rainfall and total suspended solids transport increased. The highest trace metal levels were found in the lower reaches of a creek before entering the lake and in the lake where organic matter accumulated. Copper was associated with the organic/sulphur sediment components, whereas iron and manganese were mainly mineral-bound. Zinc concentrated in the easily acid reducible phase, augmented by increasing traffic and development. At least half of the sediment-bound lead was associated with the easily acid reducible and organic/sulphur-bound phases with an overall decrease as lead has been phased out as a gasoline additive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Trace metal influx within river basins due to urban development is an ongoing concern affecting water and sediment pollution (Li et al. 2006). Trace metals are by far the most common priority pollutants in urban runoff, with copper and lead as the most prevalent metal contaminants (USEPA 1983). There is a wide array of non-point sources of metals within the urban environment, with motor vehicular traffic likely responsible for the major inputs of lead, zinc, manganese and copper (Novotny and Olem 1994; Mason et al. 1999; Davis et al. 2001; Rose et al. 2001; Couillard et al. 2004; Preciado and Li 2006). Runoff from galvanized roofs and drainpipes can be a source of zinc contamination (Good 1993). Another potential source of trace metals in the urban environment is the dry deposition of particulates upon roof tops and their subsequent remobilization during storms (Tuccillo 2006). Contaminants transported within stormwater discharges from urbanized catchment are a major cause of impairment of receiving waters (USEPA 1986; Makepeace et al. 1995; Novotny 1995).
The ultimate concern of trace metal contaminants in receiving water is their toxic impact on aquatic organisms and fish species (Bindra and Hall 1977; Morrison et al. 1989; Morrison and Revitt 1987; Kushner 1993; Sutherland and Tolosa 2000; De Carlo et al. 2004). The pollutant behaviour is mainly governed by its speciation (Morrison and Revitt 1987; Florence et al. 1992; Perin et al. 1997). Trace metals may be present in numerous physicochemical forms (soluble, adsorbed on mineral surfaces, complexed with organic matter, precipitated or entrapped in mineral phases). Exchangeable forms are usually considered as immediately bio-available species (Morrison and Revitt 1987). Much recent research has focussed on fractionation and partitioning to interpret the environmental impact to aquatic organisms (Chandra Sekhar et al. 2003; Burton et al. 2005; Glosińska et al. 2005; Boughriet et al. 2007). The partitioning of metal contaminants between specific forms is classically determined using sequential extraction (Serne 1975; Tessier et al. 1979; Lake et al. 1984; Förster 1996; El Samrani et al. 2004; Pardo et al. 2004; Preciado and Li 2006) and others have applied BCR (the European Community Bureau of Reference) three-step sequential extraction (Quevauviller et al. 1997). The mobility and bioavailability of contaminants in receiving water are important in evaluating the risk of toxicity to aquatic organisms. Understanding these processes is of special interest to local governments in order to manage the contaminants and reduce their environmental impact. However, there is limited information on the long term monitoring of such processes in relation to changes in land use due to urban development.
This study examines the extent of trace metal contamination in storm runoff, and in stream and lake sediments. It determined potentially bio-available trace metals in water and sediments and their spatial and temporal distribution over a 24-year period in the Brunette River watershed, located in a major metropolitan area. Other features of the study included: (1) determination of the distribution of trace metals in the Brunette watershed during three winter storm events; (2) collection of information on the availability of metals to aquatic organisms for base and stormwater runoff conditions using dialysis with the receiver resin technique; and (3) investigation of the bioavailability of trace metal contaminants (Cu, Fe, Mn, Pb and Zn) in the Brunette River watershed by following the hydrological gradient through the watershed, and their distribution as traffic intensity, fuel additives and land use changed from 1974 and 1998. Traditionally, total trace metals form the basis in evaluating the environmental impact on aquatic organisms. This study provides insight on the evaluation of geochemical fractionation of metals over a 24-year period due to changes in environmental activities in the watershed (traffic, impervious surfaces) that affect watershed quality and aquatic life.
2 Study Area
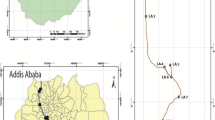
The Brunette River watershed is located in the highly urbanized area of Metro Vancouver, British Columbia, Canada, covering an area of 7200ha (Fig. 1). It is an area of high traffic density and intense land use, with impervious surfaces, such as roads and roof tops, covering more than 50% of some sub-watersheds such as that drained by Still Creek (McCallum 1995). The stormwater drainage system collects runoff from the impervious surfaces and discharges it directly to numerous streams (Still, Eagle, Stoney Creeks, etc.) in the watershed and through two lakes, Deer and Burnaby Lakes, which act as natural sediment traps (Fig. 1). The Brunette River receives discharge over a control dam from Burnaby Lake, and the runoff from the watershed is transported to the Fraser River at New Westminster, BC.
This watershed provides an excellent area for the study of spatial and temporal trace metal contamination in sediments due to urbanization. The water and sediment quality conditions in this watershed have been investigated since the early 1970’s (Hall et al. 1974, 1976; Li et al. 2006), with continuing research focused on specific contaminant relationships (McCallum 1995; Yuan 2000; Muraro 2005; Li 2007).
3 Methodology
3.1 Sampling
To investigate the dynamics of trace metals associated with stormwater runoff, three sites – a sediment trap in the Eagle Creek sub-watershed, Still Creek and the Brunette River (Fig. 1) – were selected for water sampling and field Chelex-100 resin deployment. Grab samples were collected at all three sites under baseflow (no stormwater runoff, n = 2) and during rainfall events (n = 3). Intensive water sampling was also conducted during a storm event where grab samples (n = 7) were collected every 40min in a rotation sequence at all three sites.
For the field in-situ studies, 1g of Chelex-100 resin (Na form) was placed in cellulose dialysis tubes (Spectra/Pro, 80mm × 24mm dia., molecular cut-off 1,000Da, previously washed with 0.01M EDTA), sealed with plastic clamps, and mounted in concrete blocks for placement at the three sites. The resin tubes were collected after storm events or after 1–2weeks of baseflow.
Surface sediment samples were collected at selected sites on Still (1, 2, 3, 4), Eagle Creeks and the Brunette River (Fig. 1) with an aluminum pot attached to a 3m pole. A minimum of three to five composite locations within each sampling site were sampled and the sediment sieved through a 2mm sieve into plastic bags. Sediment cores were taken from Burnaby Lake in a transect from Still Creek to the Brunette River (Fig. 1a–d) with a 1m piston corer. The core tubes were transported to the laboratory. The core was then extruded and sectioned into 20mm sections which were placed in plastic bags and frozen until analysis. Thawed stream and lake sediments were sieved through a 180μm sieve, and the <180μm fraction was dried at 104°C and disaggregated prior to digestion. Only surface sediment trace metal geochemistry for the lake sediment cores is presented in this paper.
3.2 Sample Extraction and Digestion
3.2.1 Water
Water samples were transported to the laboratory under ice in coolers and filtered through glass fiber filters (45mm, 1.2μm pore size). The dissolved phase (1–3l stormwater or 10l baseflow water) was passed through a Chelex resin column (6g of Chelex-100 resin in the sodium form in a 25 × 1.5cm dia. glass column) at a flow rate of 5–10ml/min. The resin after exchange was transferred to a 50ml polyethylene tube to which 20ml of 2M HNO3 was added followed by a 3h shake period (Eberbach Corp. shaker) to extract trace metals from the resin. The sample was centrifuged (3,000rpm for 20min, Beckman J2-21M/E centrifuge) and the eluant filtered (Whatman no. 4). The resin was then washed with 15ml of distilled water, which was combined with the acid extract. Metal species bound to colloidal material or very strongly complexed, passing through the Chelex 100 column, were determined by the difference between the total dissolved metal (TD) and the Chelex removable metal (CR). The TD and CR fractions of trace metals were digested with 5ml of conc. HNO3 to dryness on a hot plate, dissolved in 10ml of 1M HNO3, made up to 25ml with distilled water, and filtered (Whatman no. 4) prior to analysis.
The glass fibre filters containing the suspended solids were cut into small pieces and sequentially extracted with 20ml of 1M MgCl2 at pH = 7 (exchangeable phase), followed by 20ml 0.01 of acidic hydroxylamine hydrochloride (weakly reducible phase). The two phases were combined to report the results as easily reducible solids metals since the exchangeable phase was usually very low. The remaining particulates on the filter after centrifugation (10,000rpm for 30min) were digested in a porcelain crucible with 20ml of aqua regia on a hot plate and the residue dissolved in 0.05M HNO3 for analysis.
The suspended sediment weight on replicate filters was determined by Standard Methods procedures (APHA 1989).
3.2.2 Field Deployed Chelex Resins
The resin was extracted with 10ml of 1M HNO3 in 25ml plastic centrifuge tubes for 3–4h on a shaker and centrifuged at 3,000rpm for 20–30min. The resin was washed with distilled water and the combined extract analyzed. The trace metals complexed with the Chelex resin in the field are expressed as pg metal/mm2 of resin/h for a relative comparison at the three field sites since flow measurements were not taken. The Chelex resin metal binding capacity for zinc and copper were measured in the laboratory to ensure that the resin capacity was not exceeded in the field (Yuan 2000).
3.2.3 Sediments
For total metal determination, dried sediment samples (1–2g) were digested on a hot plate using either nitric acid or aqua regia (APHA, 3030, 1989). For total extractable metals, which might be considered a potentially bio-available fraction, dried sediment (2g) was extracted at room temperature with 20ml of 0.5M HCl in screw capped tubes on a mechanical shaker. The extracted samples were filtered through GFC filters and the extracts made up to 40ml with distilled water.
In 1974, the sediment geochemistry was determined by the sequential extraction method of Engler et al. (1974), whereas the 1998 analysis of stream and lake sediments followed the methodology of Tessier et al. (1979). Both methods segregate the trace metals into six geochemical fractions, but the extracting reagents differ. For comparative purposes, fractions were combined to represent four geochemical phases of trace metals, namely exchangeable phase (EP = f 1), easily acid reducible phase (EARP = f 2), organic/sulphur bound phase (OS = f3), and a residual phase (RP = f 4).
Organic matter content of the sediments was determined by loss on ignition (LOI), determined by ashing samples in a muffle furnace at 650°C (APHA 1989).
3.3 Trace Metal Analysis
Digested samples from stream water and sediments, and sediment extracts were analyzed by atomic absorption spectroscopy (Thermo Jarell Ash Video 22AA) or by inductively coupled plasma spectroscopy (Thermo Jarell Ash ICAP 61 instrument operated in the ICP-AES mode). Only copper, iron, manganese, lead and zinc are reported in this paper. Method precision was determined by duplicate or triplicate analysis of grab samples, and the accuracy of water trace metals was determined by spike recovery. For sediment determination, accuracy was evaluated by analyzing a certified reference soil.
4 Results
4.1 Metals Distribution in Runoff in the Brunette Watershed
Trace metals in water from three sites during three winter storms between November 1999 and January 2000 are presented in Fig. 2. For all metals (Cu, Fe, Mn and Zn) there is a decrease in the total metals in water samples from storm-water trap to Still Creek to Brunette River. The range of values is quite variable, possibly due to sedimentation.
The total dissolved (TD) Cu and Zn becomes a higher proportion of total metal (TM) as one moves downstream, confirming sedimentation of the suspended fraction transported in water. Chelex removable metal (i.e. complexed dissolved fraction) shown in Fig. 2 demonstrates that there is very little copper, indicating that the majority of dissolved Cu is already bound to strong complexing agents. There is only a small fraction of the iron in the dissolved and complexed fraction. At least half of the Mn and Zn are removed by Chelex resin, demonstrating that this portion is uncomplexed or that any complexes are weaker than the Chelex association.
Trace metals associated with suspended sediments (i.e. total and easily reducible) are presented in Fig. 3. There is a decrease in suspended sediment bound trace metals downstream, confirming the sedimentation suspected from the total and dissolved metal distribution in Fig. 2. In the sediment trap, less than half of the particulate bound metal (Cu, Fe, Mn and Zn) was in the easily reducible fraction. In Still Creek and Brunette River (although concentrations were lower than in the street trap), a higher proportion (up to 90%) of suspended sediment bound metal was easily reducible.
4.2 In-situ Bioavailability by Chelex Resin Adsorption
Relative resin adsorption (free or weakly complexed, dissolved-trace metals) during dry (baseflow) and wet (stormwater discharge) periods is presented in Fig. 4. In a street trap, Fe and Mn were higher during a dry period due to stagnant conditions in the trap where low dissolved oxygen (DO) and reducing conditions released Fe and Mn for adsorption by chelex, whereas with the storm runoff, the presence of O2 caused more precipitation of Fe and Mn. Cu and Zn were higher in the trap during the wet period indicating transport of dissolved metal from the street. The considerable variability (large range of values at any one station) is likely due to a variable preceeding dry period, where contaminants could collect in the street, as well, as the amount of rainfall needed to mobilize and transport those contaminants.
Less copper in the wet periods compared to dry intervals in stream and river samples is probably due to strong complexation with humic/fulvic acid materials leached from boggy sediments in the watershed which would be diluted during rainfall-runoff periods. Also illegal connections of sanitary sewers to storm drains contributes copper from a corrosive water supply (corrosion of copper pipes in houses) – more noticeable during dry low-flow periods.
Fe, Mn and Zn were higher in Still Creek during wet periods due to runoff from streets and flushing from sediment traps during runoff events. In the Brunette River, there was almost no change for Fe, but Mn and Zn were higher during wet periods.
4.3 Water Quality over a Storm Event
Trace metal distribution in water at the three sites over a rainfall event during a November storm are shown in Fig. 5. There were two periods when the rainfall exceeded 1mm/h (Table 1), but the peak flows downstream never materialized until the last hour of monitoring (Table 1). This higher flow resulted in mobilization of higher sediment concentrations, with the sediment levels decreasing during downstream transport (Table 2). The highest proportion of trace metals in the sediment trap is associated with the particulates transported in the runoff, and concentrations are highest when the higher rainfall scours these materials from the impervious surfaces and within the trap (TM-TD = SS, Fig. 5). There is very little difference in total dissolved and Chelex removable metals, so most dissolved metals were not complexed as they entered the storm drain from the street.
In Still Creek and Brunette River, most copper was in the dissolved fraction reflecting sedimentation of sediment-bound Cu during transport through the watershed. Very little of this copper was bound to Chelex, indicating that it was already complexed – probably in the humic/fulvic acids leached from boggy areas since the water was relatively highly coloured in this watershed.
Fe decreases as runoff moves along the watershed, and most of the Fe is particle-associated. There is very little free dissolved iron since very little is bound to chelex resin during the runoff event.
Mn followed the same trend as Fe in the sediment trap. Mn concentrations decreased down the watershed (trap to Still Creek to Brunette River) again reflecting sedimentation. There is a higher proportion of dissolved Mn in Still Creek, approximately half binding to Chelex. There is very little dissolved Mn in the Brunette River and most of this is Chelex-bound and therefore not complexed.
Zinc showed a similar trend to other metals in the sediment trap. Total zinc decreased from trap to Still Creek to Brunette River. Dissolved zinc predominated during the early part of storm event in Still Creek. The proportion of the Zn bound to Chelex decreased towards the middle of storm event. In the Brunette River half or less of the Zn was dissolved and most dissolved Zn was bound to the chelex resin.
4.4 Trace Metals Associated with Bottom Sediments
4.4.1 Total Trace Metals
Trace metals in the surface sediments along a hydrologic gradient through the watershed and changes that occurred over a 24year period (1974 to 1998) are presented in Table 3. Both total and acid extractable trace metals in Burnaby Lake are tabulated for the 1998 period.
In Still Creek there was an increase in sediment concentrations of all trace metals, except manganese, in the downstream direction. This is mainly attributed to the lower gradient at the last two stations (3 and 4) resulting in the deposition of finer sediments that usually bind higher concentrations of trace metals. The lower reaches of this sub-watershed also featured more commercial and industrial activity, causing more traffic and impervious surfaces (Tables 4 and 5). Lead decreased at all stations in Still Creek over this 24-year study period, attributable to the removal of lead from fuels (Li et al. 2006). Iron and manganese show similar temporal trends at all stations. Since iron and manganese occur naturally in soils, there may have been better management of erosion during construction activities in the watershed (GVSDD 1999). Copper and zinc followed the same temporal trend between 1974 and 1998, with concentrations at the upper stations (1 and 2) increasing, whereas those at the downstream stations (3 and 4) decreased. Downstream station decreases in Cu and Zn are likely attributable to the phasing out of some industrial activities in the lower reaches of this sub-watershed over this period.
In Burnaby Lake the first two stations (A and B) are located in the delta region where the coarser sediments from Still Creek are deposited. There sandy sediments have lower trace metal concentrations than the finer sediments deposited further along the lake (stations C and D).
Eagle Creek and the Brunette River, both downstream of Burnaby Lake (Fig. 1), showed decreases in both iron and manganese between 1974 and 1998 again reflecting better erosion control. However, lead did not decrease in these two tributaries over this period, even though it was removed from fuels. This is likely attributable to the slower hydrologic transport through the lower reaches of the watershed, with the surface sediments of Burnaby Lake providing a source of lead to the Brunette River.
4.4.2 Geochemical Distribution of Trace Metals – Stream Sediments
The geochemistry of the sediment-associated trace metals can affect their transport dynamics as well as their potential availability to aquatic organisms. Figure 6 presents the proportions of the trace metals associated with four geochemical phases for the stream/river sediments in 1974 and 1998.
Geochemistry of trace metals in surface stream sediment from the Brunette watershed in 1974 and 1998. See Fig. 1 for station location. Values expressed as percent of total metal
The largest proportion of copper is associated with the organic/sulphur component of the stream sediments. The percentage in this phase tends to increase downstream in Still Creek (stations 1 to 4), probably due to a decrease in the gradient and sedimentation of smaller/lighter particles higher in organic matter (Table 6). The higher proportion (although overall low levels, 3.5–11.6%) of Cu in the exchangeable phase in 1998 compared to 1974 and the lower percentage of Cu in the residual phase at all stations over this period are likely from anthropogenic sources of Cu due to traffic activities (Table 4). This exchangeable form of Cu is more mobile and easily bio-available. This source could well be copper from the wear of brake linings, accentuated by significantly higher traffic volume (FHWA 1998; Kayhanian et al. 2003). When sediment copper levels were low (1974 in Eagle Creek and Brunette River), most of this copper was in the residual phase.
Iron and manganese were predominately associated with the residual phase, reflecting their natural occurrence in soils of the watershed. The residual phase containing Fe and Mn is not readily bio-available, but could be important in the geochemical distribution of other elements due to the adsorptive properties of their oxides (Jenne 1968).
As the total iron in the watersheds decreased between 1974 and 1998 (see Table 3), the proportion in the residual phase also decreased in the stream sediments (Fig. 6). In 1998, more of the iron was distributed in the EARP and organic/sulphur bound phases, possibly affecting its mobility.
Manganese geochemistry is dominated by the residual and EARP phases, with very little manganese associated with the organic/sulphur phase. In 1998 a much higher proportion of the Mn was associated with the EARP, as well as the exchangeable phase (EP). This change in distribution was probably a consequence of the use of MMT (methylcyclo-pentadienyl manganese) as a fuel additive between the late 1970s and 2004, with manganese phosphate, sulphate and oxide combustion products contributing to these geochemical phases (Stokes et al. 1988; Blumberg and Walsh 2004).
Lead is distributed between the EARP, organic/sulphur and residual phases with at least half of the lead in these first two geochemical phases. In Still Creek between 1974 and 1998, there was a consistent increase in the proportion of lead associated with the EARP (Fig. 6), even though the total lead concentration decreased (Table 3). Organic/sulphur-bound lead is also an important component (25–38%) of lead geochemistry, with no overall consistent temporal trends over the study period. However, where lead concentrations increased between 1974 and 1998 (Eagle Creek and Brunette River), the proportion of lead in the organic/sulphur phase also increased. Except for one station (Still Creek 4), lead in the residual phase decreased from 1974 to 1998.
The largest proportion of zinc (average 50%) is associated with the easily acid reducible phase (EARP). The proportion of zinc in this geochemical phase increased over the study period (1974–1998), similar to the behaviour of lead in Still Creek. The percentage of zinc in the residual phase decreased over this period (Fig. 6). The geochemical fractions for zinc rank in order f 2> f 4 > f 3. Corrosion of galvanized components on vehicles followed by washing into urban streams is believed to be a major source of zinc contaminants on roadways (Li et al. 2006).
Comparison of all stream stations during both sampling periods reveals that the f1 (exchangeable phase) concentration for metals (Cu, Fe, Mn, Pb and Zn) is generally very low in sediments, indicating that this readily bio-available fraction of these metals is not an important contributor to organism bio-concentration or toxicity. The relative importance of these metals bound to the EARP is Zn > Mn > Pb > Cu > Fe. The relative importance of the five trace metals bound to the organic/sulphur geochemical phase is Cu > Pb > Zn > Fe > Mn, while their relative importance in the residual phase is Fe > Mn > Cu = Pb = Zn.
4.4.3 Geochemical Distribution of Trace Metals – Lake Surface Sediments
The total trace metals and the cold 0.5M HCl extractable metals during 1998 in the surface lake sediments are provided in Table 3. These data are presented from the west end of the lake (station A) where Still Creek discharges (Fig. 1) in a transect to the outlet eastern end at the Brunette River (station D). Still Creek provides more than 50% of the flow to Burnaby Lake and is the most highly urbanized sub-watershed in the drainage basin, with over half of the land surface considered impervious (Table 4). Stations A and B are in the delta area of Still Creek and have fine to coarse sandy sediments, imbedded with organic debris (LOI = 17–24%). The finer silts with higher organic matter content (LOI) accumulate further along the lake (station D, LOI = 47.5%).
Overall most trace metals in the lake surface sediments are higher at the two downstream lake stations (C and D) – Table 3. These finer sediments can provide a reservoir of trace metals to the Brunette River due to sediment scouring in the shallow lake (Z av ≤ 1m) during high rainfall periods when the lake can have a very short residence time (<1day; Hall et al. 2002). The coarser sandy sediments deposited from Still Creek (station B) contain the lowest overall concentration of trace metals in the lake surface sediment.
Research (Fytiano and Lourantou 2004; Moalla et al. 2006) indicates that the 0.5M HCl extract of sediments represents a single fraction of the trace metals that are potentially more mobile in aquatic sediments. The proportions of the trace metals in Burnaby Lake that are HCl extractable are in the order of Fe (31–45%), Mn (37–66%), Cu (46–72%), Pb (56–87%) and Zn (76–86%).
The sequential geochemical distribution of trace metals in the surface lake sediments follow a pattern which is similar to those observed for the stream sediments (Fig. 7). Copper is dominated by the organic/sulphur-bound phase (65–79%), whereas iron and manganese are dominated by their residual phases, and zinc is largely (50% or more) associated with the EARP. However, where concentrations of manganese are highest (station C and D), the exchangeable phase was dominant (40%), while only 26% was in the residual phase. It is likely that this is the area of the lake where the manganese used in MMT, the gasoline additive, was primarily deposited. At least 90% of the lead was associated with the EARP and organic/sulphur phases, with no exchangeable lead. The residual lead was only of minor importance (8–10%).
Geochemistry of trace metals in surface sediments from Burnaby Lake (1998). See Fig. 1 for station location. Value expressed as percent of total metal
A comparison of the HCl extractable metals to the sum of the three sequential metal extracts indicates that for Cu, Pb and Zn a higher proportion is extracted by the series of sequential extractions. This is especially true for Cu where 74% (average) is associated with the single organic/sulphur phase whereas only 61% (average) of the Cu is extracted with HCl. The suspect data from station D, with higher HCl extractable Cu than the total, has been excluded in these calculations. It appears that the HCl does not dissolve some of the O/S bound Cu. For iron and manganese, the proportion extracted by either method was similar and again relatively low due to their natural occurrence in the rock and soils of the watershed. The relative percentage extractions of the two methods are:
Sequential extraction (f 1 + f 2 + f 3) | Pb > Cu > Zn > Mn > Fe |
0.5M HCl extraction | Zn > Pb > Cu = Mn > Fe |
5 Discussion
5.1 Dynamics of Trace Metals in the Brunette Watershed
The mobility and deposition of trace metals in the Brunette River watershed are influenced by the land use changes and the hydrologic characteristics of the watershed. Increased urbanization over a twenty year period (Table 5) has resulted in a loss of green space areas which reduces the infiltration capacity of the land surface with more paving and roof tops (Table 4). This also increases the peak discharge of the streams and alters transport and deposition of contaminants. Superimposed on this urbanization footprint is the source control management of contaminants such as the removal of lead and then manganese as fuel additives.
The hydrologic gradient through the watershed has shown a decrease in the trace metals associated with suspended sediments (Fig. 2). These sediments are deposited where the gradient is lower, such as the lower reaches of Still Creek and the downstream end of shallow Burnaby Lake (Table 3) where a small dam helps facilitate deposition of lighter sediments (higher organic matter content). These deposition areas can act as sources of sediment contaminants for many years after source control, especially during extreme rainfall and runoff events.
5.2 Water and Sediment Quality in the Brunette River Watershed
During the monitored storm events, the total copper and zinc (Fig. 2) at all three sites far exceed the water quality criteria for the protection of aquatic life (Table 7). Even the total dissolved (TD) Cu and Zn exceeded these criteria. The Chelex resin bound dissolved metal, which should be the most bioavailable fraction of these metals, approaches the concentrations in the stream channels which are considered unsafe for aquatic life. Lead was not monitored in the stormwater since previous investigations (Macdonald et al. 1997) found levels to be very low following its removal as a fuel additive in the 1970s. No consideration has been given to the cumulative effects of these metals in stormwater.
With the exception of the upper Still Creek stations (A and B) in 1974, and copper in Eagle Creek and the Brunette River on single occasions, the total trace metals (Cu, Pb and Zn) in sediments (Table 3) exceeded the ISQGs (Interim Sediment Quality Guidelines) that have been established for Canada (CCME 2001). More contaminated areas of the watershed such as the lower two stations on both Still Creek (1974) and Burnaby Lake even exceeded the PEL (probable effects level) demonstrating severe impacts on aquatic organisms.
If only the HCl extractable trace metals in the lake sediments are considered potentially bioavailable, Cu exceeds the ISQG but is less than the PEL while both HCl extractable Pb and Zn exceed the ISQG at all stations and they even exceed the PEL at the two lower lake stations (C&D). The sequential geochemical distribution of the trace metals in Burnaby Lake sediments demonstrate that both the organic/sulphur bound Cu and Pb exceeds the ISQG while the sum of the O/S (f 3) and EARP (f 2) exceeds the ISQG at three of the stations and at the lower two stations f 1 + f 2 + f 3 exceeds even the PEL.
Similar conclusions can be reached in comparing the stream sediment criteria to the geochemistry. The O/S fraction of Cu and Pb often exceeds the ISQG especially in the lower reaches of Still Creek. For lead, the EARP + O/S (f 1 + f 2) often exceed even the PEL for this metal. For zinc the EARP (f 2) exceed the ISQG especially in lower Still Creek and this has not changed for over 20 years.
It will require laboratory bioassay studies with well characterized sediments to determine which geochemical trace metal fractions are more bioavailable and potentially toxic to aquatic organisms.
5.3 Distribution of Trace Metals in Stormwater
Very few studies have investigated the distribution of trace metals in urban watersheds as water moves through the hydrologic gradient. Recent investigations have reported dissolved and particle-associated trace metals associated with stormwater and highway runoff (Tuccillo 2006; Hallberg et al. 2007). Tuccilo (2006) reported that Cu and Zn were mainly in the dissolved phase, whereas lead was associated with particles >5 μm and iron with larger particles. In investigating highway runoff in Stockholm, Hallberg et al. (2007) reported that at least 80% of the trace metals Cu, Fe, Mn and Zn were particle-associated. However, the proportion of dissolved metal increased from <5% to 6–17% for these elements in winter, likely attributable to deicing agents releasing some particle-bound metals.
In river studies, Farag et al. (2007) reported that in the mainstream Boulder River (Montana) more than 50% of the Cu and Zn were dissolved during high-flow periods, increasing to 80–95% during low-flow periods, an obvious effect of sediment resuspension during higher flows. In the Wallkill River (New Jersey), very little iron was dissolved (4–22%) while more zinc (36–76%) and manganese (67–94%) were dissolved compared to the total leachable metals from the water (Barringer et al. 2008).
Therefore it appears that many different factors, including flow, season and activities in the watershed, can affect the trace metal distribution in streams and rivers, and thus their potential impacts upon the aquatic biota. Our investigations concluded that reaches of lower flow (shallow lake), redox conditions changes as a result of stormwater runoff, as well as complexing agents in the water can affect the dynamics of trace metals distribution between dissolved, complexed and particle-associated phases.
5.4 Trace Metal Sediment Geochemistry
Copper is predominantly associated with the organic/sulphur geochemical phase in both stream (Fig. 6) and lake sediments (Fig. 7) of the Brunette River watershed. Recent sediment contaminant research has shown a similar trend in both freshwater (Fytiano and Lourantou 2004 – lakes in N. Greece; Wang et al. 2004 – wetlands in China) and coastal marine sediments (Burton et al. 2005 – Queensland, Australia; Morillo et al. 2007 – S. Spain). As benthic invertebrates graze the organic detritus in sediments, this selective feeding could potentially ingest more of the copper bound sediments.
As with this investigation, previous sediment studies have found 70–90% of the iron firmly bound to the residual geochemical phase confirming that erosion of natural minerals is an important source (Fytiano and Lourantou 2004; Wang et al. 2004; Van der Veen et al. 2006 – River Ellie, Germany). However vehicle corrosion can also be an important source of iron in street surface contaminants (Kartal et al. 2006; Tokalioglu and Kartal 2006 – Turkey). Vehicle iron corrosion could be a factor that resulted in a reduction in the iron in Brunette River watershed sediments between 1974 and 1998 during a period when better corrosion protection became more prevalent.
Previous research has reported that a relative high proportion of the manganese (30–80%) is found in the easily or moderately reducible geochemical phases where it is present as manganese oxides or adsorbed to iron oxides (Pardo et al. 2004; Wang et al. 2004; Tlustos et al. 2005; Van der Veen et al. 2006 and Morillo et al. 2007). This component of the manganese would be extracted as the EARP (f 2), which was also a component of the Brunette watershed sediments with 25–46% in stream sediments and 21–28% in lake surface sediments. However, manganese was also predominant in the residual phase of the Brunette River sediments (20–80%) since it is an important element in the natural eroding minerals of the watershed. At two Burnaby Lake sites (C and D), 40–50% of the manganese was in the exchangeable phase (f 1) which could possibly be attributed to the use of manganese in the fuel additive MMT. Street and car park dust in Turkey as also been found to contain up to 30% exchangeable manganese (Tokalioglu et al. 2003; Tokalioglu and Kartal 2006).
Sediment geochemical studies have found considerable lead associated with the amorphous iron and manganese oxides (Pardo et al. 2004; Wang et al. 2004; Burton et al. 2005; Van der Veen et al. 2006; Morillo et al. 2007) which was also the case for the Brunette River watershed sediments [20–50% of lead found in the EARP (f 2)]. However, lead bound to organic/sulphur (f 3) can also be important and will probably depend on the organic matter characteristics of the sediments. In the high organic lake sediments (sites C and D), 50–60% of the lead was in the organic/sulphur phase (Fig. 7). Organic/sulphur bound lead has also been a prevalent geochemical phase (up to 50%) in other sediments (Tlustos et al. 2005) and street dust (Tokalioglu and Kartel 2005). As with copper, grazing of organic detritus could make the lead in this phase more readily available to benthic invertebrates.
Zinc is highly associated with the EARP (f 2) – 30–60% – in the urbanized Brunette watershed sediments and it has increased over the study period (Fig. 6) along with vehicular traffic density (Table 4) where corrosion of galvanized components appear to be an important source of the zinc. The zinc along with lead are often bound to the iron and manganese oxides in the sediment and are extracted in these acidic reducible phases (Tlustos et al. 2005; Burton et al. 2005; Van der Veen et al. 2006; Boughriet et al. 2007; Morillo et al. 2007).
A recent critical review found that the Tessier and BCR sequential extraction methodologies were the most common techniques to evaluate the geochemical properties of solid-associated trace metals (Rao et al. 2008). Thus, where comparative data are available, usually using the Tessier et al. (1979) or BCR sequential extraction techniques (Kartal et al. 2006), in both freshwater and coastal marine sediments, general geochemical distribution trends are emerging. Further research, probably under controlled laboratory conditions, is required to relate sediment geochemistry to organism contaminant bioavailability and to begin to change regulations to better reflect sediment characteristics and their binding properties with trace metals.
Single sediment extracts have been proposed as alternatives to the sequential extraction technique to evaluate potential bioavailability of trace metal contaminants in sediments, since they are less time-consuming and expensive (Sutherland 2002; Fytiano and Lourantou 2004; Moalla et al. 2006). The order of extraction of trace metals, as a percent of the total sediment trace metals, indicates that Cu, Pb and Zn are usually more extractable, demonstrating that they potentially originate from an anthropogenic source, whereas iron and manganese appear to be more firmly bound to sediments.
Sutherland (2002) reported that 0.5 M HCl extracted a slightly higher proportion of total trace metals from roadside sediments (approximately 10% more) than the sum of the three sequential extraction phases. Our research found that the sequential extraction technique (f 1 + f 2 + f 3) extracted more copper (20–40% more) and lead (10–30% more) from contaminated lake sediments than 0.5 M HCl, primarily due to the association of these two metals with the organic/sulphur bound fractions. The proportions of Fe, Mn and Zn extracted from lake sediments by the two techniques were similar.
6 Conclusions
The quality of stormwater runoff and watershed sediments provide information on the mobility and bioavailability of trace metals (Cu, Fe, Mn, Pb and Zn) in the Brunette Watershed. There was a decrease in total trace metal concentrations as the stormwater moved through the hydrologic gradient of stormwater runoff, headwater stream (Still Creek) to outflow river (Brunette). The dissolved portion of trace metals increased downstream, largely attributable to disposition in the lower reaches of Still Creek and Burnaby Lake. Chelex resin bound very little dissolved copper, indicating that it was bound to strong organic complexes. Higher concentrations of particle-associated trace metals were flushed in stormwater runoff as the rainfall intensity and total suspended solids transport increased. The highest levels of sediment-associated trace metals were found in the lower gradient reaches of Still Creek before it enters the lake and in the down-lake stations of Burnaby Lake where higher levels of organic matter accumulate. The highest concentrates of copper were associated with the organic/sulphur sediment components, whereas iron and manganese were mainly mineral-bound (residual phase). The largest proportion of zinc was in the easily acid reducible phase, this phase which has become more significant as traffic and development in the watershed have increased. At least half the sediment-bound lead was associated with the easily acid reducible and organic/sulphur bound phases, with an overall decrease in total lead as it has been phased out as a gasoline additive.
References
APHA (1989). Standard methods for the examination of water and wastewater (17th ed.). Washington, DC: American Public Health Association.
Barringer, J. L., Wilson, T. P., Szabo, Z., Bonin, J. L., Fischer, J. M., & Smith, N. P. (2008). Diurnal variations in, and influences on, concentrations of particulate and dissolved arsenic and metals in the mildly alkaline Wallkill River, New Jersey, USA. Environmetal Geology, 53, 1183–1199. doi:10.1007/s00254-007-0708-8.
Bindra, K. S., & Hall, K. (1977). Geochemical partitioning of trace metals in sediments and factors affecting bioaccumulation in bethic organisms. Draft research paper, Dept. of Civil Engineering, The University of British Columbia, Canada.
Blumberg, K., & Walsh, M. P. (2004). Status report concerning the use of MMT in gasoline. International Council on Clean Transportation. p 25.
Boughriet, A., Proix, N., Billon, G., Recourt, P., & Ouddane, B. (2007). Environmental impacts of heavy metal discharges from a smelter in Deûle-canal sediments (Northern France): concentration levels and chemical fractionation. Water, Air, and Soil Pollution, 180, 83–95. doi:10.1007/s11270-006-9252-5.
Burton, E. D., Phillips, I. R., & Hawker, D. W. (2005). Geochemical partitioning of Copper, Lead, and Zinc in benthic, estuarine sediment profiles. Journal of Environmental Quality, 34, 263–273.
Canadian Council of Ministers of the Environment (2001). Canadian sediment quality guidelines for the protection of aquatic life. Pub. No. 1299, CCME, Ottawa, Ontario.
Chandra Sekhar, K., Chary, N. S., Kamala, C. T., Suman Raj, D. S., & Sreenivasa Rao, A. (2003). Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru Lake by edible fish. Environment International, 29, 1001–1008. doi:10.1016/S0160-4120(03)00094-1.
Couillard, Y., Courcelles, M., Cattaneo, A., & Wunsam, S. (2004). A test of the integrity of metal records in sediment cores based on the documented history of metal contamination in Lac Dufault (Quebec, Canada). Journal of Paleolimnology, 32, 149–162. doi:10.1023/B:JOPL.0000029429.13621.68.
Davis, A., Shokouhian, M., & Ni, S. (2001). Loading estimates of Lead, Copper, Cadmium, and Zinc in urban runoff from specific sources. Chemosphere, 44, 997–1009. doi:10.1016/S0045-6535(00)00561-0.
De Carlo, E. H., Beltran, V. L., & Tomlinson, M. S. (2004). Composition of water and suspended sediment in streams of urbanized subtropical watersheds in Hawaii. Applied Geochemistry, 19, 1011–1037. doi:10.1016/j.apgeochem.2004.01.004.
El Samrani, A. G., Lartiges, B. S., Ghanbaja, J., Yvon, J., & Kohler, A. (2004). Trace element carriers in combined sewer during dry and wet weather: an electron microscope investigation. Water Research, 38, 2063–2076. doi:10.1016/j.watres.2004.01.029.
Engler, R. M., Branon, J. M., Rose, J., & Bighem, G. (1994). A practical selective extraction procedure for sediment characterization. A draft manuscript, US Army Corps of Engineers, Vicksburg, Miss. 15p.
Farag, A. M., Nimick, D. A., Kimball, B. A., Church, S. E., Harper, D. D., & Brumbaugh, W. G. (2007). Concentrations of metals in water, sediment, biofilm, benthic macroinvertebrates, and fish in the Boulder River Watershed, Montana, and the role of colloids in metal uptake. Archives of Environmental Contamination and Toxicology, 52, 397–409. doi:10.1007/s00244-005-0021-z.
FHWA (1998). Is highway runoff a serious problem? Office of Infrastructure R&D. Turner-Fairbank Highway Research Center, McLean, VA. http://www.fhwa.dot.gov/terp/prog.htm#I129
Florence, T. M., Morrison, G. M., & Stauber, J. L. (1992). Determination of trace element speciation and the role of speciation in aquatic toxicity. Science of the Total Environment, 125, 1–13. doi:10.1016/0048-9697(92)90377-5.
Förster, J. (1996). Patterns of roof runoff contamination and their potential implications on practice and regulation of treatment and local infiltration. Water Science and Technology, 33, 39–48. doi:10.1016/0273-1223(96)00329-0.
Fytiano, K., & Lourantou, A. (2004). Speciation of elements in sediment samples collected at Lakes Volvi and Koronia, N. Greece. Environment International, 30, 11–17. doi:10.1016/S0160-4120(03)00143-0.
Glosińska, G., Sobczyński, T., Boszke, L., Bierla, K., & Siepak, J. (2005). Fractionation of some heavy metals in bottom sediments from the Middle Odra River (Germany/Poland). Polish Journal of Environmental Studies, 14, 305–317.
Good, J. C. (1993). Roof runoff as a diffuse source of metals and aquatic toxicity in stormwater. Water Science and Technology, 28, 317–321.
GVSDD (Greater Vancouver Sewerage and Drainage District). (1999). Best management practices guide for stormwater. Appendix H: Construction Site Erosion and Sediment Control Guide. GVRD, 4330 Kingsway, Burnaby, B.C.
Hall, K. J., Koch, F. A., & Yesaki, I. (1974). Further investigations into water quality conditions in the lower Fraser River System. Technical Report No. 4, Westwater Research Centre, The University of British Columbia, Vancouver, B. C. 104p.
Hall, K., Mattu, G., Yuan, Y., McCallum, D., & Keen, P. (2002). Seasonal variability in the residence time of a shallow lake helps to regulate water quality conditions in a highly urbanized watershed. Presented at International Conference on Residence Time in Lakes: Science, Management and Education, Bolsena, Italy. Sept. 29–Oct.3, 60–64.
Hall, K. J., Yesaki, I., & Chan, J. (1976). Trace metals and chlorinated hydrocarbons in the sediments of a Metropolitan Watershed, Technical Report No. 10, Westwater Research Center, The University of British Columbia, BC, Canada.
Hallberg, M., Renman, G., & Lundbom, T. (2007). Seasonal variations of ten metals in highway runoff and their partition between dissolved and particulate matter. Water, Air, and Soil Pollution, 181, 183–191. doi:10.1007/s11270-006-9289-5.
Jenne, E. A. (1968). Controls on Mn, Fe, Co, Ni, Cu and Zn concentrations in soils and water: the significant role of hydrous Mn and Fe oxides. In R.F. Gould (ed.) Trace inorganics in water. Advances in chemistry Series 73, American Chemical Soc. Publ., Washington, DC, p. 337–387.
Kartal, Ş., Aydin, Z., & Tokalioğlu, Ş. (2006). Fractionation of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. Journal of Hazardous Materials, 132, 80–89. doi:10.1016/j.jhazmat.2005.11.091.
Kayhanian, M., Singh, A., Suverkropp, C., & Borroum, S. (2003). Impact of annual average daily traffic on highway runoff pollutant concentrations. Journal of Environmental Engineering, 129, 975–990. doi:10.1061/(ASCE)0733-9372(2003)129:11(975).
Kushner, D. J. (1993). Effects of speciation of toxic metals on their biological activity. Water Pollution Research Journal Canada, 28, 111–128.
Lake, D. L., Kirk, P. W. W., & Lester, J. N. (1984). Fractionation, characterization, and speciation of heavy metals in sewage sludge and sludge-amended soils: a review. Journal of Environmental Quality, 13, 175–83.
Li, L. Y., Mattu, G., McCallum, D., Hall, K., & Chen, M. (2006). The temporal and spatial dynamics of trace metals in sediments of a highly urbanized watershed. Journal of ASTM International, 3, 189–200.
Li, T. (2007). Trace metals in urban stormwater runoff and their management. MASc. Thesis, Department of Civil Engineering, University of British Columbia, Canada.
Macdonald, R., Hall, K. J., & Schreier, H. (1997). Water quality and stormwater contaminants in the Brunette River Watershed, British Columbia, 1994/1995. Research report, Westewater Research Center, University of British Columbia, Canada.
Makepeace, D. K., Smith, D. W., & Stanley, S. J. (1995). Urban stormwater quality: summary of contaminant data. Critical Reviews in Environmental Science and Technology, 25, 93–139.
Mason, Y., Ammann, A., Ulrich, A., & Sigg, L. (1999). Behaviour of heavy metals, nutrients, and major components during roof runoff infiltration. Environmental Science and Technology, 33, 1588–97. doi:10.1021/es980922q.
McCallum, D. W. (1995). An examination of trace metal contamination and land use in an urban watershed. M.A.Sc. Thesis, Dept. of Civil Engineering, The University of British Columbia, Canada.
Moalla, S. M. N., Soltan, M. E., Rashed, M. N., & Fawzy, E. M. (2006). Evaluation of dilute hydrochloric acid and acid ammonium oxalate as extractants for some heavy metals from Nile River sediments. Chemistry and Ecology, 22, 313–327. doi:10.1080/02757540600812289.
Morillo, J., Usero, J., & Fracia, I. (2007). Potential mobility of metals in polluted coastal sediments in two bays of southern Spain. Journal of Coastal Research, 23, 352–361. doi:10.2112/04-0246.1.
Morrison, G. M. P., & Revitt, D. M. (1987). Assessment of metal species bioavailability and geochemical mobility in polluted waters. Environmental Technology Letters, 8, 361–372.
Morrison, G. M., Revitt, D. M., & Ellis, J. B. (1989). Sources of storm loading variations of metal species in a gollypot catchment. Science and the Total Environment, 80, 267–78. doi:10.1016/0048-9697(89)90081-8.
Muraro, M. (2005). Dynamics of temporal and spatial mercury contamination in an urban watershed. M.Sc., RMES, Faculty of Graduate Studies, The University of British Columbia, Canada.
Novotny, V. (1995). Diffuse source pollution by toxic metals and impacts on receiving waters. In W. Solomons, U. Forsmer, & P. Maders (Eds.), Heavy metals: Problems and solutions (pp. 33–52). Berlin: Springer.
Novotny, V., & Olem, H. (1994). Water quality – prevention, identification, and management of diffuse pollution. Van Nostrand Reinhold, Florence, KY, USA.
Pardo, R., Helena, B. A., Cazurro, C., Guerra, C., Debán, L., Guerra, C. M., & Vega, M. (2004). Application of two- and three-way principal component analysis to the interpretation of chemical fractionation results obtained by the use of the B.C.R. procedure. Analytica Chimica Acta, 523, 125–132. doi:10.1016/j.aca.2004.07.015.
Perin, G., Fabris, R., Manente, S., Rebello Wagener, A., Hamacher, C., & Scotto, S. A. (1997). A five-year study on the heavy metal pollution of Guanabara Bay sediments (Rio De Janeiro, Brazil) and evaluation of the metal bioavailability by means of geochemical speciation. Water Research, 31, 3017–28. doi:10.1016/S0043-1354(97)00171-1.
Preciado, H. F., & Li, L. Y. (2006). Evaluation of metal loadings and bioavailability in air, water, and soil along two highways of British Columbia, Canada. Water, Air, and Soil Pollution, 172, 81–108. doi:10.1007/s11270-005-9063-0.
Quevauviller, P., Rauret, G., López-Sánchez, J.-F., Rubio, R., Ure, A., & Muntau, H. (1997). Certification of trace metal extractable contents in a sediment reference material (CRM 601) following a three-step sequential extraction procedure. Science of the Total Environment, 205, 223–234. doi:10.1016/S0048-9697(97)00205-2.
Rao, C. R. M., Sahuquillo, A., & Lopez-Sanchez, J. F. (2008). A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and related materials. Water, Air, and Soil Pollution, 189, 291–333. doi:10.1007/s11270-007-9564-0.
Rose, S., Crean, M. S., Shenheen, D. K., & Ghazi, A. M. (2001). Comparative zinc dynamics in Atlanta metropolitan region stream and street runoff. Environmental Geology, 40, 983–992. doi:10.1007/s002540100285.
Serne, R. J. (1975). Geochemical distribution of selected trace metals in San Fransisco Bay sediments. Water and Waste Management Section, Bahelle, Pacific North West Laboratories, Richland, Washington.
Stokes, P. M., Campbell, P. G. C., Schroeder, W. H., Trick, C., France, R. L., Punkett, K.J., et al. (1988). Manganese in the Canadian Environment. National Research Council of Canada, Associate Committee on Scientific Criteria for Environmental Quality. ISSN 0316-0114, NRCC No. 26193.
Sutherland, R. A. (2002). Comparison between non-residual Al, Co, Cu, Fe, Mn, Ni, Pb and Zn released by a three-step sequential extraction procedure and a dilute hydrochloric acid leach for soil and road deposited sediment. Applied Geochemistry, 17, 353–365. doi:10.1016/S0883-2927(01)00095-6.
Sutherland, R. A., & Tolosa, C. A. (2000). Multi-element analysis of road-deposited sediment in an urban drainage basin, Honolulu, Hawaii. Environmental Pollution, 110, 483–495. doi:10.1016/S0269-7491(99)00311-5.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51, 844–851. doi:10.1021/ac50043a017.
Tlustos, P., Szakova, J., Starkova, A., & Pavlikova, D. (2005). A comparison of sequential extraction procedures for fractionation of arsenic, cadmium, lead, and zinc in soil. Central European Journal of Chemistry, 3(4), 830–851. doi:10.2478/BF02475207.
Tokalioglu, S., & Kartal, S. (2005). Comparison of metal fractionation results obtained from single and BCR sequential extractions. Bulletin of Environmental Contamination and Toxicology, 75, 180–188. doi:10.1007/s00128-005-0736-6.
Tokalioglu, S., & Kartal, S. (2006). Multivariate analysis of the data and speciation of heavy metals in street dust samples from the Organized Industrial District in Kayseri (Turkey). Atmospheric Environment, 40, 2797–2805. doi:10.1016/j.atmosenv.2006.01.019.
Tokalioglu, S., Kartal, S., & Birol, G. (2003). Comparison of the extraction capabilities of three sequential extraction procedures for partitioning of heavy metals in car-park dusts. Journal of Environmental Monitoring, 5, 468–476. doi:10.1039/b300047h.
Tuccillo, M. E. (2006). Size fractionation of metals in runoff from residential and highway storm sewers. Science of the Total Environment, 355, 288–300. doi:10.1016/j.scitotenv.2005.03.003.
US Environmental Protection Agency (USEPA). (1983). Results of the Nationwide Urban Runoff Program – Vol. 1 – Final Report. PB84-185552. Washington, DC: US Environmental Protection Agency.
USEPA. (1986). Acid Digestion of sediments, sludges, and soils. Section A, Part I, Chapter Three – Metallic analytes. Method 3050. SW-846, Test methods for evaluating solid waste. Washington, DC: USEPA.
Van der Veen, A., Ahlers, C., Zachmann, D. W., & Friese, K. (2006). Spatial distribution and bonding forms of heavy metals in sediments along the middle course of the River Elbe (km287…390). Acta Hydrochimica et Hydrobiologica, 34, 214–222. doi:10.1002/aheh.200500623.
Wang, G., Liu, J., & Tang, J. (2004). Historical variation of heavy metals with respect to different chemical forms in recent sediments from Xianghai wetlands, Northeast China. Wetlands, 24, 608–619. doi:10.1672/0277-5212(2004)024[0608:HVOHMW]2.0.CO;2.
Yuan, Y. (2000). The bioavailability of trace metal contaminants in the Brunette River Watershed. M.Sc. Thesis, RMES Program, Faculty of Graduate Studies, The University of British Columbia, Canada.
Acknowledgment
The authors express their gratitude to National Science and Engineering Research Council of Canada and the former Greater Vancouver Regional District of B.C. (now Metro Vancouver) for funding this research. We would like to express our thanks to Susan Harper and Paula Parkinson at the Environmental Engineering Laboratory for their technical assistance and Jenny Ng for her secretarial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, L.Y., Hall, K., Yuan, Y. et al. Mobility and Bioavailability of Trace Metals in the Water-Sediment System of the Highly Urbanized Brunette Watershed. Water Air Soil Pollut 197, 249–266 (2009). https://doi.org/10.1007/s11270-008-9808-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9808-7