Abstract
Sixteen stations were established to assess water and sediment quality throughout the Caura/Tacarigua watershed. Samples were analyzed for Cd, Cr, Cu, Fe, Ni, Pb, Zn and Hg using atomic absorption spectrometry during the dry and wet seasons of 2005. In the dry season, Pb and Zn exceeded sediment quality guidelines for the protection of aquatic life. Highest concentrations of all metals were recorded at one station subject to a range of anthropogenic inputs in the lower watershed area; Cu exceeded the water quality guideline at this station. In the wet season, Zn exceeded the SQG at one station located south of residential areas in the lower watershed area and highest concentrations of Cr and Pb were recorded at that station. Metals in water were detected primarily at stations in the lower watershed area. Wet season concentrations of Zn in water were significantly higher than corresponding dry season concentrations. Likely sources of trace metals in the watershed include effluent from a light industrial estate, sewage/domestic waste from non-functioning sewage treatment plants and pit latrines, agricultural and urban runoff. Results of this study show that management strategies using an integrated approach are needed to minimize trace metal pollution within the watershed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace metals occur naturally in the environment but anthropogenic activities increase the bioavailable trace metal pool. Major sources of trace metals include industrial effluent, sewage and domestic waste, agricultural run-off and urban run-off. While some of these elements are essential elements, i.e. they are required as cofactors or for important biochemical functions in an organism, all can have toxic effects in organisms when present in excess (Phillips 1995). Trace metals present in the environment can bioaccumulate in aquatic organisms, posing a threat to consumers including man (USEPA 2000). This can result in neurological, systemic, carcinogenic effects, or birth defects. The Caura/Tacarigua watershed is approximately 50 km2 in size and is the fourth largest watershed in the northern range of Trinidad (Celestain 2010). Land use within the watershed is diverse. Land use in the upper to middle watershed includes areas of forest and mixed forest, agricultural areas, recreational areas, rural and squatting communities, as well as a few institutions (e.g. schools, a hospital, a horticultural site). Water is abstracted from the Caura/Tacarigua River for domestic purposes both by the national body responsible for supplying drinking water to the population, and villagers from the rural and squatting communities. The area is also used for recreational activity; it is estimated that there are over 60,000 visitors to the recreational park within the watershed annually (Celestain 2010). The lower watershed is characterized by dense residential areas including planned housing developments, various institutions (e.g. schools, an orphanage, a crematorium) and agricultural, commercial and light industrial areas. The pollution sources and activities within the watershed are considered representative of those which take place in the Caroni River Basin, the largest, most densely populated and one of the most polluted river basins in the country. This river basin is a major contributor to the country’s potable water supply and encompasses the Caroni Swamp, a Ramsar site. The swamp contains the country’s largest mangrove forest (Juman and Ramsewak 2010) and is a habitat for resident and migratory birds as well as commercially important invertebrates (Bacon 1970). The swamp also supports an ecotourism industry.
Materials and methods
Experimental protocol
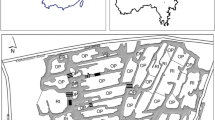
Sampling was conducted on two occasions—once in the dry season (between 11th March and 8th April 2005) and once in the wet season (between 28th June and 19th August 2005). Sixteen stations were selected for sampling, and water and surficial sediment samples were collected at each station. These were selected to (1) reflect the various types of land use within the watershed, (2) cover as wide a geographical area and as even a distribution as possible over the watershed area and (3) assess quality along the Caura/Tacarigua River as well as the contribution from tributaries to the river. Accessibility of the stations was also taken into consideration. Surficial water samples were collected by direct immersion of acid washed high-density polyethylene (HDPE) sample bottles, or where not possible, a bucket was immersed and the water collected was transferred to the sample bottles. Surficial sediment samples were collected using polypropylene scoops, homogenized and transferred to polyethylene bags. Physico-chemical parameters were also taken and GPS co-ordinates recorded at each station. The location of stations sampled and land use within the Caura/Tacarigua watershed are shown in Fig. 1. All samples were transported back to the laboratory on ice. In order to preserve the samples, water samples were acidified to pH 2 using 2 ml L−1 of concentrated HNO3 and sediment samples were frozen.
Physicochemical parameters—dissolved oxygen, conductivity, salinity, pH and temperature—were determined in water. Dissolved oxygen, conductivity and salinity were measured in situ using the YSI Model 85 Meter, which had an accuracy of ±0.3 mg L−1, ±2.5 µS cm−1 and ±0.1 ppt for the three parameters, respectively. pH and temperature were measured in situ using the YSI Model 60 Meter, which had an accuracy of ±0.1 pH unit and ±0.1 °C for the two parameters, respectively. Extractable Cd, Cr, Cu, Fe, Pb, Ni, Zn and total Hg were determined in water and sediment. Acidified water samples were shaken and allowed to stand overnight to obtain the extractable metal fraction. Total Hg was determined according to the method outlined in Environment Canada (1979). Twenty millilitres of sample was digested with 1 ml concentrated H2SO4 and 0.5 ml concentrated HNO3; 5 ml KMnO4 followed by 1 ml portions was added until the purple colour persisted for at least 15 min. Two millilitres of K2S2O8 was added and the mixture heated at 95 °C for two hours. After cooling, NH2OH HCl was used to reduce excess KMnO4. Sediment samples were dried at 60 °C and then sieved to obtain the <180 μm size fraction, in which the dependence of metal content on surface area is removed (Hawkes and Webb 1962; Oliver 1973). Total organic carbon was determined according to the method outlined in Buchanan and Kain (1971). One gramme of sample was digested in K2Cr2O7 and H2SO4 and titrated against standardised ferrous ammonia solution. The detection limit for this method was 0.01 %. Extractable metals were determined according to the method outlined in Environment Canada (1979). Ten grammes of sample was shaken with 100 ml of 0.5 N HCl acid for 16 h and filtered by suction through Whatman No. 40 filter paper. Total Hg was determined according to the method outlined in Environment Canada (1979). Five grammes of sample was digested with 10 ml concentrated H2SO4 and 5 ml concentrated HNO3. Samples were cooled, 2 ml HCl were added and the mixture digested at 60 °C for 2 h. Samples were cooled and 15 ml KMnO4 added. Five millilitres of K2S2O8 was added and the mixture allowed to stand overnight. KMnO4 was added until the purple colour persisted for at least 15 min. NH2OH HCl was used to reduce excess KMnO4.
Analyses for metals were performed using the Perkin-Elmer AAnalyst 100 Atomic Absorption Spectrometer with deuterium background correction. Cd, Cr, Cu, Fe, Pb, Ni and Zn were determined using Flame and Graphite Furnace Atomic Absorption Spectrometry. Hg was determined using Cold Vapour Atomic Absorption Spectrometry. Detection limits for water were—Cd: 0.20 μg L−1, Cr: 0.60 μg L−1, Cu: 0.66 μg L−1, Fe: 0.04 μg ml−1, Pb: 1.30 μg L−1, Ni: 2.30 μg L−1, Zn: 0.01 μg ml−1, Hg: 0.20 μg L−1. Detection limits for sediment were—Cd: 0.01 μg g−1, Cr: 0.01 μg g−1, Cu: 0.01 μg g−1, Fe: 0.45 μg g−1, Pb: 0.03 μg g−1, Ni: 0.05 μg g−1, Zn: 0.04 μg g−1, Hg: 0.01 μg g−1.
Quality control
Samples were analysed in batches with each sample analysed at least in duplicate. Procedural blanks were analysed with each batch of samples. Certified reference materials (CRMs) were used to assess the performance of the method for total Hg in sediment: Marine Sediment MESS-2 and BCSS-1 (National Research Council, Canada). Recoveries obtained were 105 and 84.5 % for MESS-2 and BCSS-1, respectively. Precision of the extractable metals in sediment method was assessed using percentage relative standard deviation (RSD) of 15 replicates of a typical marine sediment, as no CRMS are available. % RSDs obtained were as follows—Cd: 7.1, Cr: 0.7, Cu: 4.0, Fe: 9.1, Ni: 8.6, Pb: 4.5 and Zn: 5.1. High purity, 18 MΩ-cm de-ionized water (Millipore, UK) was used to prepare aqueous reagents and solutions used. Analytical reagents and compounds used in analysis were of Aristar or AnalaR grades. Glass and plastic ware for trace metals analysis were soaked overnight in 20 % HNO3, rinsed twice with distilled water and once with de-ionized water. Glassware for TOC analysis was soaked with Nochromix acid, rinsed three times with distilled water and twice with de-ionized water.
Data treatment
Data was analysed using Minitab Statistical Software (Release 16). Statistical tests were performed on the untransformed data and were described as significant when p < 0.05. Paired t tests were used to assess seasonal differences. Pearson correlation was used to assess relationships between parameters in water and between parameters in sediment. Principal Component Analysis was performed on the data for each season separately. This multivariate technique aims to reproduce the majority of the variance of the original data by a few principal components and is very useful in detecting patterns or relationships between samples (Zitko 1994).
Results and discussion
Water
Water quality guidelines (WQGs) from the Canadian Council of Ministers of the Environment (CCME 2007) were used to assess water quality parameters, as no ambient water quality guidelines are available for Trinidad and Tobago. Physical parameters were compared with WQGs for the protection of aquatic life; trace metals were compared with WQGs for the protection of aquatic life and WQGs for the protection of agricultural water uses (irrigation). These WQGs are being used solely as benchmarks and to highlight the relative toxicity of the metals as they have been developed for a temperate climate and are not necessarily applicable to a tropical environment.
Dissolved oxygen (DO) concentrations in the Caura/Tacarigua River ranged from 0.4 to 8.8 mg L−1 in the dry season and from 2.7 to 7.6 mg L−1 in the wet season (Fig. 2a). DO concentrations in the lower part of the watershed were generally below the CCME (2007) range considered protective of aquatic life (5.5–9.5 mg L−1), particularly during the dry season. pH ranged from 6.1 to 8.2 in the Caura/Tacarigua River during the dry season. Only one station (Stn 14) was more acidic than the recommended CCME (2007) range of 6.5–9.0 for the protection of aquatic life. In the wet season, pH ranged from 7.1 to 8.2 (Fig. 2b). All values were within the acceptable range. Temperature in the Caura/Tacarigua River ranged from 24.5 to 34.8 °C in the dry season and from 24.4 to 26.9 °C in the wet season (Fig. 2c). The highest temperature (34.8 °C) was recorded at Stn 14 during the dry season. The CCME (2007) water quality guideline for the protection of aquatic life is a narrative guideline on thermal additions to receiving waters, with respect to thermal stratification, the maximum weekly average temperature, and the short-term exposure to extreme temperature. Essentially, large, lengthy fluctuations from ambient conditions (such as appears to be the case with Stn 14), should be avoided. Conductivity in the Caura/Tacarigua River ranged from 158 to 710 µS cm−1 in the dry season and from 145 to 439 µS cm−1 in the wet season (Fig. 2d). These values are fairly typical of freshwater, but the highest conductivity was recorded at Stn 14 during the dry season. There is no CCME guideline for conductivity. Similar results were obtained for salinity (which is a function of conductivity). Salinity ranged from 0.1 to 0.2 ppt, except at Stn 14 in the dry season, which had a salinity of 0.3 ppt.
In the dry season, concentrations in water in the Caura/Tacarigua River ranged from <0.2 to 0.6 μg L−1 Cd, <0.6 to 11.2 μg L−1 Cr, <0.66 to 7.63 μg L−1 Cu, <1.30 to 17.34 μg L−1 Pb and <0.01 to 0.03 μg ml−1 Zn (Table 1). Fe, Ni and Hg were undetected. In the wet season, concentrations ranged from <0.6 to 5.99 μg L−1 Cr, <0.66 to 9.29 μg L−1 Cu and 0.05 to 0.40 μg ml−1 Zn (Table 1). Fe, Ni, Hg, Cd and Pb were undetected. All metal concentrations were below the available WQGs for the protection of agricultural water uses (irrigation) in both seasons. Cd, Cu, Pb and Zn exceeded the WQGs for the protection of aquatic life in the dry season, and Cu and Zn exceeded these guidelines in the wet season at stations in the lower watershed area.
Sediment
Sediment quality guidelines (SQGs) for the protection of aquatic life from the Canadian Council of Ministers of the Environment (CCME 2007) were used to assess sediment quality parameters, as no ambient sediment quality guidelines are available for Trinidad and Tobago. There is no sediment quality guideline for Total Organic Carbon. SQGs available for total metals in surficial sediment are being used solely as benchmarks and to highlight the relative toxicity of the metals for two reasons: (1) the SQGs were developed for a temperate climate and are not necessarily applicable to a tropical environment and (2) apart from Hg, sediments were analyzed for the extractable (non-residual) metal fraction; however, the SQGs are for the total metal concentration, which is the sum of the non-residual and residual metals fractions. The non-residual metal fraction is that fraction of the metal that is not part of the silicate matrix of the sediment. Analysis of this fraction is more useful in pollution studies than total metal as it tends to be more indicative of anthropogenic input (Forstner and Wittmann 1979; Doherty et al. 2000).
TOC ranged from 0.21 to 2.37 % in the dry season and from 0.15 to 2.67 % in the wet season (Fig. 3). The highest TOC concentration was recorded at Stn 3 (one of the tributaries in the upper watershed) during the wet season; however, TOC concentrations were generally higher in the lower part of the watershed. In the dry season, trace metal concentrations in sediment in the Caura/Tacarigua River ranged from 0.01 to 0.10 μg g−1 Cd, 0.20 to 6.22 μg g−1 Cr, 1.27 to 26.84 μg g−1 Cu, 0.86 to 8.57 mg g−1 Fe, 0.73 to 9.86 μg g−1 Ni, 0.37 to 42.07 μg g−1 Pb, 16.47 to 255 μg g−1 Zn and 0.01 to 0.06 μg g−1 Hg. Cd and Hg were not determined in sediments from Stns 11 to 16 in the dry season. These stations are subject to greater anthropogenic input so it is likely that maximum Cd and Hg concentrations in the watershed are higher than those recorded. Pb and Zn exceeded the SQGs for the protection of aquatic life during the dry season at Stn 14. The maximum (extractable) Cu concentration recorded was close to the SQG at this station so it is possible that total Cu concentrations would also exceed the SQG. Cd, Cr, Hg (and Cu) were below the SQGs. In the wet season, trace metal concentrations ranged from <0.01 to 0.06 μg g−1 Cd, 0.19 to 4.81 μg g−1 Cr, 1.34 to 8.29 μg g−1 Cu, 0.55 to 3.64 mg g−1 Fe, 0.74 to 4.73 μg g−1 Ni, 3.10 to 9.07 μg g−1 Pb, 4.24 to 269 μg g−1 Zn and <0.01 to 0.15 μg g−1 Hg. Zn exceeded the SQG for the protection of aquatic life during the wet season at Stn 11. Cd, Cr, Cu, Pb and Hg were below the SQGs. No SQGs are available for Fe and Ni.
Stations in the lower part of the watershed (Stns 12–16) tended to have lower DO concentrations than stations in the upper part of the watershed, particularly in the dry season. These areas are subject to greater anthropogenic input. During the dry season, there is less dilution of pollutants being discharged into the river and more of the oxygen present would be utilized during decomposition of biodegradable organic pollutants present. During the wet season, apart from increased dilution of pollutants, there would be increased river flow rates and hence increased aeration of the river. The contrast between stations in the upper part of the watershed and the lower part of the watershed was not observed with other physico-chemical parameters; however, lowest pH, and highest temperature, conductivity and salinity were recorded at Stn 14 during the dry season. This was a tributary that receives effluent from a light industrial estate in the lower part of the watershed. DO and pH were not significantly different between seasons (p > 0.05). Temperature, conductivity and salinity in the dry season were significantly higher than corresponding values in the wet season (p < 0.05), which may be due to the lack of dilution of pollutant discharges during the dry season.
Metals in water were primarily present at stations in the lower watershed area, in which there is increased land use (residential, agricultural, commercial and industrial). Zn was present throughout the entire watershed in the wet season, which suggests that there is contribution from land-based run-off. Zn concentrations in the wet season were significantly higher than concentrations in the dry season (p < 0.05), which is consistent with contribution from land-based run-off. Concentrations of other metals were not significantly different between seasons (p > 0.05). A significant correlation suggests that some relationship exists between parameters, e.g. a common source (Bastidas et al. 1999; Emmerson et al. 1997). In the dry season, significant correlations were Cr–Cu and Cd–Pb while in the wet season, Cr, Cu and Zn were all significantly correlated (p < 0.05).
Total organic carbon (TOC) is the carbon found in organic matter and can be used as an indicator of organic matter, which has an affinity for trace metals, i.e. metals tend to become concentrated on organic matter in sediments (Horowitz 1985). TOC can be due to natural sources, i.e. decomposition of plant and animal matter or anthropogenic sources such as sewage. It is likely that natural sources are contributing to the elevated TOC concentrations at Stn 3 in the upper watershed area. Apart from Stn 3, TOC tended to be higher at stations further downstream, at which there is greater anthropogenic input.
Cd and Hg were not included in statistical analyses of the sediment data, as data for stations in the lower watershed were not available during the dry season. In the dry season, correlations among all metals and correlations between all metals and TOC were significant (p < 0.05), indicating that there is some relationship between parameters, e.g. a common source. Dry season concentrations of trace metals and TOC were not significantly different from wet season concentrations (p > 0.05). In the wet season correlations between Cr, Cu, Zn and Ni and between Cu, Fe, Ni and TOC were significant, suggesting different metal sources (p < 0.05).
Principal component analysis of the dry season data showed that the first two components (PC 1 and PC 2) account for 95 % of the total variance. PC 1 accounts for 85 % and PC 2 accounts for 10 % of the total variance. Loading and score plots generated with PCA analysis are shown in Fig. 4a, b. All metals were positively correlated with PC 1 with |loading| > 0.30. Cu, Pb and Ni form a cluster and contribute most significantly to PC 1. TOC and Fe were positively correlated with and contributed significantly to PC 2 (|loading| > 0.40). Zn and Cr were negatively correlated with and contributed significantly to PC 2 (|loading| > 0.40). Cu, Pb and Ni did not contribute significantly to PC 2 (|loading| < 0.20). Different clusters of metals suggest that there may be different metal sources or that they may be transported differently. Examination of the score plot shows that there are two main groups (Group A and Group B). Group A consists of the stations in the upper watershed (Stns 1–10). These stations are subject mainly to agricultural run-off, sewage and domestic waste, and can be considered less polluted compared with the other stations. Stn 6 (a recreational bathing area) can be considered an outlier—this was largely due to the Zn concentration which was higher than at the other stations in this group. Group B consists of the stations in the lower watershed (Stns 11–16). Metal concentrations were more elevated in sediment at these stations. Stn 14 (the station at which Pb and Zn exceeded the SQGs and at which highest concentrations of all metals and TOC were recorded) was an outlier. These stations can be considered more polluted compared with the other stations sampled. Likely trace metal sources for these stations include effluent from a light industrial estate and sewage and domestic waste from at least two non-functioning sewage treatment plants serving residential areas just north of Stn 11. The lower watershed is also subject to agricultural and urban run-off (two major east–west road networks pass through this area). Apart from pollution sources within the lower watershed, these stations receive pollutants from further upstream.
Principal component analysis of the wet season data showed that the first two components (PC 1 and PC 2) account for 82 % of the total variance. PC 1 accounts for 64 % and PC 2 accounts for 18 % of the total variance. Loading and score plots generated with PCA analysis are shown in Fig. 5a, b. All metals were positively correlated with PC 1 with |loading| > 0.25. Cu and Ni form a cluster and contribute most significantly to PC 1. TOC and Fe were positively correlated with and contributed significantly to PC 2 (|loading| > 0.40). Cr, Zn and Pb were negatively correlated with and contributed significantly to PC 2 (|loading| > 0.25).Cu and Ni did not contribute significantly to PC 2 (|loading| < 0.20). As in the dry season, different clusters of metals suggest that there may be different metal sources or that they may be transported differently. Pb was much less significant along PC 1 during the wet season compared with the dry season. This was because during the wet season, there was not much variation in Pb concentrations throughout the watershed and all concentrations recorded were approximately an order of magnitude lower than the maximum concentration recorded in the dry season. In addition, during the dry season, Pb was considerably higher at the stations downstream of Stn 11 compared with stations further upstream.
Unlike the dry season, there was no marked increase in concentrations in the lower watershed area during the wet season. This may be due to increased dilution or flushing of pollutants during this season. Examination of the score plot shows that there is one main group; however, within this group there are two “sub-groups”: A and A′. Most of the stations sampled are part of Sub-group A suggesting that there is not much variation in metal concentrations at these stations. Sub-group A′ consists of Stns 2, 4 and 5 in the upstream agricultural areas, suggesting that there is some influence from land-based run-off. These stations had similar (more elevated) concentrations of Cr, Cu and Fe. Stn 3 and Stn 11 were outliers. Highest TOC, Fe, Cu and Ni concentrations were recorded at Stn 3, one of the tributaries within an agricultural area in the upper watershed. Highest concentrations of Cr, Pb and Zn were recorded at Stn 11, the station off a main road just downstream of the two non-functioning sewage treatment plants. Stn 14 (the tributary which receives effluent from the light industrial estate) can also be considered an outlier; this was due in part to the elevated concentrations of organic matter at that location.
Conclusions
The results of the study show that land-based sources and activities within the Caura/Tacarigua watershed are adversely affecting water and sediment quality. Areas in the lower part of the watershed are most affected. Likely sources of trace metals in the watershed include effluent from a light industrial estate (particularly in the dry season), sewage/domestic waste from non-functioning sewage treatment plants and pit latrines in housing areas, agricultural runoff and urban runoff. The distribution of trace metals in sediment appears to be influenced by the organic matter content of the sediment. Trace metals in water and sediment at some locations within the watershed have the potential to adversely affect aquatic life, but are unlikely to adversely affect agriculture when used for irrigation. Management strategies using an integrated approach are needed to minimize trace metal pollution within the watershed.
References
Bacon P (1970) The ecology of caroni swamp. Special Publication, Central Statistical Office, Trinidad, Trinidad
Bastidas C, Bone D, Garcia E (1999) Sedimentation rates and metal content of sediments in a venezuelan coral reef. Mar Pollut Bull 38:16–24
Buchanan J, Kain K (1971) The Determination of Organic Matter in Sediments. In: Holme W, McIntyre A (eds) Methods for the study of marine benthos. Blackwell, Oxford
Canada Environment (1979) Inland waters directorate. Analytical Methods Manual, Water Quality Branch, Ottawa, Canada
CCME Canadian Council of Ministers of the Environment (2007) Canadian Environmental Quality Guidelines for the Protection of Aquatic Life. http://ceqg-rcqe.ccme.ca. Accessed 12 Dec 2012
Celestain B (2010) Baseline assessment of Caura/Tacarigua and Maracas/St. Joseph Watersheds, Final Report submitted to the Cropper Foundation
Doherty G, Brunskill G, Ridd M (2000) Natural and enhanced concentrations of trace metals in sediments of Cleveland Bay, Great Barrier Reef Lagoon, Australia. Mar Pollut Bull 41:337–344
Emmerson R, O’ Reilly-Wiese S, MacLeod C, Lester J (1997) A multivariate assessment of metal distribution in Inter-tidal sediments of the blackwater Estuary, UK. Mar Pollut Bull 34:960–968
Forstner U, Wittmann G (1979) Metal pollution in the aquatic environment. Springer, Berlin
Hawkes H, Webb J (1962) Geochemistry in mineral exploration. Harper and Row, New York
Horowitz A (1985) A primer on trace metal- sediment chemistry. United States Geological Survey Water- Supply Paper 2277. Alexandria, VA
Juman R, Ramsewak D (2010) The state of mangrove forests in Trinidad and Tobago. Institute of Marine Affairs Research Report
Oliver B (1973) Heavy metal levels of Ottawa and Rideau River sediments. Environ Sci Techol 7:135–137
Phillips D (1995) The chemistries and environmental fates of trace metals and organochlorines in aquatic ecosystems. Mar Pollut Bull 31:193–200
USEPA (2000) United States Environmental Protection Agency. Guidance for assessing chemical contaminant data for use in fish advisories, vol. I. Fish sampling and analysis
Zitko V (1994) Principal component analysis in the evaluation of environmental data. Mar Pollut Bull 28:218–222
Acknowledgments
This project was funded by the Government of the Republic of Trinidad and Tobago. I wish to acknowledge staff of the Institute of Marine Affairs: N. Slinger-Cohen, C. Bullock, W. Beckles-Roberts, I. Moonesar (deceased), P. Nelson, R. Bickramdass and S. James from the Marine Chemistry Department, and H. Asmath, L. Gerald (deceased) and N. O’Connor from the Information Technology Department. Thanks also to residents and farmers of the Caura/Tacarigua watershed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nelson, W. Trace metals in water and sediment from a tropical watershed subject to anthropogenic inputs. Environ Earth Sci 74, 621–628 (2015). https://doi.org/10.1007/s12665-015-4066-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4066-7