Abstract
The effects of ozone in four maple species, Acer campestre, A. opalus subsp. granatense, A. monspessulanum and A. pseudoplatanus were studied in OTC under two different experimental conditions: in charcoal filtered air (CF), and in non filtered air plus 30 ppb ozone (NF+30). The four species of maple showed contrasting sensitivity to ozone as demonstrated by visible injury development, gas exchange and chlorophyll a fluorescence, and growth measurements. Plant injury index (i.e. a combination of percentage of injured leaves and leaf surface affected) was more consistently related with physiological measurements than the onset of first symptom of visible injury. Differences in ozone sensitivity among species may be partly related to higher stomatal conductances in A. opalus and A. pseudoplatanus. In these two species, ozone produced significant reductions in CO2 assimilation under saturating light conditions (A sat), stomatal conductance (g s), transpiration rate (T r) and Water Use Efficiency (WUE) (the latter also significantly declined in A. campestre) towards the end of summer, while intercellular CO2 concentrations (C i) increased significantly. In asymptomatic leaves of A. opalus, neither stomatal limitation nor photoinhibitory damage (F v/F m) could explain the observed decline of A sat, and photosynthesis was down regulated by reducing the proportion of absorbed energy used in photochemistry (Φ PSII) at expenses of the energy dispersed non-photochemically (NPQ). Leaf N content also declined significantly in A. pseudoplatanus. Plants exposed to ozone showed a tendency to decrease growth, but it was not significant within the exposure period for any of the four species. The most sensitive species were A. opalus and A. pseudoplatanus, while the species with the smallest and more coriaceous leaves, A. monspessulanum, was the most resistant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tropospheric ozone is a widespread regional air pollutant in many parts of the world. It is know that it interacts with forest ecosystems causing visible injury and other adverse effects to the plants (Krupa and Manning 1988; Krupa et al. 2001; de Vries et al. 2003; Ferretti et al. 2007). In the context of ‘global change’ (IPCC 2001), increasing levels of this gas contributing to global warming are predicted (Fowler et al. 1999; Ashmore 2005). A higher frequency of hot and sunny periods due to global warming may also result in more frequent high ozone episodes (Sanz et al. 2007a). Ozone may compromise stimulation of net primary production caused by elevated CO2 (King et al. 2005) and reduce carbon sink capacity of ecosystems (Karnosky et al. 2003). In forested areas of Europe, levels of this pollutant tend to increase towards the Mediterranean region (Sanz et al. 2007a,b), due to the fact that in Southern Europe ozone formation is particularly favored by the intense solar radiation, high temperatures, and re-circulation processes of the polluted air masses (Millán et al. 1997, 2000; Sanz and Millán 1998; Sanz et al. 2007a). These levels are high enough to produce visible injury on leaves of native vegetation (e.g. Bussotti and Ferretti 1998; Skelly et al. 1999; Innes et al. 2001; Sanz et al. 2001; de Vries et al. 2003; Paoletti 2006).
Ozone effects on plants have been reviewed from several viewpoints in the last years (e.g. Runeckles and Chevone 1992; Matyssek et al. 1995; Heath and Taylor 1997; Pell et al. 1997; Black et al. 2000; De Kok and Tausz 2001). Ozone enters the plants mainly via stomata, and inside the leaves it reacts with apoplast constituents producing reactive oxidative species (superoxide radicals, hydroxyl radicals and hydrogen peroxide) (Mehlhorn et al. 1990; Wohlgemuth et al. 2002). The initial signals produced by ozone in the leaf apoplast are translated later in responses at the tissue level (unregulated cell dead, hypersensitive response leading to programmed cell dead, accelerated senescence), in processes modulated by ethylene, jasmonic and salicylic acid levels, and the interactions among their signaling pathways (Baier et al. 2005; Fiscus et al. 2005). Ozone exposure causes increase in the activity of the enzymes associated with general plant defense mechanisms (Kangasjärvi et al. 1994), and alters the permeability of the plasma cell membranes and plant lipid patterns (Heath 1987). An impairment of photosynthetic assimilation rates (Reich and Amudson 1985), reductions in the amount and activity of Rubisco (Dann and Pell 1989), and chlorophyll destruction (e.g. Pleijel et al. 1994; Saitanis et al. 2001) are other well-known ozone effects. This pollutant also produces alterations in cells and tissues, to finally induce cell death and necrosis of the tissues leading to the development of visible injury (e.g. Mikkelsen and Heide-Jørgensen 1996; Günthardt-Goerg et al. 1997; Vollenweider et al. 2003; Gravano et al. 2004; Reig-Armiñana et al. 2004; Bussotti et al. 2005). If dose is sufficient and plant protective and repair mechanisms are overcome, growth reductions may occur (Chappelka and Chevone 1992; Chappelka and Samuelson 1998; Matyssek and Innes 1999).
Maples are important components of temperate forests, which are also present in humid parts of the Mediterranean region. Previous studies have shown that this genus host several species with a relatively high sensitivity to ozone: it is the case of the sugar maple (A. saccharum Marsh.; e.g. Gaucher et al. 2003), and red maple (A. rubrum L.; e.g. Samuelson and Kelly 1997; Schaub et al. 2003) in North America, or of sycamore (A. pseudoplatanus L.) or field maple (A. campestre L.) in Southern Europe (e.g. Innes et al. 2001; de Vries et al. 2003; Ferretti et al. 2004). In Europe, maple species are among the trees showing more typical ozone symptoms in the field (e.g. Innes et al. 2001), and plants of this genus have a high potential to be used as bioindicators. So far, information regarding the sensitivity of maple species and populations present in the Mediterranean area is rather limited or absent. In the present paper, we study the ozone sensitivities of Spanish populations of four maples: sycamore, field maple, a subspecies of Italian maple [A. opalus Mill. subsp. granatense (Boiss.) Font Quer & Rothm.] and Montpellier maple (A. monspessulanum L.). In the Iberian Peninsula, sycamore is the species with the highest water requirements, being restricted to northern humid areas. Field maple is also a predominantly northern species. The two other maples show a more southern distribution and may occur within Mediterranean vegetation. Due to their floristic interest, they are included in some regional red lists in Spain (e.g. Cabezudo and Talavera 2005). The species with the largest leaves is sycamore, while Montpellier maple, the best adapted to water stress, has the smallest and more coriaceous leaves.
The main objective of the present paper is to compare the ozone sensitivity of these four species on the basis of their foliar, physiological and growth responses, and to characterize such responses. Two hypotheses are also tested: (1) species with higher stomatal conductantes are more sensitive to ozone (Reich 1987), and (2) species with more coriaceous leaves (as an adaptation to dry Mediterranean conditions) are more tolerant to this pollutant (Paoletti 2006).
2 Materials and Methods
2.1 Organization of the Experiments
In order to achieve the above mentioned objectives, this study has been structured in four different experimental parts: (1) the species sensitivity to ozone is assessed on the basis of visible injury, and the hypothesis that in maple species higher stomatal conductances may favor injury development is tested (experiment 1). (2) Tracking of marked leaves over time (experiment 2) characterizes the sequence of physiological changes (gas exchange and chlorophyll fluorescence) produced by ozone. (3) The study of physiological responses (gas exchange, chlorophyll fluorescence and chlorophyll content) in leaves without stippling (experiment 3) was complementarily carried out, in order to characterize pre-visible injury effects. (4) Finally, the possible effects of ozone on plant growth were studied.
2.2 Plant Material
Plant seedlings (3–4 years old, about 80 cm height) were obtained from two nurseries: Acer opalus subsp. granatensis was provided by Vivero de Quart, Banc de Llavors Forestals (Valencia), and the three other species from Vivero Escuela Río de Guadarrama (Madrid). The origin was: eastern Spain (Acer campestre from the province of Valencia, and Acer opalus subsp. granatensis from the region of Els Ports-Maestrat, province of Castellón), and central Spain, province of Madrid (Acer monspessulanum and Acer pseudoplatanus). The containers (9.5 l) were filled with 50% coconut-peat, 30% peat, and 10% sand, and 10% vermiculite, soil pH being close to 7.0. A slow release fertilizer (Osmocote plus) was incorporated, with NPK 20:20:20 and additional micronutrients. Plants were irrigated using a droplet irrigation system, twice a day. For each species, 9 plants were kept in filtered air, and 12 were fumigated. Visible injury was assessed in all 12 fumigated plants (no symptoms were observed in CF plants) and growth was measured in nine plants per species and treatment. For the study of the physiological responses, however, only six plants per species and treatment were taken into account.
2.3 Open-Top Chambers and Treatments
The experiment was conducted in the ‘La Peira’ open-top chamber experimental Field (Benifaió, 39°16′14.8″N, 00°26′59.6W, 30 m of altitude), in a rural area 20 km south of the city of Valencia (eastern Spain). Plants were distributed in six OTCs with two ozone treatments: three chambers with charcoal filtered air (CF), and three chambers with non-filtered air plus 30 ppb ozone (NF+30). Plants were fumigated 8 h a day, from 10:00 to 18:00 hours CET, during the whole week. Ozone was generated from oxygen using a high-voltage electrical discharge generator (SIR s.a., Madrid, Spain). Air quality inside and outside the chambers was continuously monitored at regular intervals with an ozone monitor (Dasibi 1008-AH, Environmental Corp., Glendale, CA, USA), and nitrogen oxides monitor (Dasibi 2108, Environmental Corp., Glendale, CA, USA); these monitors were calibrated periodically. Additional meteorological data (e.g. temperature, precipitation, wind direction and speed) were also recorded for the experimental plot. The experiments started on 11 May 2004, and ended on 21 September 2004. The critical level for ozone, accumulated exposure over a threshold of 40 ppb, was calculated according to the methods described by the EU 2002/3/EC Directive (EU 2002), using mean hourly values from 08:00 hours CET to 20:00 hours CET. Ozone concentration data of the experimental site (ambient) and treatments are provided in Table 1, while accumulated AOT40 values through the experiment are represented in Fig. 2.
2.4 Visible Injury Assessment
Plants were examined every two days to record the first date of symptom onset in each individual plant. Complementarily, once a week, both the percentage of affected leaves per plant (LA), and the percentage of area affected for the symptomatic leaves (AA) were scored in each plant, using a 5% steps scale. To evaluate the whole plant injury, a Plant Injury Index (PII) was calculated combining these two parameters: \( {\text{PII}} = {{\left( {{\text{LA}} * {\text{AA}}} \right)}} \mathord{\left/ {\vphantom {{{\left( {{\text{LA}} * {\text{AA}}} \right)}} {100}}} \right. \kern-\nulldelimiterspace} {100} \).
2.5 Gas Exchange Measurements
In order to determine the stomatal conductance of the different species under ambient experimental conditions (experiment 1), stomatal conductance was measured in randomly selected, mature, healthy leaves of the upper part of the plant with a portable Delta-T AP4 porometer (Delta-T Devices, Cambridge, UK). Measurements were carried out under ambient light and temperature conditions: average PPFD of all measurements was 1099 μmol m−2 s−1 and temperature 30°C. Measurements were performed in 2 days, on 14 July and 23 September 2004, three times a day (09:00–11:00, 13:00–15:00, 17:00–19:00 hours, CET), with a total of 322 leaves measured (n = 75–86 for each species and treatment).
To compare CF and NF+30 treatments along different times, or to compare types of leaves, under fixed photon flux density (PPFD) and temperature conditions (experiments 2 and 3), gas exchange was measured with an infrared gas analyzer (IRGA) (Licor-6400, Li-cor Inc., Lincon, NE, USA). This instrument is equipped with two Peltier thermoelectric coolers to allow control temperature, and the leaf chamber is provided with a gallium arsenide phosphide (GaAsP) red-blue light source that supply photosynthetically active radiation (PAR) at the required light intensities. Block temperature of the cuvette was fixed at 25°C, and PPFD at 1000 μmol m−2 s−1. Previous determinations showed that this photon flux was saturating for the four maple species. All measurements were taken during the morning, at a constant airflow of 500 μmol air s−1. Six plants were selected per species and treatment; measurements were conducted in one mature leaf per plant, from the middle part of the crown. Plants of the different treatments were measured in alternating order to minimize shifts of environmental conditions affecting gas exchange during the measurements. In experiment 2, tracking of gas exchange in the same marked leaves was carried out at 0 (11 May), 16, 69 and 107 DSF. Relative humidity (RH) during the measurements was 50.3 ± 16.5%, and leaf-to-air water vapor pressure deficit (VPD) was 1.58 ± 0.3 kPa. In experiment 3, a complementary comparison of different type of leaves (filtered, fumigated not symptomatic, and fumigated symptomatic), was conducted after 108 days on newly selected leaves of the same age. It has to be noted that although selected asymptomatic leaves of the NF+30 treatment did not show clear visible stippling, microscopy analyses of similar leaves indicate that some alterations, including partial chloroplast degeneration, have taken place in some of these leaves (unpublished data). RH during these measurements was 62.4 ± 8.0%, and VPD was 1.4 ± 0.3 kPa.
2.6 Chlorophyll Content
Chlorophyll content was measured not destructively with a portable chlorophyll meter (SPAD-520, Minolta). This instrument uses measurements of transmitted radiation in the red and near infrared wavelengths to provide numerical values related to leaf chlorophyll content. The average of three measurements was calculated for each leaf, and two leaves were measured per plant, six plants per treatment. Complementarily, after 108 DSF, the SPAD values were determined in 40 leaf discs 1 cm diameter (area = 0.78 cm2) per species. They were later collected, rapidly transported in a cool box with ice and stored in a freezer at −80°C until analyses. Chlorophyll was extracted in 5 ml DMSO following Barnes et al. (1992), and concentrations determined with a CARY 45 UV-visible from 350–750 nm. With these data, regression analysis between the SPAD measurements and the chlorophyll content were carried out. Correlations were significant for the four species, with the following coefficients of determination and equations: A. campestre (y = 0.0601x − 0.0653, r = 0.91), A. pseudoplatanus (y = 0.046x + 0.0331, r = 0.84), A. monspessulanum (y = 0.0867x − 1.0871; r = 0.89), A. opalus (y = 0.0803x − 0.5089, r = 0.85), where y = chlorophyll concentration (μg chlorophyll/mg fresh weight), and x = SPAD absorbance (relative units). Total chlorophyll content of the leaves was derived from SPAD values using these equations.
2.7 Chlorophyll a Fluorescence Measurements
In the tracked leaves (experiment 2), modulated chlorophyll fluorescence measurements were taken at ambient temperature at the same time than gas exchange determinations, but a complementary leaf per plant was measured (n = 12 leaves per species and treatment). Measurements were carried out with a portable fluorometer (PAM-200, Walz, Effeltrich, Germany). Leaves were dark-adapted for at least 30 min prior to the measurements. After dark adaptation, the minimal fluorescence (F o) was determined using the measuring light. A subsequent application of a saturating flash of white light (0.8 s at 8000 μmol/m2 s1), raises fluorescence to its maximum value (F m). This allows the determination of the F v/F m parameter, maximum quantum efficiency of photosystem II (PSII) primary photochemistry, given by F v/F m=(F m − F o)/F m.
Comparison of symptomatic and asymptomatic leaves (experiment 3) was carried out using the saturation pulse method for the analysis of quenching components (Schreiber et al. 1986). After F v/F m determination, intermittent pulses of saturating strong white light (0.8 s at 8,000 μmol m−2 s−1) were applied in the presence of actinic light. This allows the determination of the maximum fluorescence in the light adapted state (\( F^{\prime }_{{\text{m}}} \)) after each saturating pulse, and the actinic light allowed steady-state photosynthesis and modulated fluorescence yield at this steady state (F s); the minimum fluorescence in the light-adapted state (\( F^{\prime }_{o} \)) is also measured by applying a pulse of far red-light during a brief interruption of actinic illumination. At each saturating pulse, quenching due to non-photochemical dissipation of absorbed light energy (NPQ) was determined according to the equation \( {\text{NPQ}} = {{\left( {F_{{\text{m}}} - F^{\prime }_{{\text{m}}} } \right)}} \mathord{\left/ {\vphantom {{{\left( {F_{{\text{m}}} - F^{\prime }_{{\text{m}}} } \right)}} {F^{\prime }_{{\text{m}}} }}} \right. \kern-\nulldelimiterspace} {F^{\prime }_{{\text{m}}} } \). The coefficient for photochemical quenching (q p), which represents the redox state of the primary electron acceptor of PSII, Qa, was calculated as \( {{\left( {F^{\prime }_{{\text{m}}} - F_{{\text{s}}} } \right)}} \mathord{\left/ {\vphantom {{{\left( {F^{\prime }_{{\text{m}}} - F_{{\text{s}}} } \right)}} {{\left( {F^{\prime }_{{\text{m}}} - F_{{\text{o}}} } \right)}}}} \right. \kern-\nulldelimiterspace} {{\left( {F^{\prime }_{{\text{m}}} - F_{{\text{o}}} } \right)}} \). The quantum yield of electron transfer at PSII (Φ PSII) was estimated as \( \Phi _{{{\text{PSII}}}} = {{\left( {F^{\prime }_{m} - F_{{\text{s}}} } \right)}} \mathord{\left/ {\vphantom {{{\left( {F^{\prime }_{m} - F_{{\text{s}}} } \right)}} {F^{\prime }_{m} }}} \right. \kern-\nulldelimiterspace} {F^{\prime }_{m} } \) (Genty et al. 1989), and the quantum efficiency of excitation capture by oxidized reaction centers of PSII was calculated from the equation \( \Phi _{{{\text{exc}}}} = {{\left( {F^{\prime }_{{\text{m}}} - F^{\prime }_{{\text{o}}} } \right)}} \mathord{\left/ {\vphantom {{{\left( {F^{\prime }_{{\text{m}}} - F^{\prime }_{{\text{o}}} } \right)}} {F^{\prime }_{{\text{m}}} }}} \right. \kern-\nulldelimiterspace} {F^{\prime }_{{\text{m}}} } \).
2.8 Total C and N, and C/N Ratio
For total C and N determinations, five to six mature asymptomatic leaves per species and treatment (CF, NF+30) were collected after 108 DSF, and analyzed separately. Leaves were dried at 60°C to constant weight, and after grinding up, analyzed separately with a Perkin Elmer 2400 Series II CHNS/0 elemental analyzer (Perkin Elmer, Norwalk, CT, USA). The analyzer is based on the organic analysis Pregl-Dumas combustion technique which converts sample elements to simple gases (CO2, H20, and N2) and detected as a function of their thermal conductivities.
2.9 Growth of the Stems
In 2004, plant height of nine plants was measured with a tape measure at 0 (11 May), 21, 48, 80, 104 and 133 DSF. Increases in height at the different measuring times with respect to the initial values are given as relative growth rates (RGR), because RGR expresses the rate of tree growth independent of size (Evans 1972). Relative Height Growth Rate as: RHGR = (In H2 − In H1)/(t 2 − t 1), where H = height in cm. In this case, t 1 = 0, as here we consider RGR at different measuring times always in relation to the initial values.
2.10 Statistical Analyses
For two level analyses, independent t-test were applied, and for more than two cases, one-way Analysis of Variance (ANOVA) followed by least significant difference test (LSD). Normality and homogeneity of variance requirements were previously tested, and data transformed if necessary. A probability level <0.05 was considered statistically significant. Data were analyzed using SPSS 10.0 for Windows (SPSS Inc.).
3 Results
3.1 Development of Visible Injury
At the end of the experiment (29 September), all four species displayed symptoms in the old leaves. Occasionally, symptoms were also observed in re-sprouts; they are fast growing parts of the plants, with usually a more active gas exchange activity. Leaves exhibited stippling, sometimes associated with chlorosis in the interveinal zone, with the veins and the lower side remaining unaffected. Stippling was particularly visible in A. pseudoplatanus, due to its darker colour, brown to dark brown, forming large patches, while in the three other species, stipples were paler, yellow or yellow-brown. No symptoms were observed in any of the control plants.
In Fig. 1, the AOT40 at which the onset of visible injury was recorded in each individual plant is represented, as well as the mean AOT40 of this onset for symptomatic plants of each species. At the end of the experiment, all 12 individual plants of A. opalus showed relatively abundant symptoms. In the three other species, some of the plants remained externally asymptomatic. Stippling was also widespread in eight plants of A. pseudoplatanus and, less markedly, in seven individuals of A. campestre. In A. monspessulanum, 10 plants developed a faint stippling but restricted to a few leaves, mainly from re-sprouts. One plant of A. campestre was the first to display symptoms, after 20 DSF (AOT40 = 6977 ppb·h). This AOT40 value is below the 10,000 ppb·h critical level for protection of forest trees (EU 2002), but above the recent 5,000 ppb h threshold proposed in the UNECE Mapping Manual (Mills 2004). In this species, however, there was a quite high variability in the AOT40 value for visible symptom appearance, since the rest of the plants showed injury at AOT40 of about 20,000 ppb·h, and over this threshold. A. monspessulanum was the least sensitive species: plants showed first injury at a mean AOT40 = 28,878 ppb·h, after 79 DSF as a mean. In A. opalus, the mean AOT40 value was 24,878 ppb·h, and in A. pseudoplatanus 23,834 ppb·h, with two plants distinctly more sensitive than the others in the latter species.
AOT40 values at which individual plants displayed the first symptoms (x-axis), and mean of these individual AOT40 values for each of the four species (y-axis). The number of symptomatic plants that exhibited injury along the experiment, from a total of 12 plants per species, is given in brackets. Numbers placed above or below symbols indicate some cases in which the first symptoms were observed in several individual plants (two or three plants) at the same time and AOT40. The figure refers only to plants of the NF+30 treatment, as no symptoms were observed in the CF treatment
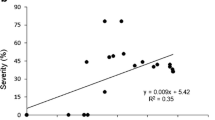
Species sensitivity has been ranked also on the basis of their Plant Injury Index (PII), an index combining the percentage of leaves of the plant affected, and the extent of visible injury (Fig. 2). This index increases progressively over time, from the onset of the first observed symptoms until the end of the experiment, in parallel with increasing AOT40 values. Consistently with results represented in Fig. 1, A. monspessulanum is confirmed as the least sensitive species to ozone as the symptoms not only appeared in general later than in the other species, but PII values were always low, i.e. only a few injured leaves, and scarcely affected, were observed in the plants. The highest PII values were calculated for A. opalus, a species in which all plants were finally symptomatic. Some of these individuals had more than 80% of the leaves affected towards the end of the experiment. Considering the PII, A.pseudoplatanus appears as more sensitive than A. campestre. The latter species developed visible injury earlier in some plants, but percentage of leaves affected remained relatively low, below 30% of the plant in most cases, while in A.pseudoplatanus some plants showed up to 80% of leaves affected.
3.2 Experiment 1: Stomatal Conductance of the Species Under the Experimental Conditions
The two species showing higher ozone injury (see previous section) at the end of the experiment (A. opalus and A. pseudoplatanus) showed significantly higher stomatal conductances than A. campestre, with A. monspessulanum placed in an intermediate position (Fig. 3).
3.3 Experiment 2: Tracking of Marked Leaves
In order to track the changes in the photosynthetic performance of the leaves, gas exchange determinations under constant PPFD and temperature were taken four times in the same leaves (Fig. 4). The first significant changes in ozone-exposed plants with regard to control (CF) were observed in A. opalus, with a 33% reduction of A sat at 69 DSF. The other significant changes in the parameters considered were detected towards the end of the experiment. After 107 days, A sat declined further in A. opalus (about a 60% reduction), and also a 50% significant reduction was observed in A. pseudoplatanus. In these two species, the impairment in CO2 assimilation was apparently associated with a significant decreases in stomatal conductance (g s; see also next section), that declined 49% in A. opalus and 37% in A. pseudoplatanus; transpiration rates (T r) were significantly reduced as well. On the contrary, intercellular CO2 concentration (C i) significantly increased in both species. Water Use Efficiency (WUE) after 107 days was also altered: fumigated plants were significantly less efficient with regard to water use than fumigated plants in all species except A. monspessulanum. In A. monspessulanum, ozone did not alter significantly any of the gas exchange parameters considered in this study, at any measuring time. Maximum efficiency of chlorophyll fluorescence (F v/F m) was significantly reduced in A. opalus (p < 0.05), while in A. pseudoplatanus differences between treatments were in the limit of significance (p = 0.06).
Experiment 2. Gas exchange and fluorescence parameters measured in the same leaves at the beginning of the experiment, and after 16, 69, and 107 days, under constant PPFD (1,000 μmol m−2 s−1) and cuvette block temperature (25°C). Data represented are NF+30/CF ratios, expressed as percentages. Significant differences between the CF and the NF+30 treatment at the different measuring times are indicated as *p < 0.05, **p < 0.01, and ***p < 0.001 (t-test, mean ± SE, n = 6)
3.4 Experiment 3: Changes in Asymptomatic and Symptomatic Leaves
As in experiment 2, final measurements included both symptomatic and asymptomatic leaves, i.e. there was a high heterogeneity in leaf response, in experiment 3 the physiological responses of the leaves with and without visible injury were characterized independently.
In ozone-exposed but still asymptomatic leaves, some early changes could be detected with regard to control leaves (Table 2). CO2 assimilation (A sat) significantly declined about 26% in A. opalus and in A. pseudoplatanus. In the first species, this reduction occurred without any negligible change in g s, while in the latter it was coupled with a 22% g s reduction (although not significant). In all species except A. monspessulanum there was a significant reduction in WUE, and in A. opalus and A. campestris, C i increased significantly. Maximum efficiency of chlorophyll fluorescence (F v/F m) remained unchanged in all four maple species. However, some significant changes in fluorescence parameters determined under actinic illumination were observed in A. opalus and A. pseudoplatanus: Φ PSII, Φ exc, and q p decreased, while NPQ increased (Table 3). Consistently but not significantly, average chlorophyll content slightly decreased in all species but A. monspessulanum. In A. pseudoplatanus, both N content decreased and C/N ratio increased significantly (Table 2).
In symptomatic leaves the tendencies observed in ozone-exposed asymptomatic leaves were in general enhanced and became significant for some of the parameters. A sat and g s and WUE declined further, C i increased, and chlorophyll content and F v/F m were significantly lowered (Tables 2 and 3).
3.5 Growth of the Stems
Under the experimental conditions, plants experienced an increase in height during the first 80 days (until 30th July), while growth became practically suppressed during August and September (data not shown). Plant height of the stems was recorded six times, but in order to summarize the results only data at 48 DSF (28 June), in the middle of the active growth period, and at the end of the treatments (133 DSF, 21 September) are presented here (Table 4). Due to the high variability in plant response, there are no significant differences in Relative Height Growth Rate (RHGR) between the two treatments (CF, NF+30). RHGR was neither significantly correlated with visible injury of the individual plants. However, with regard to control plants, there is a tendency towards a decrease of this parameter in fumigated plants of all species, except in A. monspessulanum.
4 Discussion
In this study, the sensitivity of four maple species to ozone has been assessed in several ways: visible injury, photosynthetic performance, chlorophyll and N content, and growth. A complementary study dealing with the anatomical alterations induced by this pollutant is also ongoing (unpublished data).
Ozone-induced visible foliar symptoms in maple species have been previously reported elsewhere. A. campestre shows visible injury in the field at ambient ozone levels in southern Switzerland (de Vries et al. 2003). A. pseudoplatanus is also symptomatic in Southern Switzerland (photos in Innes et al. 2001) and in Italy (Ferretti et al. 2004; Bussotti et al. 2005; the latter study also includes a microscopic description of the stipples in this species). Symptoms similar to those induced in this experiment in A. opalus subsp. granatense have been observed by us (unpublished data) in a nursery in eastern Spain. So far, A. monspessulanum has never been observed in the field showing ozone injury. Previously to this experiment, symptoms had been induced experimentally in A. campestre fumigated with ozone in OTC (Sanz et al. 2001) and in A. pseudoplatanus using continuously stirred tank reactors (CSTR; Orendovici et al. 2003). Photodocumentation on ozone visible symptoms in maple species from Europe can be accessed at the following URLs: http://www.gva.es/ceam/ICP-forests/ and http://www.ozone.wsl.ch/.
Species have been classified according to their ozone sensitivity in two different ways. (1) threshold for the onset of the first symptom in any plant, and (2) Plant Injury Index (PII) combining the percentage of affected leaves per plant, and the extent of visible injury in the affected leaves. Ranking of the two approaches are not fully coincident, as for first approach, the classification is: A. campestre>A. pseudoplatanus >A. opalus>A. monspessulanum, while, according to their PII, they are ranked as: A. opalus>A. pseudoplatanus >A. campestre>A. monspessulanum. In both cases, A. monspessulanum was the least sensitive species. A. campestre shows the most discrepant differences: it is the first species to develop symptoms (Fig. 1), but these symptoms were restricted to a relatively low number of leaves in each plant, so that PII at the end of the experiment was relatively low in comparison with other species (Fig. 2). Therefore, lower thresholds (i.e. an earlier development of symptoms) not necessarily imply a larger percentage of leaves affected in the plants at the end of the growing season, as it has been shown also in other studies (e.g. Orendovici et al. 2003). Furthermore, ranking of ozone-sensitivity of different species may also differ if instead of the date of the first symptom, the average date of injury onset for several plants is considered (Novak et al. 2003). Only one specimen of A. campestre developed symptoms below the commonly used critical level for protection of forest trees (AOT40 = 10,000 ppb·h), but above the recently proposed 5,000 ppb·h threshold (Mills 2004). The first symptom in A. pseudoplatanus was observed at AOT40 = 13,517 ppb·h, an intermediate value between the AOT40 value of 2,259 ppb·h, reported by Orendovici et al. (2003) in a CSTR experiment, and the AOT40 > 20,000 ppb·h given by Vanderheyden et al. (2001) in both open plots and in OTCs under ambient ozone levels in Southern Switzerland. These contrasting results suggest a rather high variability among populations in this species, although the different experimental conditions between the experiments might have contributed to some extent. Given the results of the present study, calculation of PII seems to be more appropriate for comparing sensitivity among species, as it describes better the state of the plants, and provides results which are more consistent with physiological measurements (see below).
Several studies have shown that O3 impacts are more closely related to ozone uptake than to external O3 exposure (Musselmann and Massmann 1999; Wieser 1997; Wieser et al. 2000), supporting the use of a flux-based concept instead of approaches based on the external O3 exposure (Matyssek et al. 2007). As flux of the pollutant to the leaf interior is predominantly controlled by stomatal aperture (Kersteins and Lendzian 1989), stomatal conductance is considered a key factor in understanding plant responses against ozone. Reich and Amudson (1985) and Reich (1987) suggest that differences among species in ozone uptake and response to ozone are related to differences in leaf conductance. In experiment 1, stomatal conductance differed significantly among species: A. opalus and A. pseudoplatanus had significantly higher g s than A. campestre, with A. monspessulanum placed in an intermediate position (Fig. 3). As ozone uptake depends in large part to stomatal conductance, and given that the ozone concentration regime was the same for all four species, the higher stomatal conductances measured in A. opalus and A. pseudoplatanus imply that both maples received the highest O3 effective doses along the experiment. Consistently with Reich’s hypothesis, the highest scores of visible injury and more clear physiological changes (see below) were recorded in these two species. In contrast, the lower injury scores observed in A. campestre may be also partly explained by its lower gs rates. However, it has to be noted than the less affected species (A. monspessulanum) was not the one with the lowest g s rates. Several other studies show that gs or internal ozone flux and deleterious effects (e.g. visible injury) are not always correlated (e.g. Taylor and Tingey 1982; Zhang et al. 2001). The mechanisms underlying the plant responses to this pollutant are rather complex as, in addition to ozone uptake, other factors are know to play important roles. In this sense, the metabolic capacity to withstand oxidative stress through repair and detoxification mechanisms may contribute importantly to determine the different plant sensitivity against ozone (Kangasjärvi et al. 1994; Matyssek et al. 2004, 2007).
Tracking of the same leaves over time (experiment 2) shows that all four species exhibited the same type of responses or trends against ozone for the studied parameters. The general tendencies of change observed in ozone-exposed plants (significant or not) were reductions in CO2 assimilation (A sat), stomatal conductance (g s), Water Use Efficiency (WUE), maximum quantum efficiency of photosystem II (F v:F m), and increases in intercellular CO2 concentrations (C i). Not surprisingly, the physiological changes observed for the different species confirmed the ranking of ozone sensitivity previously established on the basis of visible injury, as some of the measured leaves were finally symptomatic. The two species showing the highest injury, P. pseudoplatanus and A. opalus were also the most affected from a physiological viewpoint: they experienced significant reductions in A sat, g s, T r, WUE and F v/F m (strongly reduced but not significantly in A. pseudoplatanus), and an increase in C i. A. campestre was an intermediate species, as only WUE was significantly reduced. A. monspessulanum was confirmed as the most resistant species: fumigated leaves of this species did not show significant differences with regard to control ones in any of the studied parameters. In the two most sensitive species, clear reductions in A sat start to occur after 69 days (already significant for A. opalus, still not significant for A. pseudoplatanus), which is roughly coincident with the onset of visible injury in the plants (cf. Fig. 1). Novak et al. (2005) also observed that there was a correspondence between ozone-induced reductions in gas exchange and the onset of visible injury in Populus nigra, Viburnum lantana and Fraxinus excelsior exposed to ambient ozone levels in Southern Switzerland.
In experiment 3, we studied the physiological changes in leaves before stippling was clearly established. Results are overall consistent with those of experiment 2. In A. opalus and A. pseudoplatanus a significant decline in A sat and WUE, and an increase in C i (A. opalus) were already observed in externally asymptomatic leaves. In A. campestre, WUE declined and C i increased significantly, while A. monspessulanum remained unaffected. These results show that impairment of photosynthetic processes may occur before stippling appears on the leaves (Novak et al. 2003; Gravano et al. 2004), and more interestingly, that significant reductions in CO2 assimilation may occur without apparent stomatal limitations: in A. opalus there is a 26% significant reduction in A sat without appreciable changes in g s (Table 2). This is consistent with the conclusions of Reichenauer and Bolhàr-Nordenkampf (1999), who indicate that in the case of limitation of CO2 assimilation by stomatal closure, an associated decrease in C i would be expected together with an increase in WUE (Reichenauer and Bolhàr-Nordenkampf 1999). As in asymptomatic leaves of A. opalus exposed to ozone, C i significantly increased and WUE decreased, these results would support the idea that these changes in A sat are not primary due to stomatal closure. In the present case, an important reduction in g s seems to represent a further step in the sequence of deleterious ozone effects on the leaves, as it occurred in injured leaves, associated to a strong A sat decline. Stomatal closure in more affected leaves, as observed in this study, could be a secondary reaction to increased C i level (e.g. Mikkelsen 1995). It is known that ozone negatively affects of Rubisco, reducing carboxilation efficiency of the leaves (Dann and Pell 1989); an impairment of the ‘dark phase’ of photosynthesis may result in increasing C i levels, which is consistent with the enhanced C i levels observed in the maple species. Although direct effects of ozone on the stomata have been reported (Fiscus et al. 2005), the results of the present study suggest that impairment of stomatal function by ozone is not the main mechanism to explain A sat decline (although a contribution to some extent cannot be ruled out): a decrease in C i and an increase in WUE would be expected if CO2 assimilation would be mainly stomata-limited. As expected, the significant changes and tendencies observed in ozone exposed asymptomatic leaves are in general enhanced in injured leaves of all species. The observed sequence of physiological responses is similar to that described in the revision of stomatal responses of trees under elevated ozone concentrations by Paoletti and Grulke (2005).
Fluorescence results of experiment 3 indicate that in asymptomatic leaves, the observed A sat impairment occurs without a significant reduction in F v/F m, ruling out photoinhibitory damage to the PSII reaction centers as the main cause of CO2 assimilation decline. However, some fluorescence parameters under steady-state in actinic illumination experienced significant changes in the two most sensitive species, P. pseudoplatanus and A. opalus. The quantum yield of electron transfer at PSII (Φ PSII) was significantly reduced, in parallel with both reductions in quantum efficiency of excitation capture by oxidized reaction centers of PSII (Φ exc) and of the coefficient for photochemical quenching (q p). On the other hand, quenching due to non-photochemical dissipation of absorbed light energy (NPQ) increased significantly. Therefore, in these leaves there is a reduction in the proportion of absorbed energy being used in photochemistry (Φ PSII), at expenses of the energy dispersed non-photochemically (NPQ). This reduction in Φ PSII is the consequence of both reductions in the fraction of PSII reaction centers open under actinic light illumination (q p) and of a lower efficiency of excitation capture of these centers (Φ exc). As Φ PSII frequently exhibits a strong quantitative relationship with CO2 assimilation, in particular with the quantum yield of CO2 (Φ co2), these results from fluorescence are consistent with the observed A sat depression in asymptomatic leaves of the two most sensitive species. The decline in Φ PSII not necessarily implies irreversible damage of ozone on the light harvesting system or in the non-cyclic electron transport (in fact, the F v/F m is not significantly affected) as it might represent a down-regulatory process associated with an inhibition of Calvin cycle. It is well known that ozone affects Rubisco, reducing the carboxilation efficiency of the leaves (Dann and Pell 1989); this inhibition of Calvin cycle may increased excitation pressure (1 − q p) on PSII and contribute to the closure of PSII reaction centers. Similar results to those observed here in maple have been reported e.g. by Calatayud et al. (2003), who detected significant changes in q p and NPQ, without significant effects on F v/F m in spinach leaves exposed to ozone. Other authors report similar results in fluorescence parameters measured under illumination, but with an additional F v/F m decline, e.g. in poplar clones before the onset of visible injury (Lorenzini et al. 1999) or in crops such as beans (e.g. Guidi et al. 1997, 2000). In the present experiment, a significant decline in F v/F m (i.e. there is strong photoinhibitory damage), together with chlorophyll destruction, was observed only when leaves were clearly symptomatic, therefore representing a more advanced stage of damage.
In the present study, we did not observe a significant decline in chlorophyll content in fumigated asymptomatic leaves with regard to control, although the tendency in all species but A. monspessulanum is towards a reduction. An obvious loss of chlorophyll is observed only when leaves are clearly symptomatic, i.e. assimilatory tissue is partly destroyed. Decrease in chlorophyll contents in fumigated leaves is a well known response to enhanced ozone levels (e.g. Pleijel et al. 1994; Mikkelsen et al. 1995; Saitanis et al. 2001). In fumigated asymptomatic leaves of in A. pseudoplatanus, there are both a significant decrease in N and an increase in C/N content, and the same tendency although not significant was observed in A. opalus, with about 15% decrease in N content and 14% increase in C/N ratio. N is an important component of the chlorophyll structure, and stromatic enzymes, mainly Rubisco, represent the major fraction of chloroplast N (Hörtensteiner and Feller 2002). Lowered N content in mature leaves of A. pseudoplatanus may reflect not only partial chlorophyll reduction, but also deleterious effects on this enzyme (Keutgen et al. 2005). It is know that ozone modifies the carboxylating activity of this enzime (Enyedi et al. 1992) either by direct oxidation of Rubisco (Pell et al. 1994) or through suppression of messenger RNA production (Reddy et al. 1993). As mentioned above, reduced Rubisco activity may contribute to the observed A sat decline in fumigated leaves not fully explained by stomatal limitations. The effects of ozone on foliar N content has been addressed in several studies with contrasting results. A decrease in this nutrient has been reported in several studies in both trees (Samuelson et al. 1996) and crops (Keutgen et al. 2005), while other authors found increasing N accumulation with increasing ozone (Baker et al. 1994; Temple and Riechers 1995), or negligible effects (Reich et al. 1988; Schier 1990; Lindroth et al. 2002). N content of the leaves can be affected by many factors, including growth conditions of the plant, season, leaf age, or even position in the canopy (Scherzer et al. 1998). In addition, ozone induces an accelerated senescence of the leaves, reducing N content in old leaves. This N can be remobilized towards younger tissues, and loss of Rubisco in older leaves may be associated with an increase in this protein in the young leaves as a compensatory response (Brendley and Pell 1998). Therefore, plant growth conditions or compensatory responses in the leaves may be partly involved in the above mentioned different patterns of response in foliar N content of plants exposed to ozone.
One of the effects of ozone most commonly reported under controlled conditions is growth reduction (Chappelka and Chevone 1992). Reduction of availability of photoassimilates due to CO2 assimilation impairment, may limit growth of the plants. Changes of biomass have been reported in numerous seedlings of tree species after ozone fumigation in controlled conditions, including several maple species: A. saccharinum (Jensen 1983), A. saccharum (Kress and Skelly 1982; Reich et al. 1986). Also a decrease in height has been reported in A. rubrum, A. saccharinum and A. saccharum after 109 days of fumigation at 300 ppb, 5 days a week, in OTCs or in CSTR (Jensen 1973). In the present study, considerable variation was observed between individuals regarding growth, so that although fumigation produced a decrease in the average growth rate values, differences with regard to control plants were not significant. The consistency of the results, however, suggest that the applied ozone levels might be incipiently affecting growth, especially in the two species showing the highest reductions, A. pseudoplatanus and A. campestre, but that more than one growing season would be needed to detect any significant effect on this parameter. Also in agreement with injury and gas exchange results, A. monspessulanum was the least sensitive species to changes in height growth rates.
In synthesis, the four species of maple showed contrasting sensitivity to ozone as demonstrated by visible injury development, gas exchange, chlorophyll a fluorescence, and growth measurements. The most sensitive species were A. opalus and A. pseudoplatanus, while A. monspessulanum was the most resistant. Plant injury index was more consistently related with physiological measurements than thresholds for the first observation of visible injury. Under the experimental conditions, the two species with the highest g s (A. opalus and A. pseudoplatanus) where those more affected by visible injury, and also experienced the most important reductions in A sat, g s, and WUE. A. monspessulanum, the maple better adapted to dry conditions and with the most coriaceous leaves was the most resistant, despite not being the species with the lowest g s. This is consistent with the hypothesis that species or populations adapted to Mediterranean conditions, with enhanced leaf sclerophylly, and are in general more ozone tolerant: southern provenances of some species (e.g. Fagus sylvatica) are less ozone sensitive than central European ones, and Mediterranean evergreen broadleaves are know to be relatively ozone tolerant. This tolerance has been explained not only because of their low gas exchange rates (avoidance), but also by their constitutional and induced ability to tolerate oxidative stress by an active antioxidant pool (Paoletti 2006). Finally, the decline in A sat already observed in asymptomatic leaves of A. opalus could not be attributed to stomatal limitations or to photoinhibitory damage, suggesting that other causes (probably an inhibition on the ‘dark-phase’ of photosynthesis) might be initially involved in the impairment of photosynthesis under ozone stress. This reduction of the photoassimilation resulted in a tendency to decrease growth, but not significant within the exposure period.
Abbreviations
- A sat :
-
light saturated CO2 assimilation
- AOT40:
-
accumulated exposure over threshold 40 ppb
- C i :
-
intercellular CO2 concentrations
- CSTR:
-
Continuously Stirred Tank Reactors
- DSF:
-
days after starting of fumigation
- Φ exc :
-
quantum efficiency of excitation capture by oxidized reaction centers of PSII
- Φ PSII :
-
quantum yield of electron transfer at PSII
- F m :
-
maximum fluorescence
- \( F^{\prime }_{{\text{m}}} \) :
-
maximum fluorescence in the light adapted state
- F o :
-
minimal fluorescence
- \( F^{\prime }_{{\text{o}}} \) :
-
minimum fluorescence in the light-adapted state
- F s :
-
modulated fluorescence yield at steady state
- Fv/Fm:
-
maximum quantum efficiency of photosystem II (PSII) primary photochemistry
- g s :
-
stomatal conductance to water vapor
- NPQ:
-
quenching due to non-photochemical dissipation of absorved light energy
- OTC:
-
open top chamber
- PPFD:
-
photosynthetic photon flux density
- q p :
-
coefficient for photochemical quenching.
- RHGR:
-
relative height growth rate
- Tr:
-
transpiration rate
- VPD:
-
leaf-to-air water vapor pressure deficit
- WUE:
-
water Use Efficiency, calculated as A sat/T r
References
Ashmore, M. R. (2005). Assessing the future global impacts of ozone vegetation. Plant, Cell and Environment, 28, 949–964.
Baier, M., Kandlbinder, A., Golldack, D., & Dietz, K.-J. (2005). Oxidative stress and ozone: Perception, signalling and response. Plant, Cell and Environment, 28, 1012–1020.
Baker, T. R., Allen, H. L., Schoeneberger, M. M., & Kress, L. W. (1994). Nutritional response of loblolly pine exposed to ozone and simulated acid rain. Canadian Journal of Forest Research, 24, 453–461.
Barnes, J. D., Balaguer, L., Manrique, E., Elvira, S., & Davison, A. W. (1992). A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environmental and Experimental Botany, 32, 85–100.
Black, V. J., Black, C. R., Roberts, J. A., & Stewart, C. A. (2000). Impact of ozone on the reproductive development of plants. Tansley Review for New Phytologist, 147, 421–447.
Brendley, B. W., & Pell, E. J. (1998). Ozone-induced changes in biosynthesis of Rubisco and associated compensation to stress in foliage of hybrid poplar. Tree Physiology, 18, 81–90.
Bussotti, F., Agati, G., Desotgiu, R., Matteini, P., & Tani, C. (2005). Ozone foliar symptoms in woody plant species assessed with ultrastructural and fluorescence analysis. New Phytologist, 166, 941–955.
Bussotti, F., & Ferretti, M. (1998). Air pollution, forest condition and forest declines in southern europe. An overview. Environmental Pollution, 101, 49–65.
Cabezudo, B., & Talavera, S. (coordinators) (2005). Lista roja de la flora vascular de Andalucía. Junta de Andalucía, Sevilla, 83 p.
Calatayud, A., Iglesias, D., Talón, M., & Barreno, E. (2003). Effects of 2-month ozone exposure in spinach leaves on photosynthesis, antioxidant systems and lipid peroxidation. Plant Physiology & Biochemistry, 41, 839–845.
Chappelka, A. H., & Chevone, B. I. (1992). Tree responses to ozone. In A. S. Lefohn (Ed.), Surface-level ozone exposures and their effects on vegetation (pp. 271–324). Chelsea, MI: Lewis.
Chappelka, A. H., & Samuelson, L. J. (1998). Ambient ozone effects on forest trees of the eastern United States: A review. New Phytologist, 139, 91–108.
Dann, M. S., & Pell, E. J. (1989). Decline of activity and quantity of ribulose bisphosphate carboxylase/oxygenase and net photosynthesis in ozone-treated potato foliage. Plant Physiology, 91, 427–432.
De Kok, L. J., & Tausz, M. (2001). The role of glutathione in plant reaction and adaptation to air pollutants. In D. Grill, M. Tausz, & L. J. De Kok (Eds.), Significance of glutathione to plant adaptation to the environment (pp. 185–208). Amsterdam: Kluwer.
de Vries, W., Reinds, G. J., Posh, M., Sanz, M. J., Krause, G., Calatayud, V., et al. (2003). Intensive monitoring of forest ecosystems in Europe, 2003. Technical Report. EC-UN/ECE, Brussels, Geneva (ISSN 1020-6078).
Enyedi, A. J., Eckardt, N. A., & Pell, E. J. (1992). Activity of ribulose bisphosphate carboxylase/oxygenase from potato cultivars with differential response to ozone stress. New Phytologist, 122, 493–500.
EU European Union (2002). Directive 2002/3/EC of the European Parliament and of the Council of 12 February 2002 relating to ozone in ambient air. Official Journal of the European Communities, L 67/14–30.
Evans, G. C. (1972). The quantitative analysis of plant growth. Studies in Ecology 1. Great Britain: Blackwell, 734 p.
Ferretti, M., Bussotti, F., & Calderesi, M. (2004). Ozone, defoliation, of beech (Fagus sylvatica) an visible foliar symptoms on native plants on selected plots of South-Western Europe. In M. Ferretti, M.-J. Sanz, & M. Schaub (Eds.), O 3 SWE-ozone and the forests of South-West Europeern. Final Report (pp. 111–140). Jointly prepared by Corpo Forestale dello Stato, Italia; Ministerio de Medio Ambiente, Dirección General para la Biodiversidad, España; Eidgenössische Forschungsanstalt für Wald, Schnee und Landschaft – WSL, Schweiz; Office National des Forêts, France.
Ferretti, M., Calderisi, M., & Bussotti, F. (2007). Ozone exposure, defoliation of beech (Fagus sylvatica L.) and visible foliar symptoms on native plants in selected plots of South-western Europe. Environmental Pollution, 145, 644–651.
Fiscus, E. L., Booker, F. L., & Burkey, K. O. (2005). Crop responses to ozone: Uptake, modes of action, carbon assimilation and partitioning. Plant, Cell and Environment, 28, 997–1011.
Fowler, D., Cape, J. N., Coyle, M., Flechard, C., Kuylenstierna, J., Hicks, K., et al. (1999). The global exposure of forests to air pollutants. Water, Air, & Soil Pollution, 116, 5–32.
Gaucher, C., Costanzo, N., Afif, D., Mauffette, Y., Chevrier, N. & Dizengremel, P. (2003). The impact of elevated ozone and carbon dioxide on young Acer saccharum seedlings. Physiologia Plantarum, 117, 392–402.
Genty, B., Briantais, J. M., & Baker, N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of Chl fluorescence. Biochimica et Biophysica Acta, 990, 87–92.
Gravano, E., Bussotti, F., Strasser, R. J., Schaub, M., Novak, K., Skelly, K., et al. (2004). Ozone symptoms in leaves of woody plants in open-top chambers: Ultrastructural and physiological characteristics. Physiologia Plantarum, 121, 620–633.
Guidi, L., Di Cagno, R., & Soldatini, G. F. (2000). Screening of bean cultivars for their response to ozone as evaluated by visible symptoms and leaf chlorophyll fluorescence. Environmental Pollution, 107, 349–355.
Guidi, L., Nali, C., Ciompi, S., Lorenzini, G., & Franco, G. (1997). The use of chlorophyll fluorescence and leaf gas exchange as methods for studying the different responses to ozone of two bean cultivars. Journal of Experimental Botany, 48, 173–179.
Günthardt-Goerg, M. S., McQuattie, C. J., Scheidegger, C., Rhiner, C., & Matyssek, R. (1997). Ozone-induced cytochemical and ultra-structural changes in leaf mesophyll cell walls. Canadian Journal of Forest Research, 27, 453–463.
Heath, R. L. (1987). The biochemistry of ozone attack on the plasma membrane of plant cells. In J. A. Saunders, L. Kosak-Channing, & E. E. Conn (Eds.), Recent advances in phytochemistry. Phytochemical effects of environmental compounds (pp. 29–54). New York, NY: Plenum.
Heath, R. L., & Taylor, G. E., Jr. (1997). Physiological processes and plant responses to ozone exposure. In H. Sandermann, A. R. Wellburn, & R. L. Heath (Eds.), Forest Decline and Ozone: A comparison of controlled chamber and field experiments. Ecological Studies 127 (pp. 317–368). New York: Springer.
Hörtensteiner, S., & Feller, U. (2002). Nitrogen metabolism and remobilization during senescence. Journal of Experimental Botany, 53, 927–937.
Innes, J. L., Skelly, J. M., & Schaub, M. (2001). Ozone and broadleaved species. A guide to the identification of ozone-induced foliar injury. [Ozon, Laubholz- und Krautpflanzen. Ein Führer zum Bestimmen von Ozonsymptomen]. Haupt, Bern, Stuttgart, Wien.
IPCC (2001) Climate Change 2001: Synthesis Report. Geneva, Switzerland: Intergovernmental Panel on Climate Change.
Jensen, K. F. (1973). Response of nine forest tree species to chronic ozone fumigation. Plant Disease Reporter, 57, 914–917.
Jensen, K. F. (1983). Growth relationship in silver maple fumigated with O3 and SO2. Canadian Journal of Forest Research, 13, 298–302.
Kangasjärvi, J., Talvinen, J., Utriainen, M., & Karjalainen, R. (1994). Plant defense systems induced by ozone: Commissioned Review. Plant, Cell and Environment, 17, 783–794.
Karnosky, D. F., Zak, D., Pregnitzer, K., Awmack, C., Bockheim, J., Dickson, R., et al. (2003). Tropospheric O3 moderates responses of temperate hardwood forests to elevated CO2: A synthesis of molecular to ecosystem results from the Aspen FACE project. Functional Ecology, 17, 289–304.
Kersteins, G., & Lendzian, K. J. (1989). Interactions between ozone and plant cuticules 1. Ozone deposition and permeability. New Phytologist, 112, 13–19.
Keutgen, A. J., Noga, G., & Pawelzik, E. (2005) Cultivar-specific impairment of strawberry growth, photosynthesis, carbohydrate and nitrogen accumulation by ozone. Environmental and Experimental Botany, 53, 271–280.
King, J. S., Kubiske, M. E., Pregitzer, K. S., Hendrey, G. R., McDonald, E. P., Giardina, C. P., et al. (2005). Tropospheric O3 compromises net primary production in young stands of trembling aspen, paper birch and sugar maple in response to elevated atmospheric CO2. New Phytologist, 168, 623–635.
Kress, L. W., & Skelly, J. M. (1982). Response of several eastern forest trees to chronic doses of ozone and nitrogen dioxide. Plant Disease, 66, 1149–1152.
Krupa, S., & Manning, W. J. (1988). Atmospheric ozone: Formation and effects on vegetation. Environmental Pollution, 50, 101–137.
Krupa, S., McGrath, M. T., Andersen, C. P., Booker, F. L., Burkey, K. O., Chappelka, A. H., et al. (2001). Ambient ozone and plant health. Plant Disease, 85, 5–12.
Lindroth, R. L., Kopper, B. J., Parsons, F. J., Bockheim, J. G., Karnosky, D. F., Hendrey, G. R., et al. (2002). Consequences of elevated carbon dioxide and ozone for foliar chemical composition and dynamics in trembling ozone for foliar chemical composition and dynamics in trembling aspen (Populus tremuloides) and paper birch (Betula papyrifera). Environmental Pollution, 115, 395–404.
Lorenzini, G., Guidi, L., Nali, C., & Soldatini, G. F. (1999). Quenching analysis in poplar clones exposed to ozone. Tree Physiology, 19, 607–612.
Matyssek, R., Bytnerowicz, A., Karlsson, P.-E., Paoletti, E., Sanz, M. J., Schaub, M., et al. (2007) Promoting the O3 flux concept for European forest trees. Environmental Pollution, 146, 587–607.
Matyssek, R., & Innes, J. L. (1999). Ozone – a risk factor for trees and forests in Europe?. Water, Air & Soil Pollution, 116, 199–226.
Matyssek, R., Reich, P., Oren, R., & Winner, R. E. (1995). Response mechanisms of conifers to air pollutants. In W. K. Smith & T. M. Hinckley (Eds.), Ecophysiology of coniferous forests (pp. 255–308). San Diego: Academic.
Matyssek, R., Wieser, G., Nunn, A. J., Kozovits, A. R., Reiter, I. M., Heerdt, C., et al. (2004). Comparison between AOT40 and ozone uptake in forest trees of different species, age and site conditions. Atmospheric Environment, 38, 2271–2281.
Mehlhorn, H., Tabner, B. J., & Wellburn, A. R. (1990). Electron spin resonance: Evidence for the formation of free radicals in plants exposed to ozone. Physiologia Plantarum, 79, 377–383.
Mikkelsen, T. N. (1995). Physiological responses of Fagus sylvatica L. exposed to low levels of ozone in open-top chambers. Trees, 9, 355–361.
Mikkelsen, T. N., Dodell, B., & Lutz, C. (1995). Changes in pigment concentration and composition in Norway spruce induced by long-term exposure to low levels of ozone. Environmental Pollution, 87, 197–205.
Mikkelsen, T. N., & Heide-Jørgensen, H. S. (1996). Acceleration of leaf senescence in Fagus sylvatica L. by low levels of tropospheric ozone demonstrated by leaf colour, chlorophyll fluorescence and chloroplast ultrastructure. Trees, 10, 145–156.
Millán, M. M., Mantilla, E., Salvador, R., Carratalá, A., Sanz, M. J., Alonso, L., et al. (2000). Ozone cycles in the Western Mediterranean Basin: Interpretation of monitoring data in complex coastal terrain. Journal of Applied Meteorology, 39, 487–508.
Millán, M. M., Salvador, R., Mantilla, E., & Kallos, G. (1997). Photo-oxidant dynamics in the Mediterranean Basin in summer: Results from European research projects. Journal of Geophysical Research, 102(D7), 8811–8823.
Mills (2004). Mapping critical levels for vegetation. In: UNECE Convention on Long-rate Transboundary Air Pollution. Manual on Methodologies and Criteria for Modelling and Mapping Critical Loads and Levels and Air Pollution Effects, Risks and Trends. Retrieved from http://www.oekodata.com/icpmapping/.
Musselmann, R. C., & Massmann, W. J. (1999) Ozone flux to vegetation and its relationship to plant response and ambient air quality standards. Atmospheric Environment, 33, 65–73.
Novak, K., Schaub, M., Fuhrer, J., Skelly, J. M., Hug, C., & Landolt, W. (2005). Seasonal trends in reduced leaf gas exchange and ozone-induced foliar injury in three ozone sensitive woody plant species. Environmental Pollution, 136, 33–45.
Novak, K., Skelly, J. M., Schaub, M., Kräuchi, N., Hug, C. & Landolt, W. (2003). Ozone air pollution and foliar injury development on native plants of Switzerland. Environmental Pollution, 125, 41–52.
Orendovici, T., Skelly, J. M., Ferdinand, J. A., Savage, J. E., Sanz, M. J., & Smith, G. C. (2003). Response of native plants of northeastern United States and southern Spain to ozone exposures; determining exposure/response relationships. Environmental Pollution, 125, 31–40.
Paoletti, E. (2006). Impact of ozone on Mediterranean forests: A review. Environmental Pollution, 144, 463–474.
Paoletti, E., & Grulke, N. E. (2005). Does living in elevated CO2 ameliorate tree response to ozone? A review on stomatal responses. Environmental Pollution, 137, 483–493.
Pell, E. J., Eckardt, N., & Glick, R. E. (1994). Biochemical and molecular basis for the impairment of photosynthetic potential. Photosynthesis Research, 39, 453–462.
Pell, E. J., Schlagnhaufer, C. D., & Arteca, R. N. (1997). Ozone-induced oxidative stress: Mechanisms of action and reaction. Physiologia Plantarum, 100, 264–273.
Pleijel, H., Skärby, L., Ojanperä, K., & Selldén, G. (1994). Exposure of oats, Avena sativa L. to filtered and unfiltered air in open-top chambers: Effects on grain yield and quality. Environmental Pollution, 86, 129–134.
Reddy, G. N., Aeteca, R. N., Dai, Y. R., Flores, H. E., Negram, F. B., & Pell, E. J. (1993). Changes in ethylene and polyamines in relation to mRNA levels of the large and small subunits of ribulose biphosphate/oxygenase in ozone-stressed potato foliage. Plant, Cell and Environment, 120, 819–826.
Reich, P. B. (1987). Quantifying plant response to ozone: A unifying theory. Tree Physiology, 3, 63–91.
Reich, P. B., & Amudson, R. G. (1985). Ambient levels of ozone reduce net photosynthesis in tree and crop species. Science, 230, 566–570.
Reich, P. B., Schoettle, A. W., & Amudson, R. G. (1986). Effects of O3 and acidic rain on photosynthesis and growth in sugar maple and northern red oak seedlings. Environmental Pollution, 40, 1–15.
Reich, P. B., Schoettle, A. W., Stroo, H. F., & Amundson, R. G. (1988). Effects of ozone and acid rain on white pine (Pinus strobus) seedlings grown in five soils. III. Nutrient relations. Canadian Journal of Botany, 66, 1517–1531.
Reichenauer, T. G., & Bolhàr-Nordenkampf, H. R. (1999). Mechanisms of impairment of the photosynthetic apparatus in intact leaves by ozone. Zeitschrift für Naturforschung, 54c, 824–829.
Reig-Armiñana, J., Calatayud, V., Cerveró, J., García-Breijo, F. J., Ibars, A., & Sanz, M. J. (2004). Effects of ozone on the foliar histology of the mastic plant (Pistacia lentiscus L.). Environmental Pollution, 132, 321–331.
Runeckles, V. C., & Chevone, B. I. (1992). Crop responses to ozone. In A. S. Lefohn (Ed.), Surface level ozone exposures and their effects on vegetation (pp. 189–270). Chelsea, MI: Lewis
Saitanis, C. J., Riga-Karandinos, A. N., & Karandinos, M. G. (2001). Effects of ozone on chlorophyll and quantum yield of tobacco (Nicotiana tabacum L.) varieties. Chemosphere, 42, 909–917.
Samuelson, L. J., & Kelly, J. M. (1997). Ozone uptake in Prunus serotina, Acer rubrum and Quercus rubra forest trees of different sizes. New Phytologist, 136, 255–264.
Samuelson, L. J., Kelly, J. M., Mays, P. A., & Edwards, G. S. (1996). Growth and nutrition of Quercus rubra L. seedlings and mature trees after three seasons of ozone exposure. Environmental Pollution, 91, 317–323.
Sanz, M. J., & Millán, M. (1998). The dynamics of aged air mases and ozone in the western Mediterranean: Relevance to forest ecosystems. Chemosphere, 98, 1089–1094.
Sanz, M. J., Sánchez, G., Calatayud, V., Minaya, M. T., & Cerveró, J. (2001). La contaminación atmosférica en los bosques. Guía para la identificación de daños visibles causados por ozono. Organismo Autónomo de Parques Nacionales, 163 p.
Sanz, M. J., Calatayud, V., & Sanchez, G. (2007a). Measures of ozone concentrations using passive sampling in forests of South Western Europe. Environmental Pollution, 145, 620–628.
Sanz, M. J., Sanz, F., Calatayud, V., Sánchez-Peña, G. (2007b). Ozone in Spain’s National Parks and Protected Forests. TheScientificWorldJOURNAL 21, 67–77.
Schaub, M., Skelly, J. M., Steiner, K. C., Davis, D. D., Pennypacker, S. P., Zhang, J., et al. (2003). Physiological and foliar injury responses of Prunus serotina, Fraxinus americana and Acer rubrum seedlings to varying soil moisture and ozone. Environmental Pollution, 124, 307–320.
Scherzer, A. J., Rebbeck, J., & Boerner, R. E. J. (1998). Foliar nitrogen dynamics and decomposition of yellow-poplar and eastern white pine during four seasons of exposure to elevated ozone and carbon dioxide. Forest Ecology and Management, 109, 355–366.
Schier, G. A. (1990). Response of yellow-poplar (Liriodendron tulipifera L.) seedlings to simulated acid rain and ozone: 2. Effect on throughfall chemistry and nutrients in the leaves. Environmental and Experimental Botany, 30, 325–331.
Schreiber, U., Schliwa, U., & Bilger, W. (1986). Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynthesis Research, 10, 51–62.
Skelly, J. M., Innes, J. L., Savage, J. E., Snyder, K. R., Vanderheyden, D., Zhang, J., et al. (1999). Observation and confirmation of foliar ozone symptoms of native plant species of Switzerland and southern Spain. Water, Air, and Soil Pollution, 116, 227–234.
Taylor, G. E., Jr., & Tingey, D. T. (1982). Flux of ozone to Glycine max: Sites of regulation and relationship to leaf injury. Oecologia, 53, 179–186.
Temple, P. J., & Riechers, G. H. (1995). Nitrogen allocation in ponderosa pine seedlings exposed to interacting ozone and drought stresses. New Phytologist, 130, 97–104.
Vanderheyden, D., Skelly, J., Innes, J., Hug, C., Zhang, J., Landolt, W., et al. (2001). Ozone exposure thresholds and foliar injury on forest plants in Switzerland. Environmental Pollution, 111, 321–331.
Vollenweider, P., Ottiger, M., & Günthardt-Goerg, M. S. (2003). Validation of leaf ozone symptoms in natural vegetation using microscopical methods. Environmental Pollution, 124, 101–118.
Wieser, G. (1997) Ozone impact on photosynthetic capacity of mature and young Norway spruce (Picea abies (L.) Karst.): External versus internal exposure. Phyton, 37, 279–302.
Wieser, G., Häsler, R., Götz, B., Koch, W., & Havranek, W. M. (2000). Role of climate, crown position, tree age and altitude in calculated ozone flux into needles of Picea abies and Pinus cembra: A synthesis. Environmental Pollution, 109, 415–422.
Wohlgemuth, H., Mittelstrass, K., Kschieschan, S., Bender, J., Weigel, H.-J., Overmyer, K., et al. (2002). Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant, Cell and Environment, 25, 717–726.
Zhang, J., Ferdinand, J. A., Vanderheyden, D., Skelly, J., & Innes, J. (2001). Variation of gas exchange within native species of Switzerland and relationships with ozone injury: An open-top experiment. Environmental Pollution, 113, 177–185.
Acknowledgements
M.J.S. and V.C. thank the DGCN (MMA), and Conselleria de Territori i Habitatge (project FORMEDOZON, Interreg III B) for funding this study, and to Generalitat Valenciana and Bancaixa for continuous support to Fundación CEAM. This work has been carried out in connection with the activities of the Working Group of Ambient Air Quality of ICP-Forests. Esperanza Calvo is acknowledged for fruitful discussions on the article. Carmen Martín is thanked for taking care of the plants. José Reig-Armiñana and Francisco García-Breijo are also thanked for information on the anatomical traits of the plants. Ana Bucher helped with the C and N analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calatayud, V., Cerveró, J. & Sanz, M.J. Foliar, Physiologial and Growth Responses of Four Maple Species Exposed to Ozone. Water Air Soil Pollut 185, 239–254 (2007). https://doi.org/10.1007/s11270-007-9446-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9446-5