Abstract
In this study, impacts of O3 on four cultivars (‘Rose’, ‘Pink’, ‘Blush’ and ‘White’) of the polka dot plant with variegated leaves were investigated for the first time. Ozone fumigation [(120 ± 20 ppb) for 14 days (8 h day−1, from 8:30 to 16:30)] resulted in visible foliar injuries, decreased contents of pigments (chlorophyll a and b, and carotenoid), the inhibition of photosynthesis, the increase of quantum yield of non-regulated heat dissipation and fluorescence emission (Y(NO)), and the damage of cell membrane. Elevated O3 increased the content of anthocyanin (Ant). ‘White’ showed the highest, and ‘Rose’ the lowest amount of injured leaf area, indicating that the former was the most sensitive, and the latter the most tolerant to O3 stress. After O3 exposure, the highest Ant content was found in ‘Rose’, followed by ‘Pink’, ‘Blush’, and ‘White’. Levels of Ant were likely responsible for the different sensitivities to O3 due to their roles in photoprotection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Ground level ozone (O3) is currently a major air pollutant in many developed or developing countries such as China (Paoletti 2007; Tong et al. 2017). It is a threat to the well-being of humans, but also endangers crops, forests and natural vegetation, resulting in economic losses and ecological risk (Sitch et al. 2007; Avnery et al. 2011). Generally, O3 enters the leaves through stomata, then reacts with apoplastic solutes, leading to the generation of reactive oxygen species (ROS) (Brandão et al. 2017). ROS can attack the cell wall and increase membrane permeability of mesophyll cells (Vaultier and Jolivet 2015). Meanwhile, O3 also provokes signaling cascades, depresses stomatal conductance, transpiration rate, and photosynthetic rate, induces visible injury, and causes biomass loss (Xu et al. 2009; Tiwari et al. 2016).
Ozone sensitivity of plants can be judged by the capability of regulating stomata and detoxifying ROS. Some leaf pigments are considered as antioxidants in plants according to previous reports (Ruhland et al. 2007). For instance, leaf carotenoids (Car) play important roles in scavenging ROS (Maamar et al. 2015), thereby inhibiting photooxidation of photosynthetic membrane (Pellegrini et al. 2011). Carotenoids may also play a key role in protecting plants against photodamage by taking part in non-photochemical quenching (He et al. 2017).
Phenolic compounds also have great potential to cope with oxidative stress (Iriti and Faoro 2008). Among them, anthocyanin (Ant) are an important class, and their content in leaves is often associated with leaf color. Researches have shown that endogenous Ant could protect the photosynthetic apparatus from high light stress (Feild et al. 2001; Hughes and Smith 2007). In addition, under abiotic stresses, Ant serves to purge the ROS that may be generated (Gould et al. 2002). Our previous study showed that O3 sensitivities of Pak Choi cultivars with green or red leaves were significantly different due to differential content of Ant (Zhang et al. 2017). However, variegated species which are often used as foliage plant have not been investigated for the tolerance to O3 stress.
Polka dot plant (Hypoestes phyllostachya Bak.) is often cultivated in the open field as an annual plant, or indoors as house plants (Kim et al. 2012). The typical characteristic of this species is variegation, i.e. green leaves with spots, and the color of spots can range from white to pink to red due to the differential ingredients or contents of chlorophyll (Chl), Ant and Car (Kim et al. 2012). There is only limited information about responses of this species to abiotic stresses such as light (Svenson and Johnston 1994; Kim et al. 2012). As far as we know, there is no information about the effects of O3 on this species.
In the present study, the objectives were to: (1) assess O3 sensitivities of four cultivars of H. phyllostachya with different leaf colors; (2) reveal the physiological mechanisms underlying the different sensitivities.
Materials and Methods
Four cultivars (‘Rose’, ‘Pink’, ‘Blush’, ‘White’) of polka dot plant (H. phyllostachya) were selected for this experiment. Seeds were bought from China National Tree Seeds Corporation, Beijing, CN. On 24 March 2016, they were planted in plastic pots (10 cm in diameter) filled with a mixture of vermiculite and peat (1:1, v:v). Seedlings were cultivated in a greenhouse in the Horticultural Experiment Station of Northeast Agricultural University (45°74′15″N, 126°73′14″E), Harbin, CN. To avoid water deficiency, water was supplied every day throughout the experiment.
On 18 August 2016, when seedlings had four pairs of fully expanded leaves, four seedlings per each cultivar were randomly placed in six open top chambers (OTCs, 1 m in diameter and 1 m in height) which were made of transparent polymethyl methacrylate plates for treatments. After adaptation for 7 days, three OTCs were ventilated with O3 where concentrations were maintained at 120 ± 20 ppb for 14 days (8 h day−1, from 8:30 to 16:30), the other three with charcoal-filtered (CF) air. Ozone was produced by an O3 generator (CF-KG1, Beijing Sumsun EP Hi-Tech, Beijing, CN). In order to maintain the target value, O3 concentrations at the plant height were monitored using an O3 analyzer (Model 202, 2B Technologies Inc., Boulder, CO, USA). During the experiment, the daily average photosynthetic photon flux density (PPFD) inside the OTCs was about 700 μmol m−2 s−1, and the max/min temperature and relative humidity in the OTCs were 25.7/16.0°C and 66.3%/33.8%, respectively.
After 14 days of exposure, visible injuries on fully expanded leaves were immediately observed and recorded by three surveyors independently using a 5% step scale, and then the values were averaged as visible injury of the seedling (Paoletti et al. 2009). Visible injury was calculated as \({A_L} \times {A_R}\). A L means the average percentage of visible injured area in all fully expanded leaves of each seedling; A R denotes the ratio of injured fully expanded leaves to total fully expanded leaves of each seedling (Paoletti et al. 2014). Five seedlings of each cultivar in each treatment were randomly selected for observation.
Leaf chlorophyll a fluorescence measurements were carried out from 8:00 to 11:00 on the first day after the end of treatments using a PAM-2500 fluorometer (Heinz Walz, Effeltrich, DE). Five plants of each cultivar in each treatment were measured. The middle part of one leaf of the first or second pair of fully expanded leaves was chosen for measurement to avoid the main vein. Before each measurement, leaf was dark-adapted for 30 min. The minimal (Fo) and maximal fluorescence (Fm) were measured with a modulated irradiation (630 nm, 0.1 μmol (photon) m−2 s−1), and a 0.8 s saturating pulse (630 nm, 10,000 μmol (photon) m−2 s−1), respectively. An actinic light (630 nm, 199 μmol (photon) m−2 s−1) was switched on after 40 s. Then a saturating pulse was turned on every 20 s for 5 min to determine the maximal fluorescence in the irradiation-adapted state (Fm′). The formula of Fv/Fm was \(Fv/Fm={{\left( {Fm - Fo} \right)} \mathord{\left/ {\vphantom {{\left( {Fm - Fo} \right)} {Fm}}} \right. \kern-0pt} {Fm}}.\) Nonphotochemical quenching (NPQ), coefficient of photochemical fluorescence quenching based on puddle model (qP), effective quantum yield of photosystem II (PSII) (Y(II)), quantum yield of light-induced non-photochemical fluorescence quenching (Y(NPQ)) and quantum yield of non-regulated heat dissipation and fluorescence emission (Y(NO)), and electron transport rate (ETR) were calculated automatically by the PamWin-3 software (Heinz Walz, Effeltrich, DE).
Gas exchanges of the first or second pair of fully expanded leaves were measured from 8:00 to 11:30 on the second day after the end of treatments, using a portable gas exchange system (Li-6400, Li-Cor, Lincoln, NE, USA). During the measurement, leaf temperature, and light intensity were set at 35 ± 5°C and 1200 μmol (photon) m−2 s−1. Ambient CO2 concentrations were used as reference (410 ± 5 μmol mol−1). Photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (E), and the ratio of intercellular CO2 concentration/ambient CO2 concentration (Ci/Ca) were calculated.
In the morning on the third day after the end of treatments, fresh fully expanded leaves (0.2 g) were sampled and cut into uniform size, then put into test tubes filled with 20 mL of double distilled water at 25°C. After 24 h, electrolyte leakage (EL) was measured using a conductivity meter (Delta 326, Mettler-Toledo, Greifensee, CH) as EL1. Then tubes were maintained in boiling water bath (100°C) for 20 min. After cooling to 25°C, EL was measured again as EL2. The relative change of EL was calculated as \(\left( {{{E{L_1}} \mathord{\left/ {\vphantom {{E{L_1}} {E{L_2}}}} \right. \kern-0pt} {E{L_2}}}} \right) \times 100\%\).
Fully expanded leaves (0.3 g) were sampled for measuring Chl and Car according to Hao et al. (2004). Samples were cut, and ground with quartz sand, calcium carbonate powder, and ethanol (95%, v/v) in a dark room. The extracts were filtered into 25 mL brown volumetric flask, and then ethanol was added to a final volume of 25 mL. The absorbance was measured using a UV–Vis spectrophotometer (T6 New Century, Beijing, CN) at 470, 649, and 665 nm. Contents of pigments were calculated as follows: \(Chl\;a=(13.95 \times O{D_{665}} - 6.88 \times O{D_{649}}) \times 25/(0.3 \times 1000);\) \(Chl\;b=(24.96 \times O{D_{649}} - 7.32 \times O{D_{665}})\times 25/(0.3 \times 1000);\) \(Car= \left[1000 \times O{D_{470}} - 2.05 \times (13.95 \times O{D_{665}} - 6.88 \times O{D_{649}}) - 114.8 \times (24.96 \times O{D_{649}} - 7.32 \times O{D_{665}}) \right] \times 25/ (245 \times 0.3 \times 1000).\)
Ant content was measured using fresh fully expanded leaves (0.2 g). Leaves were cut into uniform size and put into test tubes, into which 10 mL 1% hydrochloric acid methanol solution were added, followed by immediate sealing. They were kept in dark for 12 h. The extracts were centrifuged at 4000×g for 10 min. Then supernatants (2.5 mL) were measured using a UV–Vis spectrophotometer (T6 New Century) at 530 and 600 nm. Optical density changes of extracts were calculated as U g−1 (FM), the unit of U was denoted as \({{\left( {O{D_{530}} - O{D_{600}}} \right)} \mathord{\left/ {\vphantom {{\left( {O{D_{530}} - O{D_{600}}} \right)} {0.1}}} \right. \kern-0pt} {0.1}}\), FM was the fresh mass of the sample (g), according to Wang et al. (2016).
Data were analyzed using SPSS (v.12, SPSS, Chicago, IL, USA). Normal distribution was checked by the Kolmogorov–Smirnov test. Two-way analysis of variance (ANOVA) was used to identify the effect of O3 treatment, cultivar, and their interactions (ns not significant, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001). Means of each parameter among different treatments were compared by the post-hoc Duncan’s test. Relative change was expressed as percentage difference between O3-exposed (O3) plants and charcoal-filtered air (CF) plants: \(\left[ {{{\left( {{O_3} - CF} \right)} \mathord{\left/ {\vphantom {{\left( {{O_3} - CF} \right)} {CF}}} \right. \kern-0pt} {CF}}} \right] \times 100\%\). In figures, each bar and error bar represent the mean of five replicates and standard error, respectively. Different letters show significant differences among bars (p ≤ 0.05).
Results and Discussion
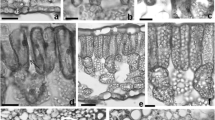
Under CF air, H. phyllostachya had irregular dot with rose, pink, blush or white color because of genetic variation (Fig. 1a, b, c, d). After O3 fumigation visible injury emerged on the adaxial surface, but not on the abaxial surface. In ‘Rose’, ‘Pink’, and ‘Blush’, only little necrosis was found on the dot area, but bronzing was observed in green area. In ‘White’, grey necrotic areas appeared by overlapping part of its white dot area firstly, and then spread to green area (Fig. 1e, f, g, h).
Images of leaf visible injury of H. phyllostachya under charcoal-filtered air (CF, 0 ppb) or acute O3 fumigation (O3, 120 ppb) for 14 days (8 h per day). a–d: ‘Rose’ (a), ‘Pink’ (b), ‘Blush’ (c) or ‘White’ (d) in CF air, respectively; e–h: ‘Rose’ (e), ‘Pink’ (f), ‘Blush’ (g) or ‘White’ (h) under O3 respectively
Leaf appearances seriously affect ornamental and economic values of garden plants, especially for foliage plants. There are several kinds of visible symptoms caused by high O3 such as chlorosis, bronzing, bleaching, flecking, and stippling (Innes et al. 2001). Some symptoms such as bronzing or flecking look like normal variegation. Therefore, careful examination is required to determine the visible injury of foliage plant with variegation. Plants in CF air were considered as control to qualify the O3-caused symptom. In this study, the main visible symptoms were irregular grey necrotic dots in ‘White’ or bronzing dots in other cultivars. It has been reported that white variegation of leaf was induced by abnormal plastids (Aoki et al. 1995). The overlap of necrotic areas and white dots probably indicated that the area without normal plastids could be damaged by O3 more easily. Significant differences of visible injuries existed among the four cultivars (Table 1). ‘White’ had the maximal injured area (56.67%), followed by ‘Blush’ (21.67%) and ‘Pink’ (16.00%), while the minimum was found in ‘Rose’ (6.00%). Necrotic areas reflect cell death in leaves (Picchi et al. 2017). The differential degrees of visible injury were probably due to the different amounts of dead cell.
There were significant interactions between O3 treatment and cultivar on the Pn, gs, E, and Ci/Ca of the polka dot plant (Fig. 2). Compared with CF air, significant decreases of Pn, gs and E, and an increase of Ci/Ca were found in seedlings under O3 fumigation (Fig. 2a, b, d). These results were in accordance with previous research (Feng et al. 2003; Zhang et al. 2010).
Gas exchange parameters (Net photosynthesis (Pn, a), stomatal conductance (gs, b), the ratio of intercellular/ambient CO2 concentration (Ci/Ca, c) and transpiration rate (E, d)) of four cultivars of H. phyllostachya exposed to charcoal-filtered air (CF, 0 ppb) or acute O3 fumigation (O3, 120 ppb) for 14 days (8 h per day)
The inhibition of Pn and the increase of Ci/Ca induced by O3 fumigation demonstrated that the decline of Pn was mainly caused by non-stomatal factors, such as biochemical processes (Zhang et al. 2010; Ismail et al. 2014). Compared with plants in CF air, ‘White’ had the highest decrease of Pn (−85.15%) under O3, while no relative loss was found in ‘Rose’. Furthermore, the gs in ‘Rose’ was the highest among all cultivars regardless of treatment. This result indicated that this cultivar had the highest uptake of O3. Therefore, the stable Pn of ‘Rose’ under O3 exposure was probably due to its ability to mitigate the induction of ROS, cell membrane damage, and decreased Chl due to O3 exposure (Biswas et al. 2008).
Significant interactions between treatment and cultivar were found in Fv/Fm and ETR (Fig. 3a, b). Under O3 fumigation, all cultivars had lower Fv/Fm and ETR, which indicated that electron transport of PSII was inhibited. Many studies have shown that the decrease of Pn caused by O3 is not related to stomatal limitation, but may be attributed to a decrease of Calvin cycle activity, damage of PSII, and obstruction of noncyclic electron transfer (Calatayud et al. 2003; Pleijel et al. 2006; Degl’Innocenti et al. 2007). Decline of Fv/Fm and ETR in the present study confirmed the inhibition of Pn was partially due to the damage of the light reaction process of photosynthesis. There were differential responses of Fv/Fm and ETR to O3 among all cultivars (Fig. 3a). The relative losses of Fv/Fm and ETR were lower in ‘Rose’ and ‘Pink’, while ‘Blush’ and ‘White’ had greater losses. There were significant interactions between treatment and cultivar in NPQ and qP (Fig. 3c, d). The increase of NPQ under O3 exposure denoted that one part of light energy absorbed by PSII dissipated in the form of heat. In addition, O3 decreased qP, which was in accordance with the finding of Ismail et al. (2014). The relative changes of NPQ and qP were significantly different among cultivars. ‘White’ had the highest relative changes while ‘Rose’ had the lowest values. The result indicated that the energy partitioning to photochemical processes could be kept at a stable level and consequently the photosynthesis was stable in ‘Rose’.
The maximal photochemical quantum yield of photosystem II (Fv/Fm, a), electron transport rate (ETR, b), The nonphotochemical quenching (NPQ, c), and coefficient of photochemical fluorescence quenching based on puddle model (qP, d) of four cultivars of H. phyllostachya under charcoal-filtered air (CF, 0 ppb) or acute O3 fumigation (O3, 120 ppb) for 14 days (8 h per day)
With regard to Y(II), there was marked interaction between O3 fumigation and cultivar (Fig. 4a). Y(II) was reduced by O3 stress. The relative losses of ‘White’ and ‘Blush’ were more than ‘Pink’ and ‘Rose’. Under O3 fumigation, Y(NPQ) and Y(NO) increased (Fig. 4b, c), but significant interaction was not found between treatment and cultivar. This result suggested that O3 stress resulted in photodamage despite the fact that heat dissipation was enhanced to alleviate the impairment of PSII reaction center (Zheng et al. 2010), and that the relative increases of heat dissipation and photodamage were similar among all cultivar.
Effective quantum yield of photosystem II (Y(II), a), quantum yield of light-induced non-photochemical fluorescence quenching (Y(NPQ), b) and quantum yield of non-regulated heat dissipation and fluorescence emission (Y(NO), c) of four cultivars of H. phyllostachya under charcoal-filtered air (CF, 0 ppb) or acute O3 fumigation (O3, 120 ppb) for 14 days (8 h per day)
There were significant interactions between treatment and cultivar on Chl a, Chl b and Car (Fig. 5a, b, d), but the interactive effects on total Chl content and Ant were not significant (Figs. 5c, 6a). Ozone fumigation significantly decreased the contents of Chl a, Chl b and Car. ‘White’ had highest relative losses, yet the lowest were found in ‘Rose’.
Chloroplasts are the most sensitive cell organelles to O3 (Saitanis et al. 2014). Our results showed that chloroplasts in ‘White’ were damaged more seriously than those of other cultivars. Previous studies proposed that Car was of importance in dissipating heat, scavenging ROS and suppressing lipid peroxidation, and the decreased content was related to the higher sensitivity to O3 stress (Brandão et al. 2017; He et al. 2017). In this study, the stable level of Car in ‘Rose’ after O3 exposure probably means a higher tolerance of this cultivar (Pellegrini et al. 2015). The contrary change trend of Car and NPQ illustrated that the decreased Car might be used for heat dissipation. Under O3 fumigation, the Ant content of all four cultivars increased (Fig. 6a). It has been pointed out that Ant could be used for preventing photoinhibition and removing free radicals (Gould et al. 2002; Zhang et al. 2017). Our results showed that ‘Rose’ and ‘White’ had the highest and lowest Ant content, respectively, regardless of treatment. Therefore, the protective roles of Ant such as photoprotection were probably highest in ‘Rose’ and lowest in ‘White’. The relative decreases of Fv/Fm, ETR and Y(II) confirmed this postulation.
Significant interaction between treatment and cultivar was observed in EL (Fig. 6b). The significant increase of EL showed cell membrane was damaged by O3 fumigation (Heath 2008). EL of ‘Rose’ under O3 exposure was not significantly different from that in CF air. However, ‘White’ had a marked increase (148.07%), followed by ‘Blush’ (77.55%) and ‘Pink’ (61.35%). Responses of EL in these cultivars to O3 had similar patterns to those of visible injury. This result confirmed that the observed visible injuries were at least partly due to cell membrane damage.
In conclusion, ‘Rose’ and ‘White’ had the highest and the lowest tolerance to O3 stress among the four cultivars. Ant probably caused the different O3 sensitivities due to its roles for the photoprotection.
References
Aoki C, Wada T, Nishimura T, Hattori K (1995) Characterization and inheritance of ‘Variegated-leaf’ mutant in Petunia hybrida. Jpn J Breed 45:31–35
Avnery S, Mauzerall DL, Liu J, Horowitz LW (2011) Global crop yield reductions due to surface ozone exposure: 1. Year 2000 crop production losses and economic damage. Atmos Environ 45:2284–2296
Biswas DK, Xu H, Li YG, Liu MZ, Chen YH, Sun JZ, Jiang GM (2008) Assessing the genetic relatedness of higher ozone sensitivity of modern wheat to its wild and cultivated progenitors/relatives. J Exp Bot 59:951–963
Brandão SE, Bulbovas P, Lima MEL, Domingos M (2017) Biochemical leaf traits as indicators of tolerance potential in tree species from the Brazilian Atlantic Forest against oxidative environmental stressors. Sci Total Environ 575:406–417
Calatayud A, Iglesias DJ, Talón M, Barreno E (2003) Effects of 2-month ozone exposure in spinach leaves on photosynthesis, antioxidant systems and lipid peroxidation. Plant Physiol Biochem 41:839–845
Degl’Innocenti E, Guidi L, Soldatini GF (2007) Effects of elevated ozone on chlorophyll a fluorescence in symptomatic and asymptomatic leaves of two tomato genotypes. Biol Plant 51:313–321
Feild TS, Lee DW, Holbrook NM (2001) Why leaves turn red in autumn. the role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiol 127:566–574
Feng ZW, Jin MH, Zhang FZ, Huang YZ (2003) Effects of ground-level ozone (O3) pollution on the yields of rice and winter wheat in the Yangtze River Delta. J Environ Sci 15:360–362
Gould KS, Neill SO, Vogelmann TC (2002) A unified explanation for anthocyanins in leaves? Adv Bot Res 37:167–192
Hao Z, Chang J, Xu Z (2004) Plant physiology experiment. Harbin Inst Technol Press, Harbin, pp 106–108 (in Chinese)
He J, Qin L, Chong EL, Choong TW, Lee SK (2017) Plant growth and photosynthetic characteristics of Mesembryanthemum crystallinum grown aeroponically under different blue-and red-LEDs. Front Plant Sci 8:1–13
Heath RL (2008) Modification of the biochemical pathways of plants induced by ozone: what are the varied routes to change? Environ Pollut 155:453–463
Hughes NM, Smith WK (2007) Attenuation of incident light in Galax urceolata (Diapensiaceae): concerted influence of adaxial and abaxial anthocyanic layers on photoprotection. Am J Bot 94:784–790
Innes JL, Skelly JM, Schaub M (2001) Ozone and broadleaved species: a guide to the identification of ozone-induced foliar injury. Haupt, Bern, pp 42–43
Iriti M, Faoro F (2008) Oxidative stress, the paradigm of ozone toxicity in plants and animals. Water Air Soil Pollut 187:285–301
Ismail IM, Basahi JM, Hassan IA (2014) Gas exchange and chlorophyll fluorescence of pea (Pisum sativum L.) plants in response to ambient ozone at a rural site in Egypt. Sci Total Environ 497–498:585–593
Kim J, Kang SW, Pak CH, Kim MS (2012) Changes in leaf variegation and coloration of English ivy and polka dot plant under various indoor light intensities. HortTechnology 22:49–55
Maamar B, Maatoug M, Iriti M, Dellal A (2015) Physiological effects of ozone exposure on De Colgar and Rechaiga II tomato (Solanum lycopersicum L.) cultivars. Environ Sci Pollut Res 22:12124–12132
Paoletti E (2007) Ozone impacts on forests. CAB Rev 2:1–13
Paoletti E, Ferrara AM, Calatayud V, Cerveró J, Giannetti F, Sanz-Sanchez MJ, Manning WJ (2009) Deciduous shrubs for ozone bioindication: Hibiscus syriacus as an example. Environ Pollut 157:865–870
Paoletti E, Castagna A, Ederli L, Pasqualini S, Ranieri A, Manning WJ (2014) Gene expression in snapbeans exposed to ozone and protected by ethylenediurea. Environ Pollut 193:1–5
Pellegrini E, Francini A, Lorenzini G, Nali C (2011) PSII photochemistry and carboxylation efficiency in Liriodendron tulipifera under ozone exposure. Environ Exp Bot 70:217–226
Pellegrini E, Francini A, Lorenzini G, Nali C (2015) Ecophysiological and antioxidant traits of Salvia officinalis under ozone stress. Environ Sci Pollut Res 22:13083–13093
Picchi V, Monga R, Marzuoli R, Gerosa G, Faoro F (2017) The ozone-like syndrome in durum wheat (Triticum durum Desf.): mechanisms underlying the different symptomatic responses of two sensitive cultivars. Plant Physiol Biochem 112:261–269
Pleijel H, Eriksen AB, Danielsson H, Bondesson N, Selldén G (2006) Differential ozone sensitivity in an old and a modern Swedish wheat cultivar––grain yield and quality, leaf chlorophyll and stomatal conductance. Environ Exp Bot 56:63–71
Ruhland CT, Fogal MJ, Buyarski CR, Krna MA (2007) Solar ultraviolet-B radiation increases phenolic content and ferric reducing antioxidant power in. Avena sativa. Molecules 12:1220–1232
Saitanis CJ, Bari SM, Burkey KO, Stamatelopoulos D, Agathokleous E (2014) Screening of Bangladeshi winter wheat (Triticum aestivum L.) cultivars for sensitivity to ozone. Environ Sci Pollut Res 21:13560–13571
Sitch S, Cox PM, Collins WJ, Huntingford C (2007) Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature 448:791–794
Svenson SE, Johnston DL (1994) Photoselective shading compounds regulate growth of Hypoestes phyllostachya Bak. HortScience 29:737–737
Tiwari S, Grote R, Churkina G, Butler T (2016) Ozone damage, detoxification and the role of isoprenoids-new impetus for integrated models. Funct Plant Biol 43:324–336
Tong L, Zhang HL, Yu J, He MM, Xu NB, Zhang JJ, Qian FZ, Feng JY, Xiao H (2017) Characteristics of surface ozone and nitrogen oxides at urban, suburban and rural sites in Ningbo, China. Atmos Res 187:57–68
Vaultier MN, Jolivet Y (2015) Ozone sensing and early signaling in plants: an outline from the cloud. Environ Exp Bot 114:144–152
Wang X, Qu BH, Lu YX, Yang HX (2016) Cloning and expression analysis of anthocyanins synthesis related gene PyANS of apple pear peel. North Hortic 5:108–112 (in Chinese)
Xu H, Chen SB, Biswas DK, Li YG, Jiang GM (2009) Photosynthetic and yield responses of an old and a modern winter wheat cultivar to short-term ozone exposure. Photosynthetica 47:247–254
Zhang L, Xu H, Yang JC, Li WD, Jiang GM, Li YG (2010) Photosynthetic characteristics of diploid honeysuckle (Lonicera japonica Thunb.) and its autotetraploid cultivar subjected to elevated ozone exposure. Photosynthetica 46:87–95
Zhang L, Xiao S, Chen Y, Xu H, Li Y, Zhang Y, Luan F (2017) Ozone sensitivity of four Pakchoi cultivars with different leaf colors: physiological and biochemical mechanisms. Photosynthetica 55:478–490
Zheng YF, Zhang JE, Wu RJ, Zhao Z, Hu CD (2010) Effects of ozone stress on photosynthesis and physiological characteristics of winter wheat in northern China. J Agro-Environ Sci 29:1429–1436 (in Chinese)
Acknowledgements
We would like to thank two anonymous reviewers for their helpful and constructive comments.
Funding
This study was financially supported by National Natural Science Foundation of China (31401895) and ‘Young Talents’ project of Northeast Agricultural University of China (14Q10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sui, J.X., Wen, M.X., Jia, L.L. et al. Effects of Elevated Ozone on Polka Dot Plant (Hypoestes phyllostachya) with Variegated Leaves. Bull Environ Contam Toxicol 99, 445–451 (2017). https://doi.org/10.1007/s00128-017-2163-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2163-x