Abstract
This study aims to evaluate the performance of different leaching schemes with respect to the mobilization of antimony and arsenic from polluted samples collected at different sites in Mansfeld District, Germany. Besides the elution by water the leaching by artificial acidic rain and by two different schemes of sequential extraction were employed for estimation of the mobilization of antimony and arsenic. The samples were characterized by X-ray fluorescence analysis for their total concentration of metalloids, metals and main constituents. It was found that both antimony and arsenic show little mobilization with de-ionized water as well as artificial acidic rain in single step batch procedures (≤ 0.13% of the total content). Although the percentage leached is very low, the concentrations in the resulting solutions are of ecotoxicological relevance. BCR procedure indicate a very strong binding of Sb and of As in the samples. Less than 20% of the total content can be leached in sum in all leaching steps, of it most under strongly oxidizing conditions. This scheme seems not suitable for a detailed investigation of possible mobilization processes under environmental conditions for the metalloids under investigation. The four-step extraction procedure by Wenzel et al. gives a more detailed pattern of the binding of antimony and arsenic. This procedure was found to be a suitable scheme for evaluating the possible mobilization processes from the samples contaminated by ore processing waste, especially by change through other ions or under reducing conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Different harmful metals and metalloids in natural systems originate from both anthropogenic and geological sources and are widely distributed in the environment. Hence, risk assessment related to environmental solids as sinks for trace elements is an important issue in environmental geology and management, agriculture and water pollution. Interaction of potential contaminants with inorganic and organic constituents of soils or sediments can lead to their immobilization and accumulation. The release of pollutants under changing environmental conditions can cause serious problems for water resources and living organisms inducing a perturbation of the ecosystem. However, mobility and bioavailability of metals and metalloids in soils and sediments depend strongly on their chemical forms and types of binding, i.e. sorption/desorption processes may significantly affect the action and toxicity of metals in a natural environment. Consequently, determination of total concentrations of these elements in solid samples is not sufficient to estimate potential risks of remobilization (and uptake of the elements by biota, which is not in focus of this study). Thus, procedures for distinguishing different binding forms of metals and metalloids are required. The identification and quantification of metals associated with predefined phases or soil compartments is defined as “fractionation analysis” according to the IUPAC recommendation (Templeton et al. 2000).
During recent years, the pollution of aquatic system by metals has attracted a lot of attention of the scientific community. Water soluble ions can easily mobilize, and maybe considered as highly available (Seguin et al. 2004). One approach, which is used to estimate the risk of the transfer of pollutants from soil/sediment to groundwater and surface water, is leaching the solid material with deionized water, as described in German standard procedure DIN 38414 – S4 (1984). This procedure simulates the initial contact of solid material with water under batch conditions. It reflects the concentration of the elements under study after attainment of an expected equilibrium (after 24 h). Some results, which evaluate this methodology – particularly the filtration of solutions – critically, were recently published (Meers et al. 2006).
To estimate the possible influence of changing pH on the mobilization of metals and metalloids the samples under investigation can be leached by simulated acidic rain (e.g. Long et al. 2006; Lager et al. 2005; Schreiber et al. 2005) or diluted hydrochloric acid (Larner et al. 2006) under batch conditions. Another possibility consists of the use of the so-called pH (stat) protocol at a fixed pH value, e.g. pH 4 for acidic conditions or pH 11 basic ones (Paschke et al. 1999; Hage and Mulder 2004).
An approach that has been widely applied is the fractionation of elements (or species) of interest into operationally defined forms under the sequential action of different extractants (Foerstner 1993). Most of these are based on the scheme introduced by Tessier et al. (1979). For more than 30 years a considerable number of sequential extraction procedures have been proposed for the fractionation of heavy metals, as summarized e.g. by Gleyzes et al. (2002), Filgueiras et al. (2002), and Smichowski et al. (2005). Selective extractants, used in the sequential extraction procedures, are aimed at the simulation of conditions whereby trace elements associated with certain components of the solids can be released. These operationally defined forms can help to estimate the amounts of metals and metalloids in different fractions that could be mobilized due to changes in chemical properties of the responsible matrix, e.g. soils, sediments, road dust.
In the literature are published some critical conclusions concerning the sequential extraction procedures. Main problems are (1) the phase overlapping, that is, the possibility of releasing metals associated to different geological phases by a single leaching agent and (2) the re-adsorption phenomenon, that is, trace element released by one extractant could associate with other undissolved solid components or freshly exposed surfaces within the time-scale of the extraction step (Bermond 2001). Depending upon the degree to which re-adsorption occurs, the contents of trace metals or metalloids bound to a given soil fraction will be underestimated, while the metal mobility in subsequent phases will be overestimated. Actually, the nominal “forms” determined by operational fractionation assists in the estimation of the amounts of elements in different reservoirs that can be mobilised under changes in the chemical properties of soil (Davidson et al. 1998).
Within the last 10 years the three-step BCR protocol, originally developed for the mobilization of ‘heavy metals’ in sediments (Ure et al. 1993), has been widely used to study the potentially mobilize element content of soils, sediments, dust, and sludge’s. It was also used for studying the leaching behavior of metals originating from mining waste (Perez and Valiente 2005; Margui et al. 2004). This scheme distinguishes between exchangeable, reducible or oxidizable leachable forms and a residual fraction. For this scheme one designed reference materials, which are available from several distributors.
Despite the fact that this protocol had been focused on the mobilization of ‘heavy metals’ we want to test it for samples collected in Mansfeld region, a former mining district in Central Germany, contaminated with metalloids (Sb, As), in presence of highly elevated concentrations of ecological harmful metals (e.g. Pb, Cu, Zn, Sn), which are also mobilized, in this work.
While arsenic has been in the focus of environmental concern for decades, elevated concentrations of antimony in polluted mining areas have recently become of interest. Both As and Sb co-occur in the environment. Both are also exist in two oxidation states As(III) / As(V) and Sb(III) / Sb(V). That means the oxidation state and form of metalloids themselves can also change during reduction and oxidation and thus during sequential extraction. These transformations primarily affect the fate and mobility of these elements.
It is generally assumed that arsenic and antimony have a similar geochemical behavior and a similar toxicity (Wilson et al. 2004; Gebel et al. 1998). Several years ago it was reported that only small amounts of Sb were found to be easily mobilizable from soils (Lintschinger et al. 1998). However, in a paper published recently (Gal et al. 2006) it is shown that Sb appears to be more mobilizable than arsenic and the pH value plays a crucial role for the mobilization of both elements.
With regard to the fractionation of the two elements of interest in this study, which should predominantly occur as anionic species, specific sequential extraction procedures have been proposed (e.g. Lombi et al. 2000; Keon et al. 2001). Wenzel et al. (2001) introduced an improved four-step sequential extraction procedure for the fractionation of arsenic based on a combination of reagents commonly used for the sequential extraction of metals and for Se and P. The sequence of extractants includes the following fractions: non-specifically sorbed, specifically sorbed, amorphous iron and aluminum oxides bound, crystalline iron and aluminum oxides bound. Because of the similarities of arsenic and antimony in binding to soil (sediment) compartments, this scheme was included into this study beside the BCR scheme as a second sequential procedure. However the procedures have been used widely to estimate the possible mobilization behavior of contaminants from soils or sediments, which is also in the focus of our investigations.
The aim of this study was to apply different leaching schemes to samples with elevated contents of antimony and arsenic and to evaluate their suitability for the description of the environmental fate of these two elements, especially possible mobilization processes, which is a worldwide task in Sb polluted mining areas (Murciego et al. 2007).
2 Materials and Methods
2.1 Samples and Reagents
The highly polluted samples originate from Mansfeld District, Germany, which has been effected by mining and smelting activities for more than 800 years. The copper ores (Kupferschiefer, a metalliferrous Permian black shale formation) had been processed for copper, silver and several other non-ferrous metals. The ore processing generated large amounts of waste dumped into tailings ponds, which are now covered. Furthermore, serious diffuse pollution by heavy metals, water, sediments, and soils at the region are impacted by elevated concentrations of arsenic and antimony (Wennrich et al. 2004; Schreck et al. 2005; Becker et al. 2001).
Two sediment samples (sample 1 and 2) and one polluted soil sample (sample 3) were taken from sites near abandoned tailings of copper ore processing waste.
Sample 1 and sample 3 were gathered between 0–30 cm and 10–20 cm, respectively. Sample 2 (0–30 cm) was overlaid by an organic horizon which was removed before sampling. After the sampling procedure the material was stored in polypropylene bottles in a refrigerator at 4°C. Before analysis the samples were dried at 80°C for 48 h, homogenized and afterwards sieved for a particle size < 63 μm. Sub-fractions of these samples were chosen for all leaching experiments. Additionally, a reference material, Chinese stream sediment GBW 07311, with elevated concentrations of arsenic as well as of antimony was included into the study for comparison.
All chemicals used were analytical grade reagents.
2.2 Characterization of Solid Samples
In order to get an overall view of the total element concentration the original materials were analyzed by energy-dispersive X-ray fluorescence spectrometry (EDXRF). Prior to determination of the total element concentration the samples were mixed with stearine wax (Hoechst) as a binder in a ratio 80:20 w/w, homogenized and subsequently pressed at 200 MPa to pellets (i.d. 32 mm). Afterwards the pellets were analyzed by EDXRF (X-LAB 2000, SPECTRO A.I.), Wennrich et al. (2004).
For the determination of total organic carbon (TOC) and total inorganic carbon (TIC) (each three replicates) about 100–150 mg of the material was incinerated in a RC-412 multiphase C/H/H2O-analyzer with IR-detector (LECO Corporation) with pure oxygen (99.5%; 2.8 b) for 200 s at 580°C and 150 s at 1,000°C for TOC and TIC, respectively.
For the determination of dissolved organic carbon (DOC) (three replicates) 7.5 g of each sample were shaken with 25 ml distilled water for 24 h at 30 rpm – modified German standard procedure DIN 38414 – S4 (1984) – and afterwards the resulting suspension was separated by centrifugation. The supernatant was filtered through a Minisart RC 15 syringe filter (Sartorius) with a pore size of 0.45 μm and analyzed by a LIQUITOC-analyzer with NDIR-detector (Foss-Heraeus).
2.3 Extraction Techniques
All applied extractions were carried out in three replicates. The grain size fraction < 63 μm of the original material was leached by operating two different extractants to perform the mobilization of Sb and As from soil/sediment in groundwater (as a single-step procedure). The German standard procedure DIN 38414 – S4 was modified a little. An amount of 7.5 g of the dried sample and 25 ml deionized water were shaken overhead (30 rpm) in polyethylene bottles for 24 h. In the second single-step extraction, 7.5 g of the dried material were eluted with 25 ml simulated acidic rain (pH = 4) using a 1:1 mixture of 5*.10−5 mol l−1 H2SO4 and 10−4 mol l−1 HNO3 in the same manner as described above. To minimize the problems of analyte losses by sorption of filter paper as reported by Meers et al. (2006) the suspensions were centrifuged, the supernatant was filtered (< 0.45 μm; cellulose acetate membrane, Sartorius) and afterwards divided into sub-samples for analysis.
Two different sequential extraction schemes were used in this study to ascertain the mobilization of Sb and As under changing conditions: the original three-step BCR procedure (Davidson et al. 1994; Thomas et al. 1994; Fiedler et al. 1994; Quevaullier et al. 1997), which had been optimized for the extraction of heavy metals, and the four-step scheme optimized for the extraction of arsenic from soil samples by Wenzel et al. (2001).
As shown in Tables 1 and 2 an amount of 1 and 5 g of each soil and sediment samples were leached using the BCR and the four-step extraction scheme, respectively. After each extraction step the suspensions were centrifuged, the supernatant was filtered (< 0.45 μm; cellulose membrane) and divided in sub-samples for analysis.
As announced before the residuals after the last step of the leaching were analyzed after drying by energy-dispersive X-ray fluorescence spectrometry (EDXRF) using a XLAB 2000 (Spectro A.I.). Only the residue of reference material (GBW 07311) after the four-step extraction scheme was analyzed by atomic absorption spectrometry after aqua regia leaching because of the low concentration of Sb and As. Here, the residue was leached using 1 ml of nitric acid (65% v/v) and 3 ml of hydrochloric acid (37% v/v), both suprapur quality (Merck), in a closed microwave digestion device (Multiwave, Perkin Elmer). The material was heated to 220°C within 20 min and held at this temperature for additional 20 min. After cooling down, the resulting solution was separated by centrifugation. The supernatant was filtered (< 0.45 μm), transferred to 50 ml polyethylene bottles and filled to volume with deionized water.
2.4 Analysis of Leachates
The concentration of the elements in all solutions and in the dissolved residue of GBW 07311 was analyzed by ICP-atomic emission spectrometry (CIROS, Spectro A.I.) – with LOD’s for Sb and As 50 μg l−1 – and by graphite furnace atomic absorption spectrometry (ZL 4100, Perkin Elmer) with LOD’s for Sb 0.4 μg l−1 and As 1.0 μg l−1 – , respectively (Wennrich et al. 2004). The measurements were performed in undiluted and diluted samples because of the wide range of concentrations of the different analytes.
3 Results and Discussion
3.1 Total Concentration of Elements
As announced before the samples chosen for these investigations were taken from a site strongly affected by mining and ore processing residues. In Table 3 the analytical results of total content determination are summarized. As can be seen from Table 3 the antimony and arsenic contents are rather high and the samples also have high concentration of harmful metals. The concentration of antimony is in the same range of other highly polluted sites (Lintschinger et al. 1998).
The very high concentrations of both As and Sb as well as the Pb, Zn and sulfur concentrations in percentage range are remarkable in these samples under study. The soil sample has a high Cu concentration as well as higher contents of some other metals, not typically for common soils. Additionally, all samples are characterized by high content of Al and Fe. This pattern is typical for the regional contamination originated from the deposit of Theisen sludge as described elsewhere (Schreck et al. 2005).
Unfortunately, there are no reference materials with such high concentrations of antimony. Therefore GBW 07311 was used in this study with certified values for total arsenic as well as total antimony. Certified values as well as XRF results are given in Table 3 for this reference material.
3.2 Carbon Content
Besides the main inorganic constituents of soils and sediments the carbon content (organic as well as inorganic) might be important for the leaching behavior of trace elements. Therefore, the samples were analyzed for the different forms of carbon. These results are given in Table 4. The total organic carbon (TOC) values and the dissolved organic carbon (DOC) values are comparable for all three samples. The only obvious difference is a higher total inorganic carbon (TIC) in the soil sample 3, which may have not a direct influence on the solubility of As and Sb. The carbonate is not a typical binding partner for arsenic and antimony compounds, but the buffer capacity may have an influence on the extractions e.g. with artificial rain water. The soluble fraction of the organic content (DOC) is very low in all cases. This means that a destruction of the organic matter like the use of H2O2 (see fraction 3 of the BCR procedure) is necessary to mobilize elements bond to this material.
3.3 Aqueous Extraction
Although it is undoubtedly important to know the total concentration of As and Sb in sediments and soils, these values do not give any information about the potential mobility of these two element under natural conditions. A first test was aimed to investigate a possible risk for ground and surface waters by polluted materials.
The contaminated samples under study as well as the reference material GB 07311 were used for the methodological investigations concerning the different leaching procedures. The content of Sb and As which were determined by EDXRF (Table 3) are declared as “total” concentration and used as reference for the further discussion of the leaching results.
Two different single-step extraction schemes were applied in accordance to the German standards procedure DIN 38414 – S4 (1984) (using deionizedwater) and an additional test applying artificial rain water (pH = 4). The results are shown in Table 5. Generally, antimony as well as arsenic seems to be little mobilizable under these two batch conditions. The dissolved portion (in comparison to the total concentration) is less than 0.13% for both elements in all samples as well as the reference material when shaking the mixtures within an exposure time of 24 h. Both elements are comparable mobilizable independent on the two leachants used. In an additional study (not shown here) a soil to leachant ratio of 1:10 was used for both extractions. It was found that the mobilized portion increases, as expected. Comparing these results by using water and ‘artificial acidic rain’ as eluents the concentrations of arsenic and antimony in the resulting solutions did not differ however clearly when using this ratio. This can be attributed to the buffer capacity of the soil and sediment samples under investigation, which results in pH values in the leachates between 6.7 and 7.5 using water and acidic rain as eluent. The buffering reaction seems to prevent an additional mobilization by applying acidic rain and on the other side the buffering reaction (dissolution of carbonates) does not seem to contribute to the mobilization of the metalloids.
Although the percentage leached is very low, the concentrations in the resulting solutions are of ecotoxicological relevance. For comparison the drinking water thresholds for arsenic and antimony, which are a criterion for estimation of the health risk, are: 10 μg l−1 for As (Council Directive 98/83/EC), and 6 μg l−1 (EPA maximum contaminant level – MCL; 2003) and 5 μg l−1 for Sb (both regulations). These thresholds were exceeded several times.
3.4 Sequential Extraction
Sequential leaching procedures may give a more complex picture regarding the binding and the mobilization behavior of the two elements under study in the samples. Different sequential extraction schemes were used for arsenic. Some of them based on conventional schemes to extract arsenic as an element that forms cations, e.g. Tessier et al. (1979), Dhoum and Evans (1998) and Van Herreweghe et al. (2003). Other schemes are linked with the anionic behavior of arsenic in soils, e.g. Van Herreweghe et al. (2003). Schemes based on the similar behavior with phosphates have been developed for As specifically, e.g. Wenzel et al. (2001).
Both different strategies were used in this study because of the possible more cationic behavior of Sb and the more anionic properties of As, having in mind that the oxidation state and form of metalloids themselves can also change during reduction and oxidation steps within the sequential leaching procedures, --the BCR procedure (see Tab. 1) under the aspect of cationic properties of As and Sb in the different matrices and the procedure proposed by Wenzel (Tab. 2).
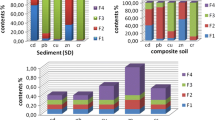
The results of the two different procedures are shown graphically in Figs. 1 and 2. The fractions are given in relation to the total concentration determined by EDXRF.
The recovery for both fractionation schemes is in the range between 80 and 120%, which is acceptable for such multi-step procedures. An outlier is the recovery of Sb from reference material in the four-step procedure (72% of total). This can be explained by the use of an aqua regia digestion during the last step instead of XRF due to concentrations below limits of detection for the EDXRF method.
3.4.1 BCR Procedure
Looking at the Sb distribution in the different fractions, a clear trend of very high concentrations in the residual fraction is observed for the BCR procedure for all samples. The same pattern of results is yielded for arsenic. Even less than 1% of As as well as Sb was mobilized from the reference material applying this scheme. If one uses this scheme for the other samples we found a different behavior. The mobilization rate is substantially higher compared with the reference material. Between 3% (sample 3) and 18% (sample 1) for As and between 6% (sample 1) and 12% (sample 3) for antimony, respectively, can be mobilized through ion exchange or under slightly reducing or oxidizing conditions from the samples. No uniform behavior for arsenic and antimony could be determined. Also clear differences can be recognized regarding the matrices, as shown in Fig. 1. Clearly visibly is, however, that the oxidizable fraction (fraction 3) is important in binding Sb as well as As with values up to 12%. This can be attributed to high concentration of sulfur and TOC in the three samples under investigation.
The TOC is in a range between 5 and 10% and the concentration of sulfur is between 1 and 8%, as been shown in Tables 3 and 4. The strong affinity of Sb to humic substances has been reported by Buschmann and Sigg (2004). Consequently, a destruction of the organic matter or oxidizing of sulfidic compounds, like it is being done in fraction 3 of the BCR procedure, can mobilize the metalloids in a remarkable amount.
3.4.2 4-Step Procedure
The four-step procedure gives a more differentiated pattern (see Fig. 2). Again, the exchange by other ions (fraction 1 and 2) is low, but much higher in comparison to the single step extraction. For example, almost 10% of Sb and about 15% of As had be exchanged by phosphate in sample 3. It is in accordance with a concentration in the solution of 0.3–0.5 and 7–8 mg l−1 for Sb and As, respectively, which is exceedingly high. That means parts of arsenic and antimony are leached in anionic form.
Most of As and Sb can be mobilized by dissolving the amorphous or poorly crystallized iron, aluminum, and manganese hydroxides (fraction 3 of the four-step procedure). About 50 and 60% of As was mobilized when extracting the two sediment samples 2 and 1, respectively. The leachable portions of Sb were distinct lower; 24 and 50%, respectively. The soil sample (sample 3) is characterized by a higher mobilization rate – about 80% of As and Sb were leached using ammonium oxalate in the third step. This is in contrast to the findings by Gal et al. (2006), who reported that Sb appears to be chemically more accessible than As despite the varied origin of the industrial soils.
A conclusion of the results is that the fourth step seems to be of minor importance for the highly polluted soil and sediments under study. The iron and aluminum hydroxides which bind the bulk of antimony as well as of arsenic seem not to be crystalline. This is in contrast to the reference material where 29% of Sb and 47% of As was found in this fraction. More detailed investigations are necessary to investigate this phenomenon. X-ray absorption fine-structure spectroscopy (e.g. XANES) would be the most suitable method for this. It will be object of further investigations.
However, there is also a quite large fraction of Sb, which can’t mobilize by leaching and is found in the residue fraction determined by XRF. For sample 2 more than 70% of the total antimony and about 30% of arsenic in the sediment is bonded in this fraction. This sample has the highest values for both Sb and As of the three selected materials as well as the highest concentrations of Zn, Pb and S.
Generally, the four-step procedure developed by Wenzel et al. (2001) was found to be much more suitable than the BCR procedure, for yielding information regarding the mobilization of antimony as well as of arsenic under changing environmental conditions. It gives a more differentiated picture than BCR procedure developed for heavy metals. However the metalloids bound to organic substances or be in a sulfidic form is not yield with this procedure, especially for the easily mobilizable fraction by exchange reactions. This fraction can be quiet large like in our case where both the antimony and the arsenic contaminations might originate from ore processing residues. From the point of view to characterize the environmental behavior, especially the mobilization of antimony and arsenic, this fraction might be of minor importance.
4 Conclusions
In general, single batch extraction procedures as with deionized water or artificial rain water could serve as suitable tools for an estimation of possible wash-out processes from soils and sediments polluted by metalloids into ground or surface waters. Under these conditions antimony and arsenic are only very little mobile in the contaminated samples under investigation. But despite this small solubility it can come to increased concentrations of both elements in the water body of the region.
For estimation of the amounts of As and Sb which could be mobilized under changing chemical conditions using the BCR scheme it was found that the easily-mobilized fraction is rather small in the samples. Up to 18 % of As and 12 % of Sb could be mobilized through ion exchange or under slightly reducing or oxidizing conditions from the samples. However strong oxidizing conditions as it is simulated by the fraction 3 of the BCR procedure are not likely under typical scenarios for creek sediments or soils in mining influenced areas.
The four-step sequential extraction developed by Wenzel et al. (2001) for the As binding form analysis was found to be also suitable for assessment of leaching of antimony bound in soils and sediments. However, the metalloids bound to organic substances or be in a sulfidic form are not mobilizable with this procedure. This fraction can be quiet large like in our case where both the antimony and the arsenic contaminations might originate from ore processing residues.
A substantial part of Sb and As is accessible by specific ion exchange against phosphate. However, the bulk of both antimony and arsenic can be mobilized by reductive resolution of the hydroxides.
References
Becker, A., Köck, W., Friese, K., Schreck, P., Treutler, H.-C., Spettel, B., et al. (2001). Lake Suesser See as a natural sink for heavy metals from copper mining. Journal of Geochemical Exploration, 74, 205–217.
Bermond, A. (2001). Limits of sequential extraction procedures re-examined with emphasis on the role of H+ ion reactivity. Analytica Chimica Acta, 445, 79–88.
Buschmann, J., & Sigg, L. (2004). Antimony (III) binding to humic substances: Influence of pH and type of humic acid. Environmental Science & Technology, 38, 4535–4541.
Council Directive 98/83/EC (1998). Quality of water intended for human consumption. Official Journal of the European Communities L 330/32.
Davidson, C. M., Duncan, A. L., Littlejohn, D., Ure, A. M., & Garden, L. M. (1998). A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land. Analytica Chimica Acta, 363, 45–55.
Davidson, C. M., Thomas, R. P., McVey, S. E., Perala, R., Littlejohn, D., & Ure, A. M. (1994). Evaluation of a sequential extraction procedure for the speciation of heavy metals in sediments. Analytica Chimica Acta, 291, 277–286.
Dhoum, R. T., & Evans, G. J. (1998). Evaluation of uranium and arsenic retention by soil from a low level radioactive waste management site using sequential extraction. Applied Geochemistry, 13, 415–420.
DIN 38414 – S4 (1984). German standards procedure: Determination of the leachability by water; German standards methods for the estimation of water, wastewater and sludge: Sludge and sediments (Group S) DIN 38414 part 4’ 1984 Fachgruppe Wasserchemie in der GDCh, Normausschuss Wasserwesen im DIN (Eds.) VCH, Weinheim.
EPA National Primary Drinking Water Standards (2003). http://www.epa.gov/safewater/consumer/pdf/mcl.pdf.
Fiedler, H. D. L., Quevauviller, P., Rauret, G., Muntau, H., Ure, A. M., Rubio, R., et al. (1994). Evaluation of a sequential extraction procedure for the determination of extractable trace metal contents in sediments. Fresenius’ Journal of Analytical Chemistry, 349, 808–814.
Filgueiras, A. V., Lavilla, I., & Bendicho, C. (2002). Chemical sequential extraction for metal partitioning in environmental solid samples. Journal of Environmental Monitoring, 4, 823–857.
Foerstner, U. (1993). Metal speciation – General concepts and applications. International Journal of Environmental Analytical Chemistry, 51, 5–23.
Gal, J., Hursthouse, A. S., & Cuthbert, S. J. (2006). Chemical availability of arsenic and antimony in industrial soils. Environmental Chemistry Letter, 3, 149–153.
Gebel, T. W., Suchenwirth, R. H. R., Bolten, C., & Dunkelberg, H. H. (1998). Human Biomonitoring of arsenic and antimony in case of an elevated geogenic exposure. Environmental Health Perspectives, 106, 33–41.
Gleyzes, C., Tellier, S., & Astruc, M. (2002). Fractionation studies of trace elements in contaminated soils and sediments: A review of sequential extraction procedures. TrAC Trends in Analytical Chemistry, 21, 451–467.
Hage, J. L. T., & Mulder, E. (2004). Preliminary assessment of three new European leaching tests. Waste Management, 24, 165–172.
Keon, N. E., Swartz, C. H., Brabander, D. J., Harvey, C., & Hemond, H. F. (2001). Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environmental Science & Technology, 35, 2778–2784.
Lager, T., Hamer, K., & Schulz, H. D. (2005). Mobility of heavy metals in harbour sediments: An environmental aspect for the reuse of contaminated dredged sediments. Environmental Geology, 48, 92–100.
Larner, B. L., Seen, A. J., & Townshend, A. T. (2006). Comparative study of oprimized BCR sequential extraction scheme and acid leaching of elements in the certified reference material NIST 2711. Analytica Chimica Acta, 556, 444–449.
Lintschinger, J., Michalke, B., Schulte-Hostede, S., & Schramel, P. (1998). Studies of speciation of antimony in soils contaminated by industrial activity. International Journal of Environmental Chemistry, 72, 11–25.
Lombi, E., Sletten, R. S., & Wenzel, W. W. (2000). Sequentially extracted arsenic from different size fractions of contaminated soils. Water Air Soil Pollution, 124, 319–332.
Long, X., Miro, M., & Hansen, E. L. (2006). On-line dynamic extraction and automated determination of readily bioavailable hexavalent chromium in solid substrates using micro-sequential injection bead-injection lab-on-valve hyphenated with electrothermal atomic absorption spectrometry. Analyst, 131, 132–140.
Margui, E., Salvado, V., Queralt, I., & Hidalgo, M. (2004). Comparison of three-stage sequential extraction and toxicity characteristic leaching tests to evaluate metal mobility in mining wastes. Analytica Chimica Acta, 524, 151–159.
Meers, E., Du Laing, G., Unamuno, V. G., Lesage, E., Tack, F. M. G., & Verloo, M. G. (2006). Water extractability of trace metals from soils: Some pitfalls. Water Air Soil Pollution, 176, 21–35.
Murciego, A. M., Sanchez, A. G., Gonzalez, M. A., Gil, E. P., Gordillo, C. T., Fernandez, J. C., et al. (2007). Antimony distribution and mobility in topsoils and plants (Cytisus striatus, Cistus Ladanifer and Dittrichia viscosy) from pollutes Sb-mining areas in Extremadura (Spain). Environmental Pollution, 145, 15–21.
Paschke, A., Morgenstern, P., & Wennrich, R. (1999). Comparison of 24 h and long-term pH(stat) leaching tests for heavy metal mobilisation from solid matrices. Acta Hydrochimica et Hydrobiologica, 27, 223–229.
Perez, G., & Valiente, M. (2005). Determination of pollution trends in an abandoned mining site by application of a multivariate statistical analysis to heavy metals fractionation using SM&T-SES. Journal of Environmental Monitoring, 7, 29–36.
Quevaullier, P., Rauret, G., Lopez-Sanchez, F. J., Rubio, R., Ure, A. M., & Muntau, H. (1997). Certification of trace metal extractable contents in a sediment reference material (CRM 601) following a three-step sequential extraction procedure. Science of the Total Environment, 205, 223–234.
Schreck, P., Schubert, M., Freyer, K., Treutler, H.-C., & Weiss, H. (2005). Multi-metal contaminated stream sediment in the Mansfeld mining district: Metal provenance and source detection. Geochemistry: Exploration Environment Analysis, 5, 51–57.
Schreiber, M., Otto, M., Fedotov, P. S., & Wennrich, R. (2005). Dynamic studies on the mobility of trace elements in soil and sediment samples influenced by dumping of residues of the flood in the Mulde River region in 2002. Chemosphere, 61, 107–115.
Seguin, V., Gagnon, C., & Courchesne, F. (2004). Changes in water extractable metals, pH and organic carbon concentrations at the soil-root interface of forested soils. Plant Soil, 260, 1–17.
Smichowski, P., Polla, G., & Gomez, D. (2005). Metal fractionation of atmospheric aerosols via sequential chemical extraction: A review. Analytical and Bioanalytical Chemistry, 381, 302–316.
Templeton, D. M., Ariese, F., Cornelis, R., Danielson, L.-G., Munau, H., van Leeuwen, H. P., et al. (2000). Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects and methodological approaches. (IUPAC Recommendations 2000). Pure and Applied Chemistry, 72, 1453–1470.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51, 844–851.
Thomas, R. P., Ure, A. M., Davidson, C. M., Littlejohn, D., Rauret, G., Rubio, R., et al. (1994). Three-stage sequential extraction procedure for the determination of metals in river sediments. Analytica Chimica Acta, 286, 423–429.
Ure, A. M., Quevaullier, Ph., Muntau, H., & Griepink, B. (1993). Speciation of heavy metals in soils and sediments – An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission the European Communities. International Journal of Environmental Analytical Chemistry, 51, 135–151.
Van Herreweghe, S., Swennen, R., Vandercasteele, C., & Cappuyns, V. (2003). Solid phase speciation of arsenic by sequential extraction in standard reference materials and industrially contaminated soil samples. Environmental Pollution, 122, 323–342.
Wennrich, R., Mattusch, J., Morgenstern, P., Freyer, K., Treutler, H.-C., Stärk, H.-J., et al. (2004). Characterization of sediments in an abandoned mining area; A case study of Mansfeld region, Germany. Environmental Geology, 45, 818–833.
Wenzel, W. W., Kirchbaumer, N., Prohaska, T., Stingeder, G., Lombi, E., & Adriano, D. C. (2001). Arsenic fractionation in soils using an improved sequential extraction procedure. Analytica Chimica Acta, 436, 309–323.
Wilson, N. J., Craw, D., & Hunter, K. (2004). Contributions of discharge from a historic antimony mine to metalloids content of river waters, Marlborough, New Zealand. Journal of Geochemical Exploration, 84, 127–139.
Acknowledgements
We are indebted to Ines Volkmann, Jürgen Steffen, Doris Sonntag and Jutta Froehlich (all UFZ) for the analytical assistance. The Deutsche Bundesstiftung Umwelt DBU (contract No. 2004/703) is greatly appreciated for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, K., Daus, B., Morgenstern, P. et al. Mobilization of Antimony and Arsenic in Soil and Sediment Samples – Evaluation of Different Leaching Procedures. Water Air Soil Pollut 183, 427–436 (2007). https://doi.org/10.1007/s11270-007-9391-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9391-3