Abstract
This review surveys schemes used to sequentially chemically fractionate metals and metalloids present in airborne particulate matter. It focuses mainly on sequential chemical fractionation schemes published over the last 15 years. These schemes have been classified into five main categories: (1) based on Tessier’s procedure, (2) based on Chester’s procedure, (3) based on Zatka’s procedure, (4) based on BCR procedure, and (5) other procedures. The operational characteristics as well as the state of the art in metal fractionation of airborne particulate matter, fly ashes and workroom aerosols, in terms of applications, optimizations and innovations, are also described. Many references to other works in this area are provided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most studies dealing with determinations of metals and metalloids in airborne particulate matter (APM) focus on the determination of total metal concentration without distinguishing the various species that are present [1, 2, 3, 4, 5]. It has become clear that this information, although needed to evaluate overall pollution levels, is insufficient, because the effect of a trace element in the environment strongly depends upon the associated form in the solid phase to which the element is bound [6].
Airborne particulate matter is introduced into the atmosphere through a variety of processes, including natural (crustal weathering, sea-salt aerosol generation, volcanism) and anthropogenic (fossil fuel combustion, industrial activity, incineration) sources [7]. Fly ashes generated in combustion processes are important carriers of hazardous substances (such as toxic metals) into the environment.
The environmental effects of aerosol particles depend upon their sizes and chemical compositions. Aerosol particles influence solar radiation transfer and cloud-aerosol interactions, and control the optical, electrical and radiative properties of the atmosphere. It is therefore crucially important to measure these two parameters [8]. Deposition, residence time, particle dispersion, transport and inhalation processes are predominantly influenced by the size of the particles. From this point of view, the first quantity sought in studies related to APM is the size distribution. Particles larger than 1.0 μm occur in the coarse mode; these are generally produced during mechanical processes and are rapidly removed (in timescales of hours to a few days) near the source by gravitational sedimentation. Small particles have a considerably longer residence time in the atmosphere and are much more efficiently transported [7]. To illustrate the importance of size distribution in APM, it is worth mentioning that the degree of respiratory penetration and retention is directly related to the aerodynamic particle size. Particles with aerodynamic diameters smaller than 1.0 μm are deposited in the alveolar regions of the lungs, where the adsorption efficiency for trace elements is 60–80% [9], and so this can affect lung physiology, especially if the particles contain biologically-available toxic metals [10].
Secondly, to evaluate the potential toxicities of trace elements, it is also necessary to know the distributions of their chemical forms, because the bioavailabilities of elements depend on their characteristic surfaces, on the strength of their bonds, and on the properties of solutions in contact with APM.
The growing interest in obtaining information about the mobilities, bioavailabilities, solubilities, metal cycles, fates, and toxicities of trace elements in environmental samples has prompted many research groups to design and investigate extraction schemes aimed at the sequential solubilization of metals bound to substrates such as soils, sediments and aerosols. Sequential chemical fractionation is therefore a way to determine the actual metal activity in the environment and provides a new perspective on analytical control [11]. As the usage of the terms “fractionation” became more widespread, a general definition was necessary. The International Union for Pure and Applied Chemistry (IUPAC) defined fractionation as the “process of classifying an analyte or group of analytes from a certain sample according to physical (e.g. size, solubility) or chemical (e.g. bonding, reactivity) properties” [12]. It should be noted that this definition does not strictly correspond to determining the chemical forms of elements, but rather to measuring broad forms of elements. Indeed, the results obtained by sequential extraction procedures are operationally defined (in other words the forms of the trace elements are defined by the scheme used for their determination).
The main objective of aerosol fractionation studies is the classification of metals in the solid matrix according to particle size and chemical bonding, in order to characterize metal mobility. Sequential extractions, although time-consuming, provide useful information about the origins, modes of occurrence, biological and physicochemical availabilities, mobilization and geological transport of trace metals [13]. In general terms, the fractions considered are exchangeable, weakly adsorbed, hydrous-oxide-bound, organically-linked, and the lattice material component. This classification provides useful information about how each element is partitioned among various compounds. A high content of an element in the first few fractions is indicative of elements of high mobility, and the environmental availability decreases with increasing fraction number. The exchangeable fraction corresponds to the form of the metal that is most available and can be released by merely changing the ionic strength of the medium. Metals bound to carbonates are sensitive to pH changes and can become mobilizable when the pH is lowered. The metal fraction bound to Fe–Mn oxides and organic matter can be mobilized with increasing reducing or oxidizing conditions in the environment. Finally, the metal associated with the residual fraction (like silicate) can only be mobilized as a result of weathering, and only long-term effects will be observed [13].
Bioavailability—the degree and rate that a substance is absorbed into a living system or is made available at the site of physiological activity—is the prime consideration in environmental risk assessment of toxicity. It depends on the intrinsic properties of the substances, the type of medium in which they occur, natural or man-made variations on the properties of that medium, and the biochemistries of the organisms at risk. The amount of potentially bioavailable trace elements in soils and sediments has been estimated from the water-soluble fraction and the fraction obtained with a dilute salt as extractant. Although both fractions were often satisfactorily correlated with the uptake of heavy metals by plants, similar studies that allow us to relate the content of an element in the soluble fraction to its bioavailabity are still lacking for APM. Environmental factors such as pH, temperature, redox potential, sailinity and the nature of the organic fraction of APM may influence the bioavailabilities of metals contained in aerosols.
Instrumental approaches based on the use of analytical techniques that directly measure the compositions of APM deposited on filters, fly ashes or dusts have also been used. These techniques have the limitation that they can only examine very small areas of the sample where the particles are generally not uniformly distributed, and phenomena of heterogeneity and encapsulation will produce erroneous evaluations. On the other hand, sequential chemical extraction methods can be applied to a larger area or to the entire filter, so non-uniform deposition or intraparticle phase variability issues are avoided.
Several sequential chemical extraction schemes have been reported in the literature. These procedures are usually based on the same principle of successive extractions of conceptually distinct lithological or authigenic fractions representing a range of elemental forms, from the most mobile to those strongly bound to the support mineral [14]. The extractant converts the metal bound in the solid phase into a soluble form. The fraction of the individual element in each phase depends on the chemical reagents used as the extractant and the chemical and physical operating parameters involved, such as pH, reagent type and concentration, time of contact, particle size, stirring system temperature, and so on.
Even though sequential chemical extraction procedures provide useful information in environmental studies and an increasing number of publications have appeared over the last few years, they also have some limitations and drawbacks. The selectivities of the chemical reagents toward specific forms of binding were not taken into account for most of the schemes reported. Also, the influence of the operational conditions should be critically evaluated when an extractant is selected, mainly because each extraction step could change the original distribution of species in the sample.
All of the fractionation schemes reported in the literature have been used to characterize pollution sources, to evaluate metal mobility and bioavailability, and to identify binding sites of metals in order to assess metal accumulation, pollution and transport mechanisms. In this review, the authors have classified the sequential chemical extraction methods for metal fractionation into five categories: (1) based on Tessier’s scheme, (2) based on Chester’s scheme, (3) based on Zatka’s scheme, (4) based on the BCR scheme, and (5) other schemes.
General considerations and sequential chemical extraction schemes
There are some factors that may influence the overall analytical procedure, and so it is important to consider the following points before selecting a fractionation scheme:
-
1.
Avoid a proliferation of unnecessary steps to minimize losses through chemical instability of the species or adsorption to laboratory ware.
-
2.
Control the risk of contamination from reagents for some ubiquitous species.
-
3.
The scheme adopted must allow the separation of the constituents attached to the APM on the surface from those incorporated in the matrix.
-
4.
Select suitable reagents with maximum extraction efficiency for each step.
-
5.
The choice of the extractant should be strictly correlated with the nature of the metal, its chemical form, and the characteristics of the matrix from which the compounds will be extracted.
-
6.
The selectivity of an extractant is influenced by its concentration, operational pH, liquid/solid ratio, shaking or stirring conditions and duration of the extraction process. Thermodynamic laws can be used to predict the efficiency of an extractant to dissolve or desorb elements from APM.
-
7.
Take into account the chemical properties of the extractant to avoid lack of selectivity.
-
8.
Study the effects of different parameters that can affect the extraction process.
-
9.
Sensitive and selective analytical techniques are necessary for quantification because elements are collected on filters in extremely low levels in most cases.
-
10.
Quantify the total concentration of each element in order to verify the mass balance.
In order to arrive at reliable results and correct evaluations, several analytical methods of separation and detection have been proposed for identifying and measuring different chemical forms of elements. The most popular methods of Tessier et al [15] and BCR [16] have been developed for metal fractionation in soils and sediments. In this regard, it is important to highlight the similarities and differences between these matrices and APM. Sediments contain high amounts of organic matter, Fe and Mn oxides and silicates, while atmospheric particles generally have significant levels of elemental carbon, sulfates, nitrates, chlorides, ammonium and organic compounds. It is evident that it is necessary to design specific fractionation schemes for APM.
Chemical fractionation procedures reported in the literature include a number of steps, between three and nine, and optimized reagents, reagent concentrations, pH values, temperatures, and extraction times. The number of fractionation steps required depends on the purpose of the study. In all of the schemes, different extractant reagents are applied in order of increasing reactivity. The sequential extraction procedure reported by Tessier et al [15] in 1979 and the BCR procedure [16] elaborated in 1993 by the European Community’s Bureau of References (now The Standards, Measurements and Testing Programme, SM&T) are the most representative procedures. Tessier’s scheme comprises four steps and was designed and widely applied to soil fractionation studies and (to a much more minor extent) to APM analysis. The fractions and the reagents used in this scheme are: exchangeable (1.0 mol l−1 magnesium chloride), bound to carbonates (1.0 mol l−1 sodium acetate), bound to Fe and Mn oxides (0.04 mol l−1 hydroxylamine chloride in 25% acetic acid), bound to organic matter (0.02 mol l−1 nitric acid + 30% hydrogen peroxide + 3.2 mol l−1 ammonium acetate in 20% nitric acid), and residual (hydrofluoric and perchloric acids). Without any doubt it is one of the most thoroughly investigated and analytically well-documented schemes. The BCR scheme allows the separation of extractable metals into three fractions: acid soluble (using 0.1 mol l−1 acetic acid), reducible (using 0.1 mol l−1 hydroxylamine hydrochloride), and oxidizable (using 8.8 mol l−1 hydrogen peroxide). It was proposed in an attempt to harmonize the different schemes and was first applied to the fractionation of Cd, Cr, Cu, Ni, Pb and Zn in sediments [16]. The Tessier and BCR schemes use (in general terms) the same extractants for the acid soluble and reducible fractions, but different concentrations and experimental conditions are applied, and consequently, the metal contents found are different. Tessier’s scheme has been subjected to different modifications, optimizations and improvements over the years in order to adapt it so that it can be used to extract metals from APM. These modifications have contributed to speeding up the conventional method and therefore improving sample throughput.
The schemes by Chester et al [17] and Zatka et al [18] were specifically designed for particulate matter. Chester’s procedure comprises three-stage sequential leaching in order to fractionate trace metals in filter-collected samples of urban and marine aerosols. Due to the carcinogenic effects of some Ni chemical forms, many specific extraction schemes for evaluating Ni occupational exposure have been proposed. Zatka et al [18] designed a method for Ni species separation in workroom air, and four fractions—soluble, sulfidic, metallic and oxidic—were considered.
Fractions obtained after sequential chemical fractionation of aerosols
Relatively labile elements with major potential bioavailabilities are first extracted with water. This first fraction contains the water-soluble species constituted by free ions and those ions that are complexed with soluble organic matter (chlorides, sulfates, nitrates, acetates, and so on). The percentage of each metal that is extracted in this fraction strongly depends on the properties of the metal. Fernández Espinosa et al [10] showed that trace metals in fine urban particles were extracted with water in the order: V > Ni > Co > Mn > Ca ≅ Cu > Cd > Mg > Ti > Fe. As a drawback, extractant water has a low buffering capacity. Other reagents have been used for this stage, such as magnesium chloride [15], ammonium acetate [17], and sodium chloride [19].
The exchangeable fraction contains metals that are weakly adsorbed onto the surface of the APM by relatively weak electrostatic interactions, and metals that can be removed by ion-exchange processes. The extraction efficiency depends on the properties of the cation, increasing in the order: H+ < Ca2+ < Mg2+ < Na+, NH +4 [20]. Acetate and chlorine salts are the reagents most used for leaching the exchangeable fraction. The metal complexing power of acetate prevents re-adsorption or precipitation of the metal ions released [13]. The pH is critical in this step; a sufficiently high pH is recommended in order to avoid protons competing or reacting with the other phases in the aerosols, but the pH should not be so high that hydroxides precipitate. Some specific reagents used in this stage are: magnesium chloride [15], sodium chloride [17], sodium acetate [21], ammonium citrate [18, 22, 23, 24] and EDTA [25]. Although ammonium acetate has been used [8, 26, 27, 28] in different fractionation schemes for APM, it has been criticized in soil fractionation studies because of difficulties in arriving at reliable analytical determinations when it is used [29].
Metals that are precipitated or coprecipitated with carbonate are collected in the acid soluble fraction [30] and are potentially bioavailable. A slightly more acidic solution is required in this step in order to decompose carbonates, liberate trace elements, and dissolve any free heavy metal carbonates. The carbonate form is a loosely-bound phase and liable to change with environmental conditions [31, 32]. Sodium acetate–acetic acid buffer (8 ml of NaOAc) used at a concentration of 1 mol l−1 and at a pH of 5 was used as extractant to release metals associated with carbonates in the Tessier et al [15] and Hansen et al [21] schemes. About 5 h are required to extract over 99.9% of the metals. At this stage, the BCR scheme uses a 0.11 mol l−1 acetic acid solution as extractant. Reducible oxides of Mn and Fe are extracted together in the reducible fraction using hydroxylamine hydrochloride in acetic acid medium (pH 2 is frequently used). At this stage, procedures differ only in minor operational details such as solid/liquid ratio, treatment time, and washing procedure. Mn(III) and Mn(IV) are reduced to soluble Mn(II) species. In a similar way, Fe(III) is reduced to the soluble Fe(II) species, and metal ions bound to oxy-compounds are also liberated into solution. A drawback commonly found is the release of substantial amounts of elements bound to organic matter, so the recovery of trace elements in the oxide fraction may be overestimated at the expense of the oxidizable fraction [33]. This mixture of reagents has also been used in other procedures to extract the acid-soluble and the reducible fractions simultaneously [10, 11, 17, 34]. Sodium pyrophosphate [33] or sodium dithionate-citrate [36] are other extractants that have been reported in the literature.
Some difficulties have been reported in accurately separating trace metals bound to organic matter. The organic fraction released in the oxidizable step is not considered to be very mobile or available and contains trace metals associated through complexation or bioaccumulation processes with organic matter. An oxidation step (hydrogen peroxide in acid medium) is usually applied to release metals bound to organic compounds and sulfides [15, 37].
More aggressive conditions are necessary to dissolve the residual/crustal trace metals held within the crystal structure of primary and secondary minerals. Silicate minerals, resistant sulfides, and small quantities of refractory organic material are the principal components found at this last stage. Some of the reagents proposed for this step are: HNO3, HClO4, HCl, HF [21, 27, 37]. The environmentally-mobile fractions bound to silicates can be interpreted as particle surface/particle matrix associations formed as a result of high temperature anthropogenic processes and low temperature crustal weathering processes.
An overall picture of the literature on this subject shows that the most popular techniques used to determine metal concentrations in the extracts have been: flame atomic absorption spectrometry (FAAS), electrothermal atomic absorption spectrometry (ETAAS), and inductively coupled plasma optical emission spectrometry (ICP OES). Inductively coupled plasma mass spectrometry (ICP-MS) has also been used by some researchers.
Table 1 summarizes the main characteristics, reagents and operating conditions of the more commonly used methods—Tessier, Chester, Zatka and BCR—that occur in the literature on the sequential chemical extraction of metals and metalloids in airborne particulate matter.
It would be obviously impracticable to cite, even briefly, all contributions on fractionation that have enriched the relevant literature to date. Nonetheless, the authors considered it important to mention some representative papers that have significantly contributed to the chemical fractionation of elements in aerosols and fly ash samples in this review. These papers, classified according to the different fractionation schemes used, are discussed briefly below.
Methods based on Tessier’s scheme
In a pioneering paper by Tessier et al [15], the authors developed and examined the merits of a method of sequential chemical extraction for partitioning trace metals into chemical forms likely to be released in solution under various environmental conditions. Cd, Co, Cu, Fe, Mn, Ni, Pb, and Zn were the elements considered, and the scheme was applied to bottom sediments because that matrix was more easily obtained in workable quantities than suspended matter. The method allows the separation of extractable metals into five fractions: exchangeable, bound to carbonates, bound to Fe and Mn oxides, and bound to organic matter and residual. The extractants used in this scheme are: (1) 1.0 mol l−1 magnesium chloride, (2) 1.0 mol l−1 sodium acetate, (3) 0.04 mol l−1 hydroxylamine + 25% acetic acid, (4) 0.02 mol l−1 nitric acid + 30% hydrogen peroxide and (5) hydrofluoric and perchloric acids. Hydroxylamine in acid medium is the most widely-used reagent for the easily reducible fraction. However, it releases significant amounts of elements bound to organic matter. For this reason, the recovery of trace elements in the oxide fraction may be overestimated [13]. Hydrogen peroxide applied to a heated medium is the reagent used in most schemes because it dissolves organic matter as a compromise between complete attack of organic matter and minimum alteration of silicates [15]. A drawback related to the use of this reagent is the possibility of metal re-adsorption into the residual fraction, which requires an additional stage with ammonium acetate at pH 2. The lack of selectivity of most reagents makes the efficient separation of trace metals bound to organic matter, sulfides and oxides using most sequential chemical extraction procedures tricky. For this reason, in the Tessier et al [15] scheme special attention was paid to the potential selectivity of each leaching solution. They evaluated the degree to which a desired sediment phase was solubilized and also the degree to which the remaining phases resisted attack.
Applications of Tessier’s scheme to atmospheric aerosols are described briefly below. The original sequential chemical extraction procedure was employed to determine the chemical forms and potential availabilities of Al, Cd, Co, Cr, Cu, Fe, Mn, Ni, P, Pb and Zn in incinerated sludge ash from an industrial urban area [14]. Metals in the extracts were determined by direct current Ar plasma (DCP). The study showed that all of the elements analyzed were relatively immobile, with residual chemical forms comprising between 70% (P) and 97.7% (Pb) of the total element concentrations. About 90% of the potentially toxic metals—Cd, Cr, Cu, Ni, Pb and Zn—was present in unavailable forms.
The importance of fractionation studies of fly ash is based on the fact that its potential toxicity is strongly related to the leachable fraction of contaminants, since the total toxic amount of fly ash is not extractable under natural environmental conditions. In this context, numerous sequential extraction procedures have been developed and applied with research or regulatory purposes and represent a valuable tool for disposal management. An extraction method that employed a kind of biosurfactant (saponins) was used for removing heavy metals from two different fly ashes [38]. Samples were treated with three triterpene-glycoside-type saponins in the pH range 4–9. Heavy metals were determined by ICP spectrometry, and ion chromatography (IC) was used for Cl− and SO 2−4 quantification. Results were compared with those from the HCl and EDTA treatment. Sequential extraction was performed on the fly ashes before and after the saponin treatment.
Procedures specifically designed for soils and sediments were adapted to the chemical fractionation of particulate matter. Nevertheless, the composition of APM and fly ash is different to that of soils and sediments, which contain high contents of organic matter, Fe and Mn oxides and silicates. For this reason, some steps of Tessier’s method have undergone various modifications over the years. Some of the modifications to the original procedure are described below.
Two fly ashes from biomass combustion were analyzed for Cd fractionation and mobility [21]. The sequential extraction procedure developed by Tessier was modified and six fractions were selected to define the distribution and partitioning of Cd: water soluble, exchangeable, precipitated as carbonate, bound in Fe and Mn oxides, organic-bound, and residual cadmium. The study demonstrated that Cd in fly ashes from straw combustion was most likely presented as soluble CdCl2, which was attributed to the high chloride content and to the neutral pH in the fly ashes. It was also found that Cd was easily to dissolve and so Cd would be mobile in the environment.
Obiols et al [19] modified Tessier’s scheme for sediments for use with particles suspended in air. This new four-step fractionation procedure was applied to samples of suspended particulate matter from an urban area of Barcelona, and it allowed the partitioning of extractable metals into four fractions: (1) soluble and exchangeable, (2) bound to carbonates and Fe–Mn oxides, (3) bound to organic matter, and (4) residual. In this scheme magnesium chloride was replaced by sodium chloride, while metal bound to carbonates and oxides were extracted in the same step. In the samples analyzed by FAAS, only Cd was predominant in the first fraction whereas the other metals were largely associated with carbonates and Fe–Mn oxides. The modification proposed by Obiols et al [19] was adopted by other researchers to investigate if metal fractionation was capable of determining and characterizing the principal pollution sources in an area using statistical analysis (principal components and cluster analysis) of pattern recognition [11]. The study showed that soil aerosols made the largest contribution to pollution, with Al and Fe as the most abundant metals acting as markers for this source. In addition, the close correlation between Cu and Pb (mainly appearing as oxides and carbonates) suggested that these metals were mainly pollutants generated by traffic.
Fernández Espinosa and his group carried out a series of comprehensive research studies based on a Tessier’s derived scheme (with several modifications) aimed specifically at the determination of trace metals in urban particles [10, 34, 37]. The objective of the optimization carried out was to find the most suitable experimental conditions for the fractionation scheme for urban particles, since the majority of the procedures used previously had been applied to sediments. The second objective was to extract the chemical forms from particles that could be biologically available to the human body. Therefore, the experimental conditions similar to those of deposition and solubilization into the lung were determined. The scheme consisted of selecting four fractions to define the distribution and partitioning of key metals: (1) soluble and exchangeable metals, (2) carbonates, oxides and reducible metals, (3) bound to organic matter, oxidizable and sulfidic metals, and (4) residual metals. Fernández Espinosa et al [34] optimized each stage of the leaching procedure for fine urban particles. The parameters investigated were: type of reagent, its concentration, pH, temperature and extraction time. A new fractionation scheme was proposed and the changes introduced were: (1) water was used instead of sodium chloride, (2) the concentration of hydroxylamine chloride was increased significantly from 0.04 to 0.25 mol l−1 and ambient temperature was selected instead of 85 °C, (3) the concentration of ammonium acetate was decreased from 3.2 to 2.5 mol l−1. The new experimental conditions of the two first stages were less aggressive than those used previously. This scheme was applied to another investigation where the chemical fractionation of 11 metals in aerosols was studied in 12 areas of the city of Seville (Spain) [10]. Urban aerosols were collected with a high-volume sampling system equipped with a cascade impactor, which effectively separated the particulate matter into six size ranges. Chemical fractionation was applied to particles of <0.61 μm and sample extractions were analyzed by ICP OES. The study showed that the metals with highest percentages in the different fractions were V (50.4%) in the soluble and exchangeable fraction, Ca (39.7%) in the carbonates, oxides and reducible fraction, Mg (59.2%) in the bound to organic matter, oxidizable and sulfidic fraction, and Fe (54.6%) in the residual fraction. Correlations between all of the variables and the results showed common sources for the metal fractions correlated with industrial activities and mainly vehicular traffic. In another study, a series of 41 samples of atmospheric particles below 0.61 μm were collected and 11 relevant metals were analyzed by ICP OES after separating the chemical forms through the scheme used for fine particles described above [39]. The data obtained were analyzed by multivariate statistical techniques and allowed the identification of the main contributing APM sources. In this context, it was observed that levels of the organic-sulfidic forms of Cd, Mn, Pb and Ti were highest on weekends and that sources from the sampling sites were influenced by local emission more than by transferred pollutions.
Methods based on Chester’s scheme
The atmosphere is recognized as being an important pathway for the transport of trace metals to the oceans [40]. Principal sources are low temperature crustal weathering, sea-salt generation and a variety of mainly high temperature anthropogenic processes, and eventually volcanic activity, rock volatilization, release from plant surfaces and forest fires. Chester et al [17] described a method for the fractionation of trace metals in filter-collected samples of marine and urban aerosols using a three-stage sequential leaching procedure. The method was specifically designed to provide information on how trace metals are distributed between loosely-held (environmentally mobile) and refractory (environmentally immobile) associations, with an additional stage for carbonate and oxide associations. The data obtained provided useful information on the source of the metals and on their potential mobility following the deposition of the aerosols to the sea surface. Concentrations of Al, Cu, Fe, Mn, Pb and Zn were determined by AAS in the three leaching solutions. In general terms, Cu, Pb and Zn were mostly found in loosely-held associations in the high-temperature-generated urban aerosols than in the low-temperature-generated crustal samples, whereas Al and Fe were generally refractory in aerosols from both populations. The importance of this method is based on the fact that it has demonstrated that aerosol fractionation data can be related to the solubility of an element in an aqueous medium, with metals in the loosely-held forms being most soluble, and that it can also provide a framework for assessing the reactivities of the elements once they have been deposited on the surface. The extractants used in this scheme are: (1) 1.0 mol l−1 ammonium acetate, (2) 1.0 mol l−1 hydroxylamine + 25% acetic acid, and (3) nitric and hydrofluoric acids. The use of acetate salts for leaching the soluble and exchangeable fractions presents a drawback to enhancing the extraction of transition metals because of the complexing power of acetate. Nevertheless, solutions of ammonium salts have the advantage of avoiding possible interference effects when FAAS and ETAAS are used for detection over alkaline and alkaline-earth salts. No information on either the reproducibility or accuracy of the three-step sequential leaching technique was reported.
Hlavay and his group performed several comprehensive studies on the chemical fractionation of elements in aerosol samples from Hungary [8, 27, 28]. Twelve environmentally-significant trace elements collected in Teflon filters were separated in three fractions (mobile, carbonate and oxide-associated and bound to silicate) to determine the elemental distribution patterns in a moderately polluted city and a regional background sampling site [27]. By using AAS for element quantification, it was found that Pb was the most abundant in the environmentally mobile fractions while Cd was the most abundant in environmentally immobile fractions. In another approach, the fractionation of aerosols according to particle size and chemical bonding was reported for the first time [28]. Eight particles size ranges were considered. The study revealed that several species of Cd, Cr and Pb were concentrated over the particle size range 0.18–0.71 μm. Metals followed different pattern distributions depending on the particle size, which is important from the point of view of the atmospheric transport of metals. It is important to highlight that Cd, Cr and Pb were adsorbed on sub-micrometer diameter particles and so they could be deposited in the pulmonary area. On the other hand, Cd compounds have mainly been found in environmentally mobile fractions, whereas As compounds accumulated in the environmentally immobile portions. Other investigations reported the distributions of elements in atmospheric aerosols collected on membrane filters over different size ranges [8]. Environmentally mobile fractions, fractions bound to carbonates and oxides, and fractions bound to silicates and organic matter (environmentally immobile) were obtained. The concentrations of ten elements were determined by ETAAS. Dry deposition rates and enrichment factors were calculated for the fine and coarse fractions as well as the mobile portion of the chemical bonding of the elements. The study demonstrated that the pollution load effectively characterizes the background area that the aerosols are transported from over long ranges from both anthropogenic and natural sources.
A capillary electrophoresis (CE) method with direct UV detection has been developed and validated to determine the distributions of metals in particulate matter following a sequential extraction procedure based on Chester’s method [26]. The work was performed in two experimental stages. In the first stage, CE conditions were established for the determination of Cd(II), Cu(II), Fe(II), Mn(II) and Zn(II) ions in high-strength matrices. Ions were separated using a background electrolyte consisting of 200 mmol l−1 ammonium acetate (pH 5.5), 0.5 mmol l−1 1,10-phenanthroline, 10 mmol l−1 hydroxylamine hydrochloric and 20% acetone. In the second stage, the reliability and applicability of the CE method was checked using NIST 1648 (urban air particulate matter) certified reference material.
Methods based on Zatka’s scheme
Many studies have been conducted to determine the Ni species in the workplace, in order to characterize employees’ exposure to Ni-containing aerosols. In work-room air, Ni was found at μg m−3 and mg m−3 levels. The identification and measurement of Ni species is of prime importance, because some compounds are carcinogenic whereas others are not [41]. Zatka et al [18] developed an approach to Ni fractionation that exploits the differences in chemical properties of the various Ni phases found in dusts from Ni-producing factories and workplaces. The scheme of fractionation differentiates among four groups of similarly reacting Ni compounds rather than individual species. This four-fold categorization of the wide variety of Ni compounds is quite satisfactory because a workplace dust sample from the Ni-producing or -using industry contains only a limited number of different Ni-bearing species. The authors recommend the use of quartz fiber filters for particle collection, and to avoid the use of glass fiber filters due to their natural surface alkalinity, which can cause soluble Ni compounds to form basic salts that are only partly soluble. Three selective leaches utilizing, in order, ammonium citrate (soluble Ni), hydrogen peroxide-ammonium citrate (sulfidic Ni), and bromine–methanol (metallic Ni) solutions as well as a final step with nitric and perchloric acids (oxidic Ni) were proposed. To leach soluble Ni compounds with ammonium citrate is preferable to procedures that use water in this step due to the buffering and chelating properties of this reagent. Undesirable hydrolysis of higher valent ions, such as Fe3+, and changes of pH during leaching are also minimized [18].
It is important to note that the leach selectivity and extraction efficiencies of the reagent solutions used were examined using pure commercial Ni compounds. The authors recommended paying special attention to the possibility of incomplete leaching of the species in any group because this would affect results in the following groups. The advantage of this approach is the specificity and that it was developed to overcome the intraparticle heterogeneity and encapsulation phenomena common in industrial dust, and the non-homogeneous distribution of individual species on aerosol filters.
A study was undertaken by Andersen et al [22] to investigate if workers from a Ni refinery were exposed to soluble Ni species. Since the method proposed by Zatka et al [18] was tedious and time-consuming, it was only applied by them to two phases: soluble and insoluble Ni. For residue digestion, a mixture of 4:2.1 of HNO3, HClO4 and H2SO4 was used. Compared with the method of Zatka et al [18], the latter was the sum of the sulfidic, metallic and oxidic Ni. Other metals, namely Co, Cu, Fe were determined by FAAS, and X-ray fluorescence (XRF) was used to analyze the sulfate in the ammonium citrate solution. Water soluble Ni was found in all samples ranging from 5–35%, and the sulfate in the solution correlated almost stoichiometrically to the total metal content. Dust composition based on X-ray diffraction analysis (XRD) was also reported. Some other investigations into the presence of Ni in industrial aerosols are also worth mentioning. Stationary dust and production samples were subject to the first two steps of Zatka’s method. Andersen and Svenes [24] reported the XRD spectrometric analysis of Ni extracts derived from the lungs of two refinery workers and from three stationary air samples collected in the workplace. Stationary dust samples and production samples were subjected to the first two steps of Zatka’s leaching scheme [18]. Nickel and other metals such as Co, Cr, Cu and Fe were determined in the extracts by ICP OES with a high resolution axial spectrometer [24]. The residue was analyzed by XRD. The study may indicate that the Ni remaining in the lungs some years after exposure was trevorite, and that this may be biologically inert.
Since there was no commercially-available sampler that could provide samples of sufficient quantity to enable chemical speciation for the species fractions of interest as well as particle size distribution information, a new instrumentation based on a modified version of the Andersen cascade impactor was developed by Vincent et al [23]. A porous foam media top stage was incorporated to give particle classification over the upper end of the inhalable range. The results obtained after chemical fractionation of the respirable, thoracic and inhalable fractions showed that the four Ni species groups were consistently uniform across the full range of particle-size distribution. ICP OES was used to analyze each of the four extracted fractions for Ni content.
Füchtjohann et al [42] designed a method based on Zatka’s scheme to distinguish between the four Ni species mentioned above in APM collected on cellulose nitrate filters. EDTA solution (instead of ammonium citrate), a mixture of diammonium citrate and hydrogen peroxide, and a KCuCl3 solution (instead of the mixture of anhydrous methanol and bromine) were used as leaching agents. For reduction of blanks, a new microscale filter holder placed in a closed FIA system was employed. Concentrations of the various Ni species in the low ng m−3 range down to the detection limits (0.1–0.3 ng ml−1) were determined by ETAAS and ICP-MS. The mean fraction of total Ni (sampling period of one month) was found to contain 36±20% of soluble, 6±4% of sulfidic, 11±15% of metallic, and 48±18% of oxidic Ni. These authors examined the selectivity of the method proposed. The degree of separation was evaluated by measuring the solubility of the sulfidic, metallic and oxidic species in each extraction phase. Even in the worse case, a co-extraction of lower than 3% was observed, which demonstrates the very good selectivity of each leaching step. No information on the compositions of the individual particles that were leached was reported.
Methods based on the BCR scheme
The BCR procedure has been proposed to harmonize and improve the extractants and procedures for chemical speciation analysis of heavy metals in soils and sediments. The BCR protocol was then applied to different kind of matrices such as sewage sludge [43, 44, 45, 46], soils [47, 48, 49, 50, 51] and river sediments [52, 53, 54]. The BCR scheme was used to certify the extractable trace metal contents of a sediment reference material (CRM 601). Although the procedure offered a means to ensure compatibility between data in these fields, some difficulties concerning reproducibility remained, and a new project was conducted to determine the cause of poor reproducibility in the extraction scheme prior to the certification of a new sediment and soil reference material [55]. The BCR scheme has shown lower matrix effects for a number of elements in comparison with the Tessier scheme regarding the analytical techniques used for element quantification (FAAS, ETAAS, ICP OES). Less attention has been paid to fractionation studies on APM using the BCR scheme, and some of these studies are restricted to only one metal.
A study was undertaken by Hlavay et al to monitor the natural environment by performing chemical fractionation on aerosols and sediments [56]. The reason for collecting precipitate and aerosol samples at the same time was to determine the distribution of elements in dry and wet depositions. The elemental concentrations of the solutions were determined by ETAAS and the method was successfully applied to describe environmentally mobile and stable fractions of toxic metals. Cadmium compounds were relatively common in the environmentally mobile fractions and arsenic compounds had accumulated almost evenly among portions.
The original BCR extraction protocol was modified and applied in order to study the partitioning of metals in two fly ash certified reference materials [57]. A first step, using water as extractant, was used to evaluate the risk of environmental pollution by emission of water-soluble species from coal fly ashes. The metal concentrations in the extracts were measured by ICP OES. Of the trace elements analyzed, B, Cr and Sr compounds were relatively easily extracted by water. The authors recommended incorporating more aggressive steps into the BCR scheme, since coal fly ashes are generated in a very aggressive combustion process.
Dabek-Zlotorzynska et al [58] determined the distribution of Cd, Cu, Fe, Mn and Zn species in APM after sequential small-scale extraction using BCR reagents. The work focused on (1) optimizing the experimental conditions for sensitive measuring of trace metals in the BCR extractants and (2) speeding up the BCR extraction by finding suitable sonication conditions. With the exception of Fe, the results achieved from ultrasonic extraction for all metals were reasonably comparable with those obtained using the conventional BCR procedure. It is important to remark that the overall extraction time was reduced considerably, from 48 to 2 h.
Table 2 illustrates the similarities and differences between the four main extraction schemes and their modified forms in regard to fractions and reagents used.
Other schemes
In an attempt to investigate the binding of heavy metals in thermally treated (sintering, vitrification) residues from waste incineration, a six-step sequential extraction procedure was developed [59]. Fly ashes separated from flue gas by electrostatic precipitation were treated in an assay furnace for 2 h and then the samples were cooled at ambient temperature. Subsequently, the thermally-treated fly ashes were leached following a procedure that enabled identification of the following fractions: (1) exchangeable cations (ammonium acetate solution), (2) carbonate fraction (sodium acetate solution), (3) easily reducible phase (hydroxylamine chloride solution), (4) reduction resistant phase (ammonium oxalate/oxalic acid solution), (5) sulfidic fraction (hydrogen peroxide and then ammonium acetate solution), (6) residual fraction (nitric acid). The sequential leaching study showed that, with the exception of Cr and Ni, the thermal treatment of fly ashes largely inertizes metals (Cd, Cu, Pb and Zn). The content of each metal in the different fractions was discussed.
Other authors have reported investigations aimed at studying the fractionation of airborne Ni in occupational exposure [60, 61, 62]. Samples of a standard fly ash (NIST-SRM 1633 coal fly ash) were subject to a highly specific nine-step procedure, and the Ni was quantified by voltammetry and characterized by cumulative extraction plots and dissolution kinetics [60]. The study was applied to two field samples of oil fly ash and it showed different Ni bioavailabilities.
A mild sequential extraction procedure utilized for size-fractionated aerosol samples collected on-filter, based on the solubility of As in water and phosphate solutions has been reported [63]. Aerosols were collected by sequential filtration into a coarse (about 10–2 μm) and a fine (<2 μm) fraction through membrane filters. The total amount of As was determined in a quarter of each filter by instrumental neutron activation analysis (INAA). Another part of each filter was treated with a specific sequential extraction procedure aimed to differentiate between water-extractable, phosphate-extractable and refractory chemical forms. Water-extractable forms were further differentiated into anionic As species by high performance liquid chromatography (HPLC)-hydride generation (HG)- atomic fluorescence spectroscopy (AFS). The As content in water depended on the aerosol size fraction (12% in the coarse fraction and 50% in the fine fraction) while their did not seem to be any significant dependency of the phosphate-extractable As on the aerosol size fraction (only 10–15%) in either size fraction. The remaining amount (about 78% of the coarse As and about 40% of the fine As) was considered to be refractory or environmentally immobile.
Thomassen et al [64] reported a specific four-step chemical fractionation procedure for characterizing workroom aerosols collected in plants producing Mn alloy. The following Mn compounds were quantified by ICP OES after extraction: (1) water soluble Mn using 0.01 M ammonium acetate, (2) Mn0 and Mn2+ using 25% acetic acid, (3) Mn3+ and Mn4+ using 0.5% hydroxylamine hydrochloride in 25% acetic acid, and (4) insoluble Mn digested in aqua regia and hydrofluoric acid. The authors reported that essentially complete dissolution of pure Mn compounds with well-defined stoichiometries occurred in the respective leaching steps, with detectable amounts <1% in others. Recoveries for the mixed quality control sample were in the range 92–97%. It was found that the predominant oxidation state in the inhalable aerosol fraction was Mn0 and Mn2+, independent of where the samples were collected.
Profumo et al [65] developed a series of sequential extraction procedures for separating inorganic metallic compounds of one element in emissions and working area particulate matter. The reagents selected depended on the metal partitioned. The sequential extraction of inorganic Cd species in particulate matter comprised the following compounds: Cd(II) salts, CdO, Cd(O), CdS, CsSe and Cd-aluminosilicates. The reagents used in each step when a synthetic mixture was evaluated were as follows: (1) water, (2) citrate buffer solution, (3) ammonium nitrate solution and, (4) nitric acid. Selective sequential solubilization procedures were developed depending on the presence of other concomitant metals. FAAS and GFAAS were used for Cd determination in the fractions. The fractionation of Se using a selective sequential scheme focused on the following species: Se(−II), Se(0) and Se(IV) [66]. These compounds are expected to be present in atmospheric particulates from the industrial production of metallic Se from the anodic sludges that arise from electrolytically refining Cu (which are particularly rich in Ag and Cu(I) selenide). A three-step sequential extraction procedure was used: (1) 0.1 mol l−1 sodium hydroxide and 0.5 mol l−1 sodium carbonate (for Se(IV) and/or Se(VI)); (2) 0.1 mol l−1 sodium sulfide (for Se0), and (3) 70% nitric acid (for Se(−II)). AAS and differential pulse cathodic stripping voltammetry (DPCSV) were used for the quantitative determination of selenium. Be(0), soluble Be(II) inorganic compounds, BeO and insoluble Be silicates were determined after a specific sequential extraction procedure [25] by ICP OES and ETAAS. The reagents used and the species extracted in the four steps were: (1) 0.01 mol l−1 hydrochloric acid (for Be(II) from soluble salts), (2) 0.1 mol l−1 copper sulfate (for Be(0) from metal), (3) concentrated nitric and sulfuric acids (for Be(II) from BeO), and (4) concentrated nitric and hydrofluoric acids (Be(II) from silicates). In a 2003 study, Profumo et al [67] developed a fractionation scheme to separate and determine Ni(0), Ni(II) soluble salts (such as sulfate and chloride), and Ni insoluble compounds (such as Ni oxide and sulfide) in APM from emissions and workplace air. The reagents used and the species extracted in each step are as follows: (1) water (for Ni (II) from soluble salts), (2) 0.01 mol l−1 ferric chloride and 0.1 mol l−1 hydrochloric acid (for Ni(0)), (3) 70% nitric acid and 37% hydrochloric acid (for Ni(II) from insoluble compounds) and (4) 48% hydrofluoric acid (for Ni(II) from highly insoluble compounds). Nickel was determined in each fraction by FAAS. The fractionation method was applied to the determination of Ni in fly ash derived from a solid waste incinerator and three reference materials from NIST: coal fly ash SRM 1633b, urban particulate matter 1648, and Washington dust 1649, followed by an evaluation of matrix spiking and recovery analysis. Recoveries from the different fractions ranged from 83 to 125%.
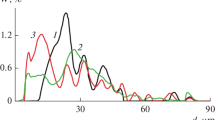
Table 3 shows sequential chemical extraction methods for metal partitioning that are used specifically for aerosols and fly ashes. Only relevant papers published over the period 1986–2004 are included. The table also includes sample characteristics, sample collection, elements analyzed and analytical techniques used. Finally, Fig. 1 shows the number of sequential chemical fractionation studies published for each element for the period 1986–2004.
Conclusions
This review has surveyed the various published schemes used for the sequential chemical fractionation of airborne particulate matter. Despite the limitations mentioned, sequential chemical extraction procedures provide a valuable tool for distinguishing between trace element fractions of different particle sizes and solubilities.
The lack of uniformity of the schemes rules out any comparison between results or procedure validations. For this reason, programs should be initiated by official organizations to harmonize and optimize the different sequential procedures in order to facilitate data comparisons as well as to optimize the operating conditions, and to introduce on-line procedures aimed at reducing reagent consumption and the time involved in the different steps. More studies on problems associated with re-adsorption/redistribution of elements among phases, robustness, and reproducibilities of sequential extraction procedures are also needed.
Development and validation of more specific methods of chemically fractionating the metals and metalloids in APM is urgently required in order to design better models for assessing and predicting the transport and fate of metals and metalloids in the atmospheric environment. There is room for improvement in the quality assurance associated with these studies; in particular there is a need for interlaboratory testing in regard to proficiency testing schemes and method performance studies. Furthermore, it goes without saying that there is both a need and a demand for size-fractionated APM reference materials whose extractable contents have been certified by this type of extraction procedure. The availability of data on the distribution of potentially toxic elements in APM will be a useful tool for developing environmental protection regulations and improved air quality monitoring strategies for health protection.
Abbreviations
- AFS:
-
Atomic fluorescence spectroscopy
- APM:
-
Airborne particulate matter
- ASV:
-
Anodic stripping voltammetry
- CE:
-
Capillary electrophoresis
- CPEV:
-
Carbon paste electrode voltammetry
- DCP:
-
Direct current plasma
- DPCSV:
-
Differential pulse cathodic stripping voltammetry
- ETAAS:
-
Electrothermal atomic absorption spectrometry
- FAAS:
-
Flame atomic absorption spectrometry
- HG:
-
Hydride generation
- HPLC:
-
High performance liquid chromatography
- IC:
-
Ion chromatography
- ICP MS:
-
Inductively coupled plasma mass spectrometry
- ICP OES:
-
Inductively coupled plasma optical emission spectrometry
- INAA:
-
Instrumental Neutron Activation Analysis
- XRD:
-
X-ray diffraction analysis
- XRF:
-
X-ray fluorescence
References
Boevski Iv, Daskalova N, Havezov I (2000) Spectrochim Acta 55B:1643–1657
Yang KX, Swami K, Husain L (2002) Spectrochim Acta 57B:73–84
Bocca B, Petrucci F, Alimonti A, Caroli S (2003) J Environ Monit 5:563–568
Goodarzy F, Sanei H, Duncan WF (2001) J Environ Monit 3:515–525
Smichowski N, Gómez DR, Dawidowski LE, Giné MF, Sánchez Bellato AC, Reich SL (2004) J Environ Monit 6:286–294
Ure AM, Davidson CM (2001) Chemical speciation in the environment. Blackie, Glasgow, UK
Nriagu JO (1989) Nature 338:47–49
Bikkes M, Polyák K, Hlavay J (2001) J Anal Atom Spectrom 16:74–81
Infante R, Acosta IL (1991) Atmos Environ 25B:121–131
Fernández Espinosa AJ, Ternero Rodríguez M, Barragán de la Rosa FJ, Jiménez Sánchez JC (2002) Atmos Environ 36:773–780
Fernández AJ, Ternero M, Barragán FJ, Jiménez JC (2000) Chemosphere 2:123–136
Templeton DM, Ariese F, Cornelis R, Danielsson L-G, Muntau H, Van Leeuwen HIP, Lobinski R (2000) Pure Appl Chem 72:1453–1470
Filgueiras AV, Lavilla I, Bendicho C (2002) J Environ Monit 4:823–857
Fraser JL, Lum KR (1983) Environ Sci Technol 17:52–54
Tessier A, Campbell PGC, Bisson M (1979) Anal Chem 7:844–851
Ure AM, Quevauviller PH, Muntau H, Griepink B (1993) Int J Environ Anal Chem 51:135–151
Chester R, Lin FJ, Murphy KJT (1989) Environ Technol Lett 10: 887–900
Zatka VJ, Warner JS, Maskery D (1992) Environ Sci Technol 26:138–144
Obiols J, Devesa R, Sol A (1986) Toxicol Enrivon Chem 13:121–128
Pickering WF (1986) Ore Geol Rev 1:83–146
Hansen HK, Pedersen AJ, Ottosen LM, Villumsen A (2001) Chemosphere 45:123–128
Andersen I, Berge SR, Resmann F (1998) Analyst 123:687–689
Vincent JH, Ramachandran G, Kerr SM (2001) J Environ Monit 3:565–574
Andersen I, Svenes K (2003) J Environ Monit 5:202–205
Profumo A, Spini G, Cucca L, Pesavento M (2002) Talanta 57:929–934
Dabek-Zlotorzynska E, Aranda-Rodriguez R, Buykx SEJ (2002) Anal Bioanal Chem 372:467–472
Hlavay J, Polyák K, Bódog I, Molnár Á, Mészáros E (1996) Fresen J Anal Chem 354:227–232
Hlavay J, Polyák K, Molnár Á, Mészáros E (1998) Analyst 123:859–863
Arunachalam J, Emons H, Krasnodebska B, Mohl C (1996) Sci Total Environ 181:147–159
Clevenger TE (1990) Water Air Soil Pollut 50:241–250
Stone M, Marsalek J (1996) Water Air Soil Pollut 87:149–159
Li X, Shen Z, Wai OWH, Li YS (2001) Mar Pollut Bull 42:215–223
Ahnstrom ZS, Parker DR (1999) Soil Soc Am 63:1650–1658
Fernández Espinosa AJ, Ternero Rodríguez M, Fernández Alvárez F, Barragán de la Rosa FJ, Jiménez Sánchez JC (2001) Toxicol Environ Chem 82:59–73
Clevenger TE, Saiwan Ch, Kolrtyohann SR (1991) Environ Sci Technol 25:1128–1133
Anderson BJ, Jenne EA (1970) Soil Sci 109:163–169
Fernández AJ (1998) Doctoral Thesis. University of Seville, Spain
Hong KJ, Tokunaga S, Ishigami Y, Kajiuchi T (2000) Chemosphere 41:345–352
Fernández Espinosa AJ, Ternero Rodríguez M, Fernández Alvárez F (2004) Atmos Environ 38:873–886
Chester R, Murphy KJT (1990) Metals in the marine atmosphere. In: Furnace R, Rainbow P (eds) Heavy metals in the marine environment. CRC, Boca Raton, FL
Wang XW, Imbra RJ, Costa M (1988) Cancer Res 48:6850–6854
Füchtjohann L, Jakubowski N, Gladtke D, Klockow D, Broekaert JAC (2001) J Environ Monit 3:681–687
Pérez Cid B, Fernández Alborés A, Fernández Gómez, Falqué López E (2001) Analyst 126:1304–1311
Conte RN, de Loos-Vollebregt MTC (2000) Anal Bioanal Chem 367:722–726
Pérez-Cid B, Lavilla I, Bendicho C (1998) Anal Chim Acta 360:35–41
Pérez-Cid B, Lavilla I, Bendicho C (1999) Fresen J Anal Chem 363:667–672
Campos E, Barahoma E, Lachica M, Mingorance MD (1998) Anal Chim Acta 369:235–243
Chen B, Shan X, Shen DQ, Mou SF (2001) Fresen J Anal Chem 357:941–945
Davidson CM, Duncan AL, Littlejohn, Ure AM, Garde LM (1998) Anal Chim Acta 363:45–55
Száková J, Tlustos, Balik J, Pavliková D, Vanek V (1999) Fresen J Anal Chem 363:494–595
Petit MD, Rucandio MI (1999) Anal Chim Acta 401: 283–291
Pardo P, López-Sánchez JF, Rauret G (1998) Anal Chim Acta 376:183–195
Polyák K, Hlavay J (1999) Fresen J Anal Chem 363:587–593
Belazi A, Davidson CM, Keating GE, Littlejohn D, McCartney M (1995) J Anal Atom Spectrom 10:233–240
Rauret G, López-Sánchez JF, Sahuquillo, Rubio R, Davidson C, Ure A, Quevauviller Ph (1999) J Environ Monit 1:57:61
Hlavay J, Polyák K, Weisz M (2001) J Environ Monit 3:74–80
Smeda A, Zyrnicki W (2002) Microchem J 72:9–16
Dabek-Zlotorzynska E, Kelly M, Chen H, Chakrabarti CL (2003) Anal Chim Acta 498:175–187
Wunsch P, Greilinger C, Bieniek D, Kettrup A (1996) Chemosphere 32:2211–2218
Wong JL, Qian J, Chen CH (1997) Anal Chim Acta 349:121–129
Wong JL, Wu T-G (1991) Environ Sci Technol 25:306–309
Liu A, Wong JL (2000) J Hazard Mater 74:25–35
Šlejkovec Z, Salma I, van Elteren JT, Zemplén-Papp É (2000) Fresen J Anal Chem 366:830–834
Thomassen Y, Ellingsen DG, Hetland S, Sand G (2001) J Environ Monit 3:555–559
Profumo A, Spini G, Cucca L, Zecca E (1998) Talanta 47:605–612
Profumo A, Spini G, Cucca L, Mannucci B (2001) Talanta 55:155–161
Profumo A, Spini G, Cucca L, Pesavento M (2003) Talanta 61:465–472
Acknowledgements
This work is part of CNEA-CAC Project P5-PID 36-2 and 36-4. Financial support was provided by the Agencia Nacional de Promoción Científica y Tecnológica through Project PICT-2000 N° 13-08772.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smichowski, P., Polla, G. & Gómez, D. Metal fractionation of atmospheric aerosols via sequential chemical extraction: a review. Anal Bioanal Chem 381, 302–316 (2005). https://doi.org/10.1007/s00216-004-2849-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-004-2849-x