Abstract
The accumulation of the heavy metals in the soil received a particular interest because of their toxicity and retention time in the soil which is slower than in other compartments of the biosphere. Knowledge of the total concentration of metals in soils and sediments is frequently insufficient to ascertain environmental risk. Simple and sequential extractions are useful tools for estimating the mobility of metals. In this study we were interested in highly toxic metals such as cadmium, lead, chromium, copper, and zinc in the soil of a controlled dump and the witness soil as well as the sediment of this dump in order to assess the mobility of these metals and their toxicity. The physicochemical parameters pH, organic matter, total calcium, cation exchange capacity, and total nitrogen were determined on the samples. Results show that average contents of heavy metals exceed the threshold recommended by the AFNOR NF U 44-041 standard. The results of the sequential extraction of heavy metals in the composite samples of soil and sediment according to the Community Bureau of Reference method show that cadmium is mainly associated with the exchangeable fraction (for sediment 77.7 % and for soil 40 %). Cadmium is therefore mainly associated with the mobile fraction and the risk of its transfer is high. Zinc is mainly bound to the metallic oxyhydroxides and carbonates, while Cu and Pb are mostly bound to organic matter and metallic oxyhydroxides in the proportions of 96.12 % (Cu) and 84.38 % (Pb), respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The soil is a natural resource that fulfills several vital functions for humans. It is an important pole of the water–soil–plant–atmosphere compartment. The role of soil and its functions depend closely on the nature and amount of these minerals and organic constituents that determine its properties and its physicochemical, mineralogical, and biological behavior (Mojahid 2007).Like the largest cities in Africa, Marrakech knew this last year’s an important industrial and population growth, resulting in an increase of waste production.
Some authors such as Mehdi et al. (2007), showed that discharges have a negative impact on the environment. The huge quantity and heterogeneity of urban waste (electronic waste, batteries, paper cartons, etc.) makes urban discharges a place of accumulation of heavy metals. During the last decades, heavy metals stemming from garbage dumps are very dangerous for the health of both humans and animals (Wang et al. 2003). For this reason, it is essential to measure the contents of heavy metals in the soil (Sirvens 2006). The determination of the total concentration of metals gives no information on their various forms. One of the most crucial properties of these metals which differentiates them from other toxic pollutants is that they are not biodegradable in the environment. Therefore, the determination and monitoring of specific chemical forms of heavy metals in the environmental samples such as airborne particulates, waters, biological materials, soils, or sediments are extremely important (Tokaliǒglu et al. 2000). The occurrence of metals in soils or sediments results from natural weathering processes affecting soils and rocks potentially with additional anthropogenic inputs. The fate of these metals in the environment depends on several factors, such as soils or sediment properties, (e.g., metal source, loading rate, soil pH, redox potential, texture, organic matter, and mineral composition), as well as external factors, such as chemical and biological processes. Metals can be bound in various ways. For example, they may be adsorbed on clay surfaces, or iron and manganese oxyhydroxides, and or, also present in the lattice of residual primary mineral phases (e.g., silicates) and or, secondary mineral phases, such as carbonates, sulfates, and oxides. Metals may also be bound in amorphous materials, such as iron and manganese oxyhydroxides, or complexed with organic matter (Gismera et al. 2004).
Heavy metals in the soil are distributed in the solid and liquid phases, and their speciation in every phase depends on several factors. Complexation by organic and inorganic ligands, the oxidation–reduction reactions, adsorption, ion exchange, and dissolution–precipitation phenomena are the main processes that determine the metal content in each phase. Therefore, these processes control the mobility and the bioavailability of heavy metals in grounds (Elass et al. 2003).
Speciation techniques have been widely used for the determination of heavy metals in sediments in the past two decades. These sequential extraction procedures are a useful tool for solid speciation of particulate elements, to study the origin, the fate, the biological and physicochemical availability, and the transport of sorbed elements. As many chemical extraction procedure have been proposed in the literature (Alvarez et al. 2006).
In our study, a sequential extraction method has been applied for heavy metals, in accordance with many chemical extraction procedures that are proposed in literature and by Community Bureau of Reference (BCR) (Tokalioglu et al. 2000):
-
Exchangeable and bound to carbonates Trace metals are adsorbed on the sediments or on their essential components namely clays, Fe and Mn hydrated oxides, and humic acids. The adsorption of trace metals is related to changes in water ionic composition which probably affects sorption–desorption processes. It is known that the carbonates of sediment contain significant trace metal concentrations and the concentrations are sensitive to changes of pH.
-
Reducible or bound to Fe and Mn oxides It is well known that Fe and Mn oxides are present as cement, concretions, or nodules between particles or only as a coating on particles. These oxides bind the trace metals and have strong scavenging efficiency for trace metals, but they are thermodynamically unstable under the anoxic circumstances.
-
Oxidizable or bound to organic substances The trace metals may be associated with various forms of organic material such as living organisms, detritus, or coatings on mineral particles. The complexation and peptization characteristics of the natural organic substances are well known. The organic substances may be broken up freeing the soluble trace metals in the natural waters under oxidizing conditions.
-
Residual The residual solid contains mainly primary and secondary minerals which occlude the trace metals within their crystal structures.
In Marrakech most of the dumps have been operated since 1987 by the Urban Community, to bury its municipal waste; it was selected based on the criteria that do not meet the requirements imposed by environmental protection and waste control that are developing at Morocco (Hakkou 2001). The landfill site is located on the right bank of the river “Oued Tensift”, on the national road "to Safi". It extends over an area of 14 ha and includes only one equipment as a fence 2 m high (urban district of Marrakech, June 2006). The dump of Marrakech is an uncontrolled discharge. Waste is deposited anarchically in the site without compaction or covering layer. Emissions of greenhouse gases and also nauseating odors are a source of nuisance for neighboring populations. They present risks to health and contribute to the degradation of water quality of the groundwater and the Oued Tensift. The present work consists of making an evaluation of heavy metals (lead, cadmium, copper, zinc, chromium, and arsenic) in the soil and the sediments of the final discharge of Marrakesh. The sequential and simple extractions were done to determine the risks of environmental pollution of these metals.
Materials and methods
Sampling
The municipal solid wastes from the Marrakech city are disposed in this landfill. Samples were taken from the soils (S1, S2, S3, S4, S5) and sediments (SD) coming from the decomposition of fermentable wastes that were collected from various localities of discharge from Morocco.
All the samples were air-dried, then passed through a 2-mm sieve before analysis and stored at 4 °C in polypropylene bottles. The use of air-dried materials in this study, does not present a problem, because the sediment samples were collected in oxic conditions (Kersten and Forstner 1986; Rapin et al. 1986).
Physical and chemical characteristics
Chemical properties such as pH, cation exchange capacity, organic matter, conductivity, total nitrogen, and limestone total of the concerned soils and sediments are also analyzed:
-
pH measurement The pH was determined in a solution of 10 g of waste in 25 mL of distilled water. After 30 min of agitation with the help of a magnetic agitator, the solution was left to rest for 2 h and the pH was measured afterwards with a pH meter, using the electrode-type CRISON pH 25 (norm ISO 10390, NF-X31-117).
-
Cation exchange capacity (CEC) The CEC is the maximum amount of cations that 100 g of dry soil can absorb. It was determined using the method of ammonium acetate CH3COONH4 (1 M, pH 7). Thirty milliliters of 1 M CH3COONH4 were added to 5 g of soil. The suspension was shaken for 2 h and then centrifuged (15 min, 6000 rpm). After centrifugation and filtration, the filtrate was transferred into a 100-mL flask and two other volumes of 30 mL ammonium acetate were added successively after 30 min of agitation and centrifugation. The final filtrates were completed to 100 mL with ammonium acetate solution.
-
Organique matter (OM) The content of OM was determined by calcination of the sample at 550 °C. A mass of 10 g was charred at 550 °C for 4 h in the electric oven of type Nabertherm controller P 320.
-
Electrical conductivity (EC) Measurement of the EC was performed on the extracts' salts of the samples. The extraction method is based on the displacement of the salts by stirring a suspension of the soil sample for 2 h. The suspension is left to stand before the conductivity measurement. The measuring device is a conductivity meter LF 330/SET of WTW brand.
-
Total nitrogen (NTK) was determined by the Kjeldahl method.
-
Total limestone The carbonates were determined according to AFNOR X 31-103. The method consists in solubilizing the carbonate (CaCO3) and the sample is attacked with concentrated hydrochloric acid solution. The volume of CO2 released is measured using the Calcimeter Bernard.
Single extractions
Total concentration of metals [cadmium (Cd), copper (Cu), zinc (Zn), chromium (Cr), and lead (Pb)] in the soil and sediment was determined by acid attacks using AFNOR method AFNOR X 31-151 which consists in the mineralization of soil and sediment samples; this operation is done by the calcination of 0.5 g of soil (finely crushed) in a muffle oven at 450 °C for 2 h. The obtained residue is dissolved in a beaker Teflon with 10 mL of hydrofluoric acid 50 %, and dried into dryness on a sand bath. The dissolution of the residue is made by 7.5 mL of hydrochloric acid and 2.5 mL of nitric acid for 2 h with lime. The resulting solution is adjusted to 10 mL by distilled water and kept in polyethylene pipe, then analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES).
Sequential extractions
A sequential extraction procedure for determination of the speciation of extractable heavy metals, proposed by (BCR) (Ure et al. 1993), has been applied to soils and sediments, based on the successive attacks of the samples by specific reagents on every fraction: Exchangeable and bound to carbonate fractions, the reducible fraction (metals bound to hydrated oxides of iron and manganese), oxidizable fraction (metals bound to organic matter), and the residual fraction. The heavy metals' contents were determined in the four fractions with the help of an ICP-AES. This method was chosen among several procedures because it was checked for selectivity, reproducibility, and repeatability of the different steps (Leleyter and Baraud 2006; Leleyter and Probst 1999) and it was commonly used in literature (Bur et al. 2009; N’guessan et al. 2009).
Sequential extraction was performed using the four-stage procedure (Table 1); for the exchangeable and acid soluble fractions (F1), about 1 g sample was extracted at room temperature with 40 mL of 0.11 M CH3COOH (quality from PROLABO) during 16 h. For the reducible fractions (F2), residue from (F1) was extracted at room temperature with 40 mL of 0.1 M NH2OH. HCl (quality from FLUKA) was extracted at pH 2 for 16 h. The extraction of the oxidizable portion (F3) was done by adding to the residue from (F2), 10 mL of 8.8 M H2O2 (pH 2) (quality from Riedel–de Haen). After 1 h, the mixture was heated at 90 °C for 1 h. And another amount of 10 mL of 8.8 M H2O2 was added and heated again at 90 °C during 1 h. The mixture was then cooled, 50 mL of 1 M CH3COONH4 was added and agitated for 16 h at room temperature. The residual fraction (F4) was extracted by regal water. After each extraction, the mixture was centrifuged (20 min, 10,000 rpm) and then the supernatant was filtered through a 0.45-μm membrane filter.
We are dedicated to determine the contents of metals which have a high toxicity, namely Pb, Cd, Cu, Zn, and Cr. The contents of heavy metals in the supernatants of the four fractions were determined by ICP-AES. The contents are expressed in mg/kg of dry matter. Our results are compared with those given by the AFNOR NF U 44-041.
Chemical analysis
Elemental analyses of the digests are carried out using (ICP-AES). The quality control of ICP-AES analyses was assessed by the analysis of blank reagents and calibration standards, prepared with commercially available solutions. Accuracy of ICP-AES measurements was determined with various certified reference materials (Rousseau et al. 2009).
Statistical analysis
The descriptive statistical parameters were calculated with the commercial statistics software package SPSS version 19.0 for Windows. Pearson’s correlation coefficient analysis, principal component analysis (PCA), and cluster analysis (CA) are the most common multivariate statistical methods used to identify the relationship among heavy metals in environmental studies and their possible sources (Franco-Uría et al. 2009; Lu et al. 2010). In the study, PCA was performed with Varimax rotation with Kaiser Meyer-Olkin normalization (KMO = 0.46). Clustering and calculations were performed using the Ward's method and by the Euclidean distances (Franco-Uría et al. 2009).
Results and discussion
The disparity of the results of soil samples and (SD) show the heterogeneity of the waste type (organic, medical, industrial…) (Kolédzi et al. 2011). The pH of the soil is a parameter which influences adsorption, the retention, and the movement of heavy metals in the ground (De Matos et al. 2001). As the organic acids are degraded, the pH increases to an almost neutral level, buffered by the bicarbonate system (Bozkurt et al. 1999). The pH of the samples is slightly alkaline except that of (SD) which is acidic. This alkalinity may be due to certain elements present as ions called exchangeable base (Ca2+, Mg2+, Na+, and K+), which increases the pH of the medium (Bodjona et al. 2012). It can also refer to bacterial hydrolysis of nitrogen with the production of ammonia (NH3) associated with protein degradation and decomposition of organic acids in the massif.
This observed pH is characteristic of the soil rich in OM. Also, the pH depends on the content of carbonates, therefore the soil is supposed to contain a significant content of CaCO3 and MnCO3. The alkaline pH increases the capacity of adsorption of oxides, manganese, and iron hydroxides which become goods and strong natural adsorbent (Bozkurt et al. 1999). Metals are thus adsorbed or coprecipitated. The OM content is low compared to those found in the literature; in fact our values are less than 59 % cited by ADEME (1999). But (SD) presents a peculiarity with high contents of OM (69.61 %) and nitrogen (5.2 %). This is probably due to intense farming activity in the region engendering waste rich of OM. The OM of the soil has an important role in the mobility of the metallic cations by complexation reactions which modify the accumulation and the toxicity of heavy metals. Some works (Bjerre and Schierup 1985) showed that the transfer of Cd is lower in soils with high OM content. Indeed, the increase of the organic load is accompanied by an increase in the (CEC) of the soil and reducing the metal transfer toward plants. What proves a relatively high CEC (17 meq/100 g) in the sample SD (Table 2).
Total content of metals
The total extracted fractions comprise both the residual and the non-residual portion of that fraction bound to silica. Table 3 shows that Cu, Zn, Pb, Cd, and Cr contents are equal to 425–526, 574–1134, 321–356, 0.72–4, and 99.69–576 ppm, respectively. These levels are far above the average concentrations in the limit values (150 ppm for the Cu, 150–300 ppm for Zn, 50–300 ppm for Pb, 1–3 ppm for Cd, and 2.677 ppm for Cr) (Kouame et al. 2006). The value obtained for Cr lies in the range of the average concentration of limit values. The soil is heavily polluted with Cd, Pb, and Zn that are part of the most dangerous industrial and municipal waste (Hasan 2007).
The concentrations of heavy metals in discharge of Marrakech (Table 4) are higher than those of discharge in literature like Al Ain (Howari 2004), Akouédo (Kouame et al. 2006), Sydney and Mall (New Jersey) (Suh et al. 2004). This difference in contents is probably due to the beginning of migration of the metals contained in the soil toward the depth of the discharge. For both samples of soil and sediment, Zn contents are very high. These results are in agreement with those of (Aulin and Neretnieks 1997) who estimate that the concentrations in Zn are 5–127 times higher in landfills than in the natural soil. These high contents are due to its use in numerous applications (electronic, chemical, electricity, pharmaceutical products, medical equipment, cosmetics, etc.) (Malayeri 1995). Except the Cr content in soil samples (S1 and S5, Table 2), all the tenures of heavy metals are above the threshold recommended by the standard AFNOR NF 44-041 U. These high contents may be due to the heterogeneous nature of household waste which is the source of metal pollution (Kolédzi et al. 2011), related to the rejection of materials that may contain Pb Cu, Cd, Zn, Cr, etc. Although these contents are higher than the norm, it is still insufficient to pronounce on the state of discharge pollution by these elements, since the total concentration of a potentially toxic element provides little information about the actual toxicity (Elass et al. 2003). To better assess the pollution due to these metals in the landfill, it is important to proceed with their sequential extraction to determine the chemical form in which each element is present and therefore predict their probable mobility in soil.
Sequential extraction
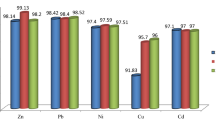
Splitting Cd, Pb, Cr, Cu, and Zn in the composite soil (obtained by homogenization of five soils S1, S2, S3, S4, and S5), sediment (SD) of the landfill, and witness soil is performed by BCR method. This method has enabled us to identify four fractions: the fraction bound to carbonates (F1), the fraction bound to metal (oxy) hydroxides (F2), the fraction bound to organic matter (F3), and the residual fraction (F4). Results are shown in, Fig. 1.
The distribution of heavy metals in various fractions (Fig. 1) is the same in the soil samples and those of sediments. In the exchangeable and acido-soluble fraction (F1), the Pb is present in 40 %, the Cd in 5 %, the Cu in 8 %, the Zn in 58 %, and the Cr is almost nonexistent in the samples of soils and sediments. The strong presence of the lead and the Zn in F1 is due to the fact that these two elements are often strongly adsorbed by carbonates and oxides and therefore have a high affinity vis-à-vis these fractions. Often, the reactions of dissolution/precipitation of carbonates favor trapping metallic ions in crystal forms (Blanchard 2000), this can be the cause of the strong presence of these elements in this fraction F1. Pb, Cd, and Zn are in high proportion in the reducible fraction (F2), respectively 43, 83, and 42 %. The disposition of Cd and Zn in F2 fraction constitutes serious environmental problems because of their high mobility (Yobouet et al. 2010). The Cu and Cr are plentiful in the oxidizable fraction (F3). The abundance of the Cu in F3 is due to the stability of the complexes formed by its ions with the OM of the soil by the association between the metallic cations and the functional groupings of humic substances. The classification made by Alloway (1995) relative to the stability of the complexes formed between the metal cations and the functional groups of the humic substances (Cu2+ > Fe2+ > Pb2+ > Ni2+ > Co2+ > Mn2+ > Zn2+) confirms the strong presence of Cu in the oxidizable fraction. The basic pH of the samples would probably also resulted in the dissolution of OM and the consecutive formation of organometallic complexes (Chaignon 2001). Since, variations of pH especially in the presence of organic and inorganic ligands have a considerable influence on the mobility of heavy metals.
The strong presence of Cr (90 %) in the oxidizable fraction have a very high risk to the environment and human health, because the Cr3+ is oxidized to Cr6+, which is more mobile and more toxic (Hamilton and Wetterham 1988). Trivalent chromium (Cr3+) is relatively little reactive and less toxic because of its slow kinetics of ligand exchange. Furthermore, the modification of the degree of oxidation of the ligands or the elements being bound with the metal, indirectly influences the solubility of heavy metals. Under reducing conditions, the sulfates are reduced to sulfides which gladly trap the chalcophile elements such as Zn, Pb, Cd, etc. (Deneux-Mustin et al. 2003).
All the studied metal elements are almost nonexistent in the residual fraction (F4). The absence of the metals studied in the residual fraction shows the risk of pollution of these elements in the environment, since the residual fraction contains metals which are unlikely to be released under normal environmental conditions.
The metals present in the soil matrix, behave differently according to their position in the different fractions. In some soil compartments, for example the oxidizable and residual fractions, metals are tightly bound, making them difficult to mobilize. On the other hand in other fractions which are exchangeable and acid-soluble (F1) and reducible (F2), metals are weakly adsorbed and thus can be easily mobilized.
However, quantifying the portion of metals contained in F1 and F2 supposedly mobilized in the samples of soil and sediment, allows estimating the risk of immediate pollution. This portion is very high for Pb, Cd, and Zn, respectively 83, 88, and 100 %. This estimate lets us to say that Pb, Cd, and Zn have a very high risk of pollution at the final Marrakech dump. A metallic element retained on the surface of a material (physisorption, complexation, precipitation, chemisorption) will be dissolved more quickly and therefore present a greater risk of toxicity if it is inserted into the crystal lattice of the material (Benard 2003).
Descriptive statistics
Pearson’s correlation analysis
Obtained correlation coefficients are presented in Table 5 as the linear correlation matrix. From this table, the heavy metals Cu, Pb, and Zn are positively correlated among themselves. Content of Cr is correlated with Zn and Cd. No correlation is observed between Cd and studied metals except Zn. According to Suresh et al. (2011) and Suresh et al. (2012), if the correlation coefficient between the metals is higher, metals have common sources, mutual dependence, and identical behavior during the transport. The absence of correlation among the other metals suggests that the contents of these metals are not controlled by a single factor.
Correlation analysis was conducted on heavy metal concentrations and all physicochemical parameters. Table 5 shows intensively significant correlations which were observed between (Zn, Pb, and Cu, r > 0.9), (Cd, Cr, and Zn, r > 0.9) and a significantly positive was also detected between Cd and Cr, r = 0.998.
A significantly positive correlation at P < 0.05 was found between the Pb–C.E (r = 0.771). On the other hand, a highly positive correlation was found between OM and NTK (r = 0.992), C.E and C.T (r = 0.775, P < 0.05). pH has a significantly negative correlation with OM and NTK at the 0.01 probability level (Fig. 2).
Multivariate statistical analysis results
Principal component analysis
PCA is an effective method to determine human impacts on a spatial scale (Facchinelli et al. 2001). In this study, using PCA (VARIMAX rotation mode) we identified three principal components (PC) for total metal concentrations and soil characteristics, representing 92.33 % of the total variance of the original dataset before and after rotation (Table 6). In addition, before applying PCA results to interpretations, Kaisere–Meyere–Olkin (KMO) and Bartlett’s sphericity tests were employed to examine the validity of PCA (Zhou et al. 2007). The first principal components (PC1) explains 32.77 % of the total variance and loads heavily on Cu, Zn, Cd, and Cr. PC 2, characterized by pH, OM, and NTK, explains 32.24 % of the total variance. PC3 is loaded by Pb, CT, and CE, accounting for 27.32 % of the total variance. The relations among the heavy metals based on the three factors are illustrated in Fig. 3 in three-dimensional space.
Hierarchical cluster analysis
CA is a multivariate technique, whose primary purpose is to classify the objects of the system into categories or clusters based on their similarities, and the objective is to find an optimal grouping for which the observations or objects within each cluster are similar, but the clusters are dissimilar from each other. The similarity of objects is quantified by distances in the multivariate space. The graphic representation of those distances is performed using dendograms where the magnitude of distances between two objects is represented by the vertical length of lines that connect both objects. The hierarchical clustering was enforced on the standardized data applying Ward’s method. The results of hierarchical clustering for the studied heavy metals are presented in Fig. 3. In this dendrogram, all 11 parameters are grouped into three statistically significant clusters. Cluster 1 consists of the parameters such as C.E, Cd, pH, CT, NTK, CEC, and OM; Cluster 2 consists of Cu–Pb–Cr; (3) Zn.
Conclusion
Through the various levies points of the soil samples and sediments at the final Marrakech discharge, we were able to know the evolution of physicochemical parameters and make an assessment of present heavy metals. It appears that the results differ from one sample to another considering the heterogeneity of the waste. Moreover, we found an alkaline pH in all samples except those of (SD) and the OM contents were rather high. This engendered strong accumulation of Cu and Cr in the oxidizable fraction. We noted that the increase of the organic load comes along with an increase in the (CEC) of the soil through the results of physicochemical parameters. Except for the content of Cr in soil samples (S1 and S5), all heavy metal contents are superior to the threshold recommended by the AFNOR standard NF U 44-041.
The results of the sequential extraction allowed finding an important proportion of Pb, Cd, and Zn in the fractions where they are easily mobilizable. This estimate allows us to say that these three elements have a very high risk of pollution at the final Marrakech discharge; this study on the final Marrakech dump allows envisaging a rehabilitation procedures of the landfill in order to preserve a healthy environment.
References
ADEME (1999) Les installations de stockage de déchets ménagers et assimilés: techniques et recommandations. ADEME Editions, Paris
Alloway BJ (1995) Heavy metals in soils, 2nd edn. Blackie Academic, Professional, London
Alvarez JM, Lopez-Valdivia LM, Novillo J, Obrador A, Rico MI (2006) Comparison of EDTA and sequential extraction tests for phytoavailability prediction of manganese and zinc in agricultural alkaline soils. Geoderma 132:450–463
Aulin C, Neretnieks I (1997) A material balance for an Industrial Landfill. In: Proceeding Sardinia, 5th international waste management and landfill symposium, vol 3. Cagliari, Italy, pp 173–180
Benard A (2003) Le plomb et le chrome dans les ciments: Spéciation et modélisation du transfert au cours de la lixiviation. Thèse de doctorat, Université de droit, d’économie et des sciences de Marseille, France
Bjerre GK, Schierup HH (1985) Influence of waterlogging on availability and uptake of heavy metals by oat grown in different soils. Plant Soil 88:45–56
Blanchard C (2000) Caractérisation de la mobilisation potentielle des polluants inorganiques dans les sols pollués. Thèse de doctorat, Institut national des sciences appliquées de Lyon, France
Bodjona B, Kili AK, Tchegueni S, Kennou B, Tchangbedji G, El Meray M (2012) Evaluation de la quantité des métaux lourds dans la décharge d’Agoè (Lomé-Togo): cas du plomb, cadmium, cuivre, nickel et zinc. Int J Biol Chem Sci 6(3):1368–1380
Bozkurt S, Moreno L, Neretnieks I (1999) Long-term fate of organics in waste deposits and its effect on metal release. Sci Total Environ 228:135–152. doi:10.1016/S0048-9697(99)00047-9
Bur T, Probst JL, N’guessan M, Probst A (2009) Distribution and origin of lead in stream sediments from small agricultural catchments draining Miocene molassic deposits (SW France). Appl Geochem 24:1324–1338
Chaignon V (2001) Biodisponibilité du cuivre dans la rhizosphère de différentes plantes cultivées. Cas de sols viticoles contaminés par des fongicides. Thèse: Ecole doctorale, Sciences de l’Environnement : Système Terre, Université d’Aix-Marseille, France
Deneux-Mustin S, Roussel-Debet S, Mustin C, Henner P, Munier-Lamy C, Colle C, Berthelin J, Garnier-Laplace J, Leyval C (2003) Mobilité et transfert racinaire des éléments en traces: influence des micro-organismes du sol. TEC & DOC, Paris
De Matos AT, Fontes MPF, Da Costa LM, Martinez MA (2001) Mobility of heavy metals as related to soil chemical and mineralogical characteristics of Brazilian soils. Environ Pollut 111:429-435
Elass K, Laachach A, Azzi M (2003) Etude de la biodisponibilité des métaux lourds dans les sols agricoles irrigués par des eaux pollués. Rev Francophone d’Ecologie Ind 32:1–6
Facchinelli A, Sacchi E, Mallen L (2001) Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ Pollut 114:313–324
Franco-Uría A, Lôpez-Mateo C, Roca E, Fernaíndez-Marcos ML (2009) Source identification of heavy metals in pasturel and by multivariate analysis in NW Spain. J Hazard Mater 165:1008–1015
Gismera MJ, Lacal J, da Silva P, Garcia R, Sevilla MT, Procopio JR (2004) Study of metal fractionation in river sediments. A comparison between kinetic and sequential extraction procedures. Environ Pollut 127:175–182
Hakkou R (2001) La décharge publique de Marrakech: caractérisation des lixiviats, étude de leur impact sur les ressources en eau et essais de traitement. Thèse de Doctorat d’État, Université Cadi Ayyad, Marrakech, Maroc
Hamilton JW, Wetterham KE (1988) Chromium. In: Seiler HG, Sigel H, Sigel A (eds) Handbook on toxicity of inorganic compounds. Marcel Dekker, New York, pp 239–241
Hasan HAH (2007) Role of rock phosphate in alleviation of heavy metals stress on Fusarium oxysporum. Plant Soil Environ 53(1):1–6
Howari F (2004) Heavy metal speciation and mobility assessment of arid soils in the vicinity of Al Ain landfill, United Arab Emirates. Int J Environ Pollut 22(6):721–731
Kersten M, Forstner U (1986) Chemical fractionation of heavy metals in anoxic estuarine and coastal sediments. Water Sci Technol 18:121–130
Kolédzi KE, Baba G, Feuillade G, Matejka G (2011) Caractérisation physique des déchets solides urbains à Lomé au Togo, dans la perspective du compostage décentralisé dans les quartiers. Rév Déchets Sci et Techniques Mars 59
Kouame IK et al (2006) Mobilité relative des métaux lourds issus de la décharge d’Akouédo et risque de contamination de la nappe du Continental Terminal (Abidjan-Côte d’Ivoire). Afrique Sci 2:39–56
Leleyter L, Baraud F (2006) Selectivity and efficiency of the acido-soluble extraction in sequential extraction procedure. Int J Soil Sci 1:168–170
Leleyter L, Probst JL (1999) A new sequential extraction procedure for the speciation of particulate trace elements in river sediments. Int J Environ Anal Chem 73:109–128
Lu XW, Wang LJ, Li LY, Lei K, Huang L, Kang D (2010) Multivariate statistical analysis of heavy metals in street dust of Baoji, NW China. J Hazard Mater 173:744–749
Malayeri EB (1995) Décontamination des sols contenant des métaux lourds à l’aide de plantes et microorganismes. Thèse de Doctorat, Université de Nancy I, France
Mehdi MM, Belabbed BE, Djabri L, Hani A, Laour R (2007) Caractérisation de la décharge publique de la ville de Tiaret et son impact sur la qualité des eaux souterraines. Courrier du Savoir 08:93–99
Mojahid Y (2007) Physic-chimie, mineralogy et dynamique du phosphore et du potassium dans quelques sols Marocains. these de doctorat Université Mohamed V Agdal Rabat, Maroc
N’guessan YM, Probst JL, Bur T, Probst A (2009) Trace elements in stream bed sediments from agricultural catchments (Gascogne region, S-W France): where do they come from? Sci Total Environ 407:2939–2952
Rapin F, Tessier A, Campbell PGC, Carignan R (1986) Potential artifacts in the determination of metal partitioning in sediments by a sequential extraction procedure. Environ Sci Technol 20:836–840
Rousseau C, Baraud F, Leleyter L, Gil O (2009) Cathodic protection by zinc sacrificial anodes: impact on marine sediment metallic contamination. J Hazard Mater 167:953–958
Sirven JB (2006) Détection de métaux lourds dans les sols par Spectroscopie d’Emission sur Plasma Induit par Laser (LIBS). Thèse de doctorat, Université de Bordeaux, France, pp 6–7
Suh J-Y (2004) Spatial distribution of heavy metals in soils and groundwater at the 2000 Olympic Games site Sydney, Australia. J Soil Groundw Environ 9(1):70–78
Suh JY, Birch GF, Hughes K, Matthai C (2004) Spatial distribution and source of heavy metals in reclaimed lands of Homebush Bay: the venue of the 2000 Olympic Games, Sydney, New South Wales. Aust J Earth Sci 51:53–67
Suresh G, Ramasamy V, Meenakshisundaram V, Venkatachalapathy R, Ponnusamy V (2011) Influence of mineralogical and heavy metal composition on natural radionuclide contents in the river sediments. Appl Radiat Isot 69:1466–1474
Suresh G, Sutharsan P, Ramasamy V, Venkatachalapathy R (2012) Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxicol Environ Saf. doi:10.1016/j.ecoenv.2012.06.027
Tokaliǒglu S, Kartal S, Elçi L (2000) Determination of heavy metals and their speciation in lake sediments by flame atomic absorption spectrometry after a four-stage sequential extraction procedure. Anal Chim Acta 413:33–40
Ure AM, Quevauviller PH, Munteau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European Communities. Int J Environ Anal Chem 51:135–151
Wang QR, Cui YS, Liu XM, Dong YT, Christie P (2003) Soil contamination and plant uptake of heavy metals at polluted sites in China. J Environ Sci Health Part A 38:823–838
Yobouet YA, Adouby K, Trokourey A, Yao B (2010) Cadmium, copper, lead and zinc speciation in contaminated soils. Int J Eng Sci Technol 2(5):802–812
Zhou F, Guo HC, Liu L (2007) Quantitative identification and source apportionment of anthropogenic heavy metals in marine sediment of Hong Kong. Environ Geol 53:295–305
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kennou, B., El Meray, M., Romane, A. et al. Assessment of heavy metal availability (Pb, Cu, Cr, Cd, Zn) and speciation in contaminated soils and sediment of discharge by sequential extraction. Environ Earth Sci 74, 5849–5858 (2015). https://doi.org/10.1007/s12665-015-4609-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4609-y