Abstract
A critical stage in the establishment of new individuals is seedling emergence and litter is a main factor affecting this stage. Recent research found that adults of Chuquiraga avellanedae are over-dispersed. Among several mechanisms, this pattern might be due to the negative influences of adults on seedlings through root competition. We performed field and glasshouse experiments to evaluate (i) the effects of C. avellanedae leaf litter and root presence on the emergence of conspecific seedlings, and (ii) the effects of leaf litter type (C. avellanedae litter, inert litter, no litter) and seed burial depth (seed at the surface or buried) on the emergence of C. avellanedae and Nassella tenuis (dominant grass) seedlings. The field experiment demonstrated root competition from adult plants on shrub seedlings, reducing seedling emergence. However, the effect of root competition did not differ between microsites (under-shrubs vs. between-shrubs). We dismiss the effect of allelopathy because inert litter (i.e., plastic leaves) had the same negative effect as C. avellanedae litter, indicating a mechanical effect. The glasshouse experiment revealed a species-specific response of seedling emergence. C. avellanedae litter limits the emergence of conspecific seedlings but was neutral with regard to the emergence of grass seedlings (N. tenuis). No differences in root competition between microsites and reduction of shrub seedlings by litter suggest that the over-dispersed pattern found for C. avellanedae is caused, at least partially, by litter effects on seedling emergence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A critical stage for the establishment of new individuals is seedling emergence (Fay and Schultz 2009). It is generally accepted that desert shrubs facilitate the establishment of new individuals by providing suitable microhabitats (Flores and Jurado 2003). However, in some cases, beneficial effects of shrubs are counteracted by negative effects such as root competition or allelopathy (Talamo et al. 2015). Litter is a main factor affecting seedling emergence because of its effects on the chemical and physical environments (Facelli and Pickett 1991b). Litter can exhibit both positive and negative effects on plants (Xiong and Nilsson 1999; Loydi et al. 2013, 2015). Increasing water and nutrient availability are among the positive effects (Carson and Peterson 1990; Brearley et al. 2003). In contrast, litter can negatively affect seedling emergence due to allelopathy (Bonanomi et al. 2006), acting like a mechanical barrier (Bosy and Reader 1995), reducing photon flux density (Facelli and Pickett 1991a; Jensen and Gutekunst 2003) and soil-seed contact (Chambers 2000; Liu et al. 2016).
Biotic interactions drive the structure and dynamics of plant communities (Armas and Pugnaire 2005). In arid ecosystems, plant cover is low and has a patchy pattern (Aguiar and Sala 1999). The balance between facilitation and competition is one of the factors affecting that patchiness (Aguiar and Sala 1994; Barbier et al. 2008). Nutrient enrichment, protection against herbivores, and ameliorated microclimate have been proposed as mechanisms behind positive interactions (Aguiar and Sala 1999; Gomez-Aparicio 2008; Nogueira et al. 2018; Vaz et al. 2019). Given the scarcity of water in these ecosystems, competition mostly refers to root competition between emerging seedlings and adult plants (Bisigato and Bertiller 2004a). Jurena and Archer (2003) found that the early growth of shrub seedlings was lower in treatments without root exclusion than in treatments where the roots of neighbors were excluded.
In north-eastern Patagonia the characteristic vegetation is a mosaic of herbaceous and shrub steppes where Chuquiraga avellanedae Lorentz (quilembai) is the dominant shrub and Nassella tenuis (Phil.) Barkworth (flechilla) is the dominant grass (Beeskow et al. 1995). Adults of C. avellanedae exhibit an over-dispersed spatial pattern (i.e., they are more separated in space than expected by chance), suggesting that at some stage of their life cycle they are subjected to negative plant interactions (Casalini and Bisigato 2018). This species produces a large mass of leaf litter with a high concentration of secondary metabolites (Campanella and Bertiller 2008), which may inhibit seedling emergence, and competition between emerging seedlings and established vegetation may also affect seedling recruitment (Bisigato and Bertiller, 2004a, b). Our first objective was to evaluate the effects of C. avellanedae leaf litter and root presence on the emergence of conspecific seedlings. We performed a field experiment to test the hypothesis that shrub emergence was negatively affected by C. avellanedae litter and/or root presence. Our second objective was to evaluate the effects of leaf litter type (C. avellanedae litter, inert litter, no litter) and seed burial depth (seed at the surface or buried) on the emergence of C. avellanedae and N. tenuis (grass) seedlings, and whether the effects are similar for the two species. We performed a glasshouse experiment to test the hypothesis that the effect of shrub litter on seedling emergence is allelopathic, that the burying of the seeds favors the emergence of seedlings, and that the effect of litter is similar for the two species.
Material and methods
Site description
The study site is located in north-eastern Patagonia (Argentina), 60 km southeastern Puerto Madryn (42°55′S, 64°33′W). The annual mean temperature is 12.7 °C and mean precipitation is 259.3 mm (1995–2004 and 2011–2014) (Campanella et al. 2016). The mean annual wind velocity at 10 cm above ground level is 4.6 m s−1 (Beeskow et al. 1995). Continuous grazing is practiced in paddocks greater than 2500 ha with a mean stocking rate of 0.3 sheep ha−1 (Chartier and Rostagno 2006). Wild herbivores, such as guanacos (Lama guanicoe), maras (Dolichotis patagonum) and lesser rhea (Rhea pennata pennata) are present but at very low densities. C. avellanedae is considered an unpalatable species (Siffredi 2012). Dominant soil in the study area is a Xeric Calciargid with a Xeric Haplocalcid as the subdominant soil (Chartier and Rostagno 2006). The characteristic vegetation is a mosaic of herbaceous and shrub steppes where C. avellanedae is the dominant shrub and N. tenuis is the dominant grass. In fact, these species represent 86.3% and 45.5% of total shrub and total grass cover, respectively (Beeskow et al. 1995).
Chuguiraga avellanedae description
Chuquiraga avellanedae Lorentz (Asteraceae) is an evergreen shrub typical of the Patagonian region (Bisigato et al. 2016). It has a hemispherical shape and reaches 1 m in height (Bertiller et al. 1991). This species exhibits a peak in biomass growth in September (Campanella and Bertiller 2009). The reproduction period occurs in the austral summer (Campanella and Bertiller 2008). Fruit maturation and achene dispersion take place during February (Bertiller et al. 1991). Leaf litterfall occurs throughout the year with a peak in July. Mean leaf litter production is 92 g m−2 canopy year−1 (Campanella and Bertiller 2010). Cover of C. avellanedae litter, visually estimated in 7 × 7 cm quadrats, was higher under C. avellanedae canopies than in-between shrubs. In fact, litter was almost absent from between-shrubs areas (under-shrubs: 81.5% ± 2.5%, between-shrubs: 0.7% ± 0.2%). The litter depth under C. avellanedae adults in the field was 1.11 ± 0.08 cm thick (mean ± SE), measured with a ruler. The wind rarely moves the litter once it reaches the ground below C. avellanedae plants because of its dense canopy (Campanella and Bertiller 2008).
Field experiment
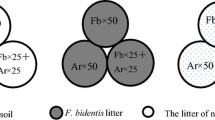
We performed a field seed sowing experiment to evaluate the effect of litter and root competition on seedling emergence. We conducted an experiment with two crossed factors: microsite (under-shrub with accumulated litter and between-shrubs without litter but with sparse grass cover) and root competition (with and without). We randomly selected 4 sites of the herbaceous steppe with shrubs community at a mean distance of 370 m ± 43 (SE) from each other (range = 320–459 m). At each site, we randomly chose six shrubs (Fig. 1). For each shrub, two microsites were selected: (1) under-shrub (with high litter accumulation) and (2) between-shrubs (with no litter). In each microsite, we randomly located one circular micro-plot of 10 cm diameter (12 circular micro-plots in each site). At half of the micro-plots, we inserted a PVC tube of 10 cm diameter and 20 cm length into the soil, to exclude root competition from neighboring plants (Bisigato and Bertiller 2004b). The final experimental layout was 2 microsites × 2 levels of root competition × 4 sites × 3 micro-plots. Ten seeds per micro-plot were sown in May at a depth of 1.5 cm (Cipriotti and Aguiar 2015). In under-shrub microsites, we carefully removed the litter layer, buried the seeds, and then returned the litter layer to its place. Soil characteristics between the two microsites (under-shrubs and between-shrubs) did not differ (Table S1). Monitoring was performed with a monthly frequency from June to December. Emergence was calculated as the proportion of seedlings emerged from sown seeds.
Schematic representation of the field experiment in one of the four selected sites. The distances among sites were 370 m. In a distance ranging between 3 and 5 m, six shrubs were randomly chosen. At each shrub, two microsites were defined: (1) under-shrub with high litter accumulation (indicated with gray ovals) and (2) between-shrubs, without litter accumulation. Between-shrubs microsites were located 50 cm outside the edge of the canopy. In each microsite, we delimited one micro-plot (indicated with small ovals), i.e., 12 micro-plot in each site. At half of the micro-plots, we pressed into the soil a PVC tube of 10 cm diameter and 20 cm length (indicated with cylinders), to avoid root competition from neighboring plants
Glasshouse experiment
We performed a glasshouse experiment to evaluate the possible allelopathic effect that litter has on seedling emergence. In this experiment, we also included seeds of the dominant grass species, N. tenuis. We conducted a fully-crossed experiment with 3 factors: species (two levels: C. avellanedae and N. tenuis), litter type (3 levels: C. avellanedae litter, inert litter and no litter) and seed burial depth (two levels: on the surface (seeds placed on top of soil or litter layer, depending on the treatment) and sown at 1.5 cm depth). The glasshouse experiment had 6 pots per treatment resulting in 72 pots in total (2 species × 3 litter type × 2 depth of sown × 6 pots). Pots were 8 cm in diameter and 10 cm in height. All pots were filled with dry soil. The soil was collected from the top 10 cm of several inter-shrub areas, sieved through a 2-mm mesh and homogenized. We decided to use inter-shrub soil to control for the possible accumulation of litter leachates in soil. In litter addition treatments including C. avellanedae and inert litter, we added 1 cm litter layer. Pots were randomly arrayed in the glasshouse and re-randomized periodically during the experiment.
Natural leaf litter was collected in May below 10 C. avellanedae plants. In the case of inert litter, we used plastic that mimicked the size and shape of natural leaf litter (Liu et al. 2016). Enhanced emergence in both natural and plastic litter with respect to controls without litter indicates positive physical effects (such as reduced evaporation), while decreased emergence in both kinds of litter is a sign of negative physical effects (e.g., acting as a mechanical barrier). Higher emergence in natural litter than in plastic litter can be attributed to positive chemical effects (such as nutrient release from litter) while the opposite suggests negative chemical (e.g., allelopathic compounds) or biological (e.g., fungus or pests) effects (Facelli and Pickett 1991b; Rotundo and Aguiar 2005).
Seeds were collected in the previous summer (February). Seed germination was evaluated in Petri dishes at the laboratory. The mean germination percentages were 83% and 69.2% for C. avellanedae and N. tenuis, respectively. We used 20 seeds per pot in the case of C. avellanedae while for N. tenuis we used 30 seeds per pot. Emergence was calculated as the percent proportion of seedlings emerged from sown seeds per pot. Seedlings were removed after emergence to avoid competition among emerged and emerging seedlings. The experiment started in September and lasted two-and-a-half months, which was after three weeks without emergence. Every day we checked the pots for emergence. Pots were maintained at field capacity through regular watering.
Statistical analysis
In the field experiment, differences in emergence (proportional data) were evaluated by Generalized Linear Models (GLM) using a binomial distribution with logit link function. Microsite and root competition were included as fixed factors in the model. Following the hierarchical structure of the design, we used a Generalized Linear Mixed Model (GLMM) including the micro-plots nested within sites as a random factor. However, we decided to remove the random effect because it did not significantly improve the model (χ2 = 0.37, P = 0.95). In the glasshouse experiment, we used Generalized Linear Models (GLM; binomial errors, logistic link). Species were separately analyzed. Litter type (with three levels) and seed burial depth (with two levels) were considered as fixed factors. In the two cases, we first fitted a full model taking into consideration all main effects and their interactions. We then reduced the full models by successive removal of non-significant interactions. The adequacy of the models was checked (Zuur et al. 2009) and analyses were performed in R (v. 3.5.0, R Core Team 2018), using the lme4 package (Bates et al. 2015).
Results
Field experiment
Seedling emergence was lower under-shrubs than in-between shrub areas without litter accumulation (Z = 3.92, P < 0.0001, Fig. 2; Table S2). C. avellanedae emergence was greater in the treatments without root competition in both microsites (Z = − 3.44, P < 0.0001). The interaction between microsite and root competition was not statistically significant (Z = 1.16, P = 0.17).
Glasshouse experiment
Emergence from buried seeds was greater in both species (Z = − 4.23, P < 0.0001 and Z = − 17.75, P < 0.0001 for C. avellanedae and N. tenuis, respectively; Fig. 3 and Tables S3 and S4) than unburied seeds. In N. tenuis, seedling emergence was greater for inert litter than in the treatment with Chuquiraga litter (Z = − 1.71, P < 0.0001) (Fig. 3a). However, no differences in emergence were found between the treatment without litter and both litter treatments (Z = 1.71, P = 0.08 and Z = 1.32, P = 0.18 for inert and Chuquiraga litter, respectively). By contrast, seedling emergence by the shrub species was lower in litter treatments than in the treatment without litter (Z = 2.70, P = 0.007 and Z = 3.22, P = 0.001 for inert and Chuquiraga litter, respectively; Fig. 3b). As we did not find differences in the emergence of C. avellanedae between the two types of litter (Chuquiraga and inert litter, Z = − 0.61, P = 0.54) and emergence was lower than without litter, we discard the effect of allelopathy and conclude that the effect of litter is mechanical.
Discussion
Both experiments indicate that C. avellanedae litter suppresses conspecific seedling emergence. Other studies have reported suppression of conspecifics by plant litter in mangroves (Chapman and Feller 2011), grasslands (Hovstad and Ohlson 2009), sclerophyllous forests (Cavieres et al. 2007), and crops (Singh et al. 1999). Different mechanisms have been proposed to explain litter effects on conspecific seedlings: the presence of allelopathic/toxic compounds such as cyanohydrin (Cavieres et al. 2007) and fragmented self-DNA (Mazzoleni et al. 2015), the formation of a mechanical barrier (Facelli and Pickett 1991b), and changes in light quantity and/or quality (Facelli and Pickett 1991a). Likewise, indirect effects of litter such as those mediated by a high abundance of pathogens and/or herbivores were reported by some authors (Facelli and Pickett 1991c). We discard the effect of allelopathy and pathogens or herbivores because inert litter had the same effect as the Chuquiraga litter and because we found no evidence of fungi or insect attack on emerging seedlings. Likewise, shading did not limit seedling emergence since our glasshouse experiment showed the greatest emergence when seeds were buried. Thus, C. avellanedae litter had a mechanical effect on seedlings instead of chemical effects. This result is consistent with the general finding that the effect of litter on emergence is physical rather than biological or chemical (Xiong and Nilsson 1999; Liu et al. 2016), especially in litter comprising leaves of evergreen species because of longer residence time (Facelli and Picket 1991b; Xiong and Nilsson 1999).
The glasshouse experiment revealed a species-specific response to litter effects on seedling emergence. C. avellanedae litter limited the emergence of its own seedlings but was neutral with regard to the emergence of the grass seedlings. This species-specific interaction may be due to differences in the growth rate of seedlings (Westoby et al. 1996). Other studies have revealed species-specific responses to litter that can be attributable to traits such as seedling morphology. In general, grasses are less prone to suppression by litter (Facelli and Pickett 1991b; Barrit and Facelli 2001).
At first glance, strong conspecific negative effects of litter are maladaptive. Similar results in other species have been attributed to the competitive pressure between parent and offspring in polycarpic species (Hovstad and Ohlson 2009). When this conflict is present, it selects for mechanisms that enhance spatial and temporal dispersal. Indeed, achenes of C. avellanedae have a pappus that enables long-distance dispersion (Andersen 1993).
More emergence from buried seeds is consistent with previous research findings that seedling emergence is greater when seeds are buried in the soil than when are on the soil surface or within the litter layer (Fowler 1986a; Facelli and Pickett 1991b; Ghermandi 1995; Chambers 2000; Rotundo and Aguiar 2005). Several studies have demonstrated the importance of good soil-seed contact for seedling emergence (Fowler 1986a; Chambers 2000; Liu et al 2016). Moreover, seed size/shape also determines the ability of seeds to germinate and emerge; small-seeded species do better on the soil surface in comparison to heavier seeded species (Merino-Martín et al. 2017). Consistently, N. tenuis presents smaller seeds and higher emergence on the soil surface with respect to C. avellanedae (average seed weights were 1.13 ± 0.05 mg vs. 14 ± 0.2 mg, respectively).
Our field experiment proved the existence of root competition by adults on shrub seedlings, causing reduced seedling emergence. However, this competition was equally important at both microsites. Inter-shrub areas on the steppe are colonized by tussock grasses (mostly Nassella tenuis). In this community, the average distance among grasses is 20 cm (Chartier et al. 2013). Since the root system of N. tenuis is very shallow (first 30 cm of soil, Bertiller et al. 1991), this species has the potential to compete with shrub seedlings (Busso 1997). Evidence of root competition between established plants and seedlings has been previously reported (Aguiar et al. 1992; Callaway et al. 1996; Rey Benayas et al. 2007). In fact, root competition is a frequent phenomenon in arid ecosystems (Fowler 1986b; Goldberg and Barton 1992; Cipriotti and Aguiar 2005), and Cipriotti and Aguiar (2015) found that it is as intense under shrub-dominated patches as between them.
In contrast to our results, most previous studies carried out in Patagonia showed no effect (Defossé et al. 1997; Bosco et al. 2015) or a positive effect (Rotundo and Aguiar 2005; Franzese et al. 2009; Bosco et al. 2015, 2018) of litter on seedling emergence and establishment. Most of these studies evaluated the effects of grass litter on grass emergence and establishment. Only Bosco et al. (2018) and Franzese et al. (2009) evaluated litter effects on shrub emergence and establishment. These researchers found that the effects of litter are species-specific. While the emergence of Larrea divaricata and Senecio bracteolatus were enhanced by litter (Bosco et al. 2015; Franzese et al. 2009, respectively), the emergence of Atripex lampa and Schinus johnstonii were reduced and unaffected, respectively (Bosco et al. 2015). Litter quantity is not the reason for the difference between our study and the previous one because we used 1 cm depth litter as in Bosco et al. (2015). Thus, the contrast between our results and those previously obtained in Patagonia can only be due to differences in litter type. Chuquiraga avellanedae has both the highest leaf mass area (216.27 g m−2) and leaf area (61.89 mm2) among shrubs in NE Patagonia (Campanella and Bertiller 2008). Large, heavy leaves can be a more difficult mechanical barrier for seedlings to traverse than small and light leaves (Winn 1985). In support of the latter, Xiong and Nilsson (1999) found that grass litter had weaker effects than tree and forb litter on seedlings.
Over-dispersed (or regular) patterns in desert shrubs have been commonly ascribed to competition (Fowler 1986b). Our results demonstrate the existence of an alternative mechanism: negative conspecific effects of plant litter. Some authors have noted that competition can be confounded by negative litter effects (Xiong and Nilsson 1999). However, as far as we know, this mechanism has not been previously proposed as influencing intra-specific spatial patterns and over-dispersed distributions.
Although our work shows unequivocal evidence of conspecific effects of C. avellanedae litter on seedling emergence, future studies should consider aspects not contemplated in this work. For example, it remains to be determined if early differences in emergence are maintained over time. Likewise, as climate can influence interactions among plants (Cipriotti and Aguiar 2015), our field experiment should be repeated under different climatic conditions. Annual precipitation during the year of the study was 32% below long-term average. However, lower precipitation during the field experiment agrees with climate change scenarios for northern Patagonia which indicates a reduction in total precipitation by the end of the twenty-first century (IPCC 2007).
In conclusion, our field experiment proved the existence of root competition by adult plants on shrub seedlings, reducing seedling emergence. However, the effect of root competition did not differ between microsites. On the other hand, both experiments showed that litter reduces the emergence of C. avellanedae. These results, no differences in root competition between microsites and reduction of shrub seedlings by litter, suggest that the over-dispersed pattern found for C. avellanedae is due, at least partially, to mechanical effects of litter on seedling emergence. Considering that shrubs are long-lived and their litter decomposes at slow rates, their negative effect on seedling emergence could have important consequences for plant population and vegetation dynamics in temperate herbaceous steppes.
References
Aguiar MR, Sala OE (1994) Competition, facilitation, seed distribution and the origin of patches in a Patagonian steppe. Oikos 70:26–34
Aguiar MR, Sala OE (1999) Patch structure, dynamics and implications for the functioning of arid ecosystems. TREE 14:273–277
Aguiar MR, Soriano A, Sala OE (1992) Competition and facilitation in the recruitment of seedlings in Patagonian steppe. Funct Ecol 6:66–70
Andersen MC (1993) Diaspore morphology and seed dispersal in several wind-dispersed Asteraceae. Am J Bot 80:487–492
Armas C, Pugnaire FI (2005) Plant interactions govern population dynamics in a semi-arid plant community. J Ecol 93:978–989
Barbier N, Couteron P, Lefever R, Deblauwe V, Lejeune O (2008) Spatial decoupling of facilitation and competition at the origin of gapped vegetation patterns. Ecology 89:1521–1531
Barritt AR, Facelli JM (2001) Effects of Casuarina pauper litter and grove soil on emergence and growth of understorey species in arid lands of South Australia. J Arid Environ 49:569–579
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Beeskow AM, Elissalde NO, Rostagno CM (1995) Ecosystem changes associated with grazing intensity on the Punta Ninfas rangelands of Patagonia, Argentina. J Range Manag 48:517–522
Bertiller MB, Beeskow AM, Coronato F (1991) Seasonal environmental variation and plant phenology in arid Patagonia (Argentina). J Arid Environ 21:1–11
Bisigato AJ, Bertiller MB (2004a) Temporal and micro-spatial pattering of seedling establishment. Consequences for patch dynamics in the southern Monte. Argentina Plant Ecol 174:235–246
Bisigato AJ, Bertiller MB (2004b) Seedling recruitment of perennial grasses in degraded areas of the Patagonian Monte. J Range Manage 57:191–196
Bisigato AJ, Hardtke LA, del Valle HF, Bouza PJ, Palacio RG (2016) Regional-scale vegetation heterogeneity in northeastern Patagonia: environmental and spatial components. Community Ecol 17:8–16
Bonanomi G, Sicurezza MG, Caporaso Esposito A, Mazzoleni S (2006) Phytotoxicity dynamics of decaying plant material. New Phytol 169:571–578
Bosco T, Bertiller MB, Carrera AL (2015) Micro-environmental conditions affect grass and shrub seedling emergence in denuded areas of the arid Patagonian Monte, Argentina. Flora 210:66–71
Bosco T, Bertiller MB, Carrera AL (2018) Abiotic factors affect the recruitment and biomass of perennial grass and evergreen shrub seedlings in denuded areas of Patagonian Monte rangelands. J Environ Manage 218:118–128
Bosy JL, Reader RJ (1995) Mechanisms underlying the suppression of forb seedling emergence by grass (Poa pratensis) litter. Funct Ecol 9:635–639
Brearley FQ, Press MC, Scholes JD (2003) Nutrients obtained from leaf litter can improve the growth of dipterocarp seedlings. New Phytol 160:101–110
Busso CA (1997) Towards an increased and sustainable production in semi-arid rangelands of central Argentina: two decades of research. J Arid Environ 36:197–210
Callaway RM, DeLucia EH, Moore D, Nowak R, Schlesinger WH (1996) Competition and facilitation: contrasting effects of Artemisia tridentata on desert vs Monte pines. Ecology 77:2130–2141
Campanella MV, Bertiller MB (2008) Plant phenology, leaf traits, and leaf litterfall of contrasting life forms in the arid Patagonian Monte, Argentina. J Veg Sci 19:75–85
Campanella MV, Bertiller MB (2009) Leafing patterns and leaf traits of four evergreen shrubs in the Patagonian Monte, Argentina. Acta Oecol 35:831–837
Campanella MV, Bertiller MB (2010) Leaf litterfall patterns of perennial plant species in the arid Patagonian Monte, Argentina. Plant Ecol 210:43–52
Campanella MV, Rostagno CM, Videla LS, Bisigato AJ (2016) Interacting effects of soil degradation and precipitation on plant productivity in NE Patagonia, Argentina. Arid Land Res Manag 30:79–88
Carson WP, Peterson CJ (1990) The role of litter in an old-field community: impact of litter quantity in different seasons on plant species richness and abundance. Oecologia 85:8–13
Casalini AI, Bisigato AJ (2018) Stress-gradient hypothesis and plant distribution along ecotonal gradients. Austral Ecol 43:807–816
Cavieres LA, Chacón P, Peñaloza A, Molina-Montenegro M, Arroyo MTK (2007) Leaf litter of Kageneckia angustifolia D. Don (Rosaceae) inhibits seed germination in sclerophyllous montane woodlands of central Chile. Plant Ecol 190:13–22
Chambers JC (2000) Seed movements and seedling fates in disturbed sagebrush steppe ecosystems: implications for restoration. Ecol Appl 10:1400–1413
Chapman SK, Feller IC (2011) Away-field advantage: mangrove seedlings grow best in litter from other mangrove species. Oikos 120:1880–1888
Chartier MP, Rostagno CM (2006) Soil erosion thresholds and alternative states in northeastern Patagonian Rangelands. Rangel Ecol Manag 59:616–624
Chartier MP, Rostagno CM, Videla LS (2013) Selective erosion of clay, organic carbon and total nitrogen in grazed semiarid rangelands of northeastern Patagonia, Argentina. J Arid Environ 88:43–49
Cipriotti PA, Aguiar MR (2005) Interspecific competition interacts with the spatial distribution of a palatable grass to reduced its recruitments. Rangel Ecol Manag 58:393–399
Cipriotti PA, Aguiar MR (2015) Is the balance between competition and facilitation a driver of the patch dynamics in arid vegetation mosaics? Oikos 124:139–149
Defossé GE, Bertiller MB, Robberecht R (1997) Effects of topography, soil moisture, wind and grazing on Festuca seedlings in a Patagonian grassland. J Veg Sci 8:677–684
Facelli JM, Pickett STA (1991a) Plant litter: light interception and effects on an old-field plant community. Ecology 72:1024–1031
Facelli JM, Pickett STA (1991b) Plant litter: its dynamics and effects on plant community structure. Bot Rev 57:1–32
Facelli JM, Pickett STA (1991c) Indirect effects of litter on woody seedlings subject to herb competition. Oikos 62:129–138
Fay PA, Schultz MJ (2009) Germination, survival, and growth of grass and forb seedlings: effects of soil moisture variability. Acta Oecol 35:679–684
Flores J, Jurado E (2003) Are nurse–protege interactions more common among plants from arid environments? J Veg Sci 14:911–916
Fowler NL (1986a) Microsite requirements for germination and establishment of three grass species. Am Midl Nat 115:131–145
Fowler NL (1986b) The role of competition in plant communities in arid and semiarid regions. Ann Rev Ecol Syst 17:89–110
Franzese J, Ghermandi L, Donaldo B (2009) Post-fire shrub recruitment in a semi-arid grassland: the role of microsites. J Veg Sci 20:215–259
Ghermandi L (1995) The effect of the awn on the burial and germination of Stipa speciosa (Poaceae). Acta Oecol 16:719–728
Goldberg DE, Barton AM (1992) Patterns and consequences of interspecific competition in natural communities: field experiments with plants. Am Nat 139:771–801
Gomez-Aparicio L (2008) Spatial patterns of recruitment in Mediterranean plant species: linking the fate of seeds, seedlings and saplings in heterogeneous landscapes at different scales. J Ecol 96:1128–1140
Hovstad KA, Ohlson M (2009) Conspecific versus heterospecific litter effects on seedling establishment. Plant Ecol 204:33–42
IPCC (2007) Technical summary. In: Solomon S, Qin D, Manning M, et al. (eds.) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Jensen K, Gutekunst K (2003) Effect of litter on establishment of grassland plant species: the role of seed sized and successional status. Basic Appl Ecol 4:579–587
Jurena PN, Archer S (2003) Woody plant establishment and spatial heterogeneity in grasslands. Ecology 84:907–919
Liu G, Wan L, He F, Tong Z, Liu Z, Li X (2016) Effects of litter, seed position, and water availability on establishment of seedlings for two semiarid grass species. Plant Ecol 217:277–287
Loydi A, Eckstein RL, Otte A, Donath TW (2013) Effects of litter on seedling establishment in natural and semi-natural grasslands: a meta-analysis. J Ecol 101:454–464
Loydi A, Donath TW, Otte A, Eckstein RL (2015) Negative and positive interactions among plants: effect of competitors and litter on seedling emergence and growth of forest and grassland species. Plant Biol 17:667–675
Mazzoleni S, Bonanomi G, Incerti G, Chiusano ML, Termolino P, Mingo A, Senatore M, Giannino F, Cartenì F, Rietkerk M, Lanzotti V (2015) Inhibitory and toxic effects of extracellular self-DNA in litter: a mechanism for negative plant-soils feedbacks? New Phytol 205:1195–1210
Merino-Martín L, Courtauld C, Commander L, Turner S, Lewandrowski W, Stevens J (2017) Interactions between seed functional traits and burial depth regulate germination and seedling emergence under water stress in species form semi-arid environments. J Arid Environ 147:25–33
Nogueira C, Nunes A, Bugalho MN, Branquinho C, McCulley RL, Caldeira MC (2018) Nutrient addition and drought interact to change the structure and decrease the functional diversity of a Mediterranean Grassland. Front Ecol Evol 6:155
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
Rey Benayas JM, Fernández A, Aubenau A (2007) Clipping herbaceous vegetation improves early performance of planted seedlings of the Mediterranean shrub Quercus coccifera. Web Ecol 7:120–131
Rotundo JL, Aguiar MR (2005) Litter effects on plant regeneration in arid lands: a complex balance between seed retention, seed longevity and soil-seed contact. J Ecol 93:829–838
Siffredi GL (2012) Guía de evaluación del pastoreo de cuadros INTA EEA Bariloche. Propastizal, Ley Ovina Río Negro
Singh HP, Batish DR, Kohli RK (1999) Autotoxicity: concept, organisms, and ecological significance. Crit Rev Plant Sci 18:757–772
Talamo A, Barchuk AH, Garibaldi LA, Trucco CE, Cardozo S, Mohr F (2015) Disentangling the effects of shrubs and herbivores on tree regeneration in a dry Chaco forest (Argentina). Oecologia 178:847–854
Vaz PG, Bugalho MN, Fedriani JM, Branco M, Lecomte X, Nogueira C, Caldeira MC (2019) Unravelling associations between tree-seedling performance, herbivory, competition, and facilitation in high nature value farmlands. J Environ Manage 232:1066–1074
Westoby M, Leishman M, Lord J, Poorter H, Schoen DJ (1996) Comparative ecology of seed size and dispersal. Phil Trans R Soc Lond B 351:1309–1318
Winn AA (1985) Effects of seed size and microsite on seedling emergence of Prunella vulgaris in four habitats. J Ecol 73:831–840
Xiong S, Nilsson C (1999) The effects of plant litter on vegetation: a meta-analysis. J Ecol 87:984–994
Zuur AF, Ieno EN, Walker N et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We are especially grateful to Lina Videla and Cristian Barrionuevo for their assistance during field and laboratory work. Anonymous reviewers and Michael John Lawes provided thoughtful comments on the manuscript. Ana Casalini and Gustavo Pazos helped with data analysis. This work was supported by the National Research Council of Argentina CONICET (PIP 11220170100981). This paper was written within the framework of the PUE-IPEEC-2016 22920160100044. MVC and AJB are CONICET researchers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Michael John Lawes.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Campanella, M.V., Bisigato, A.J. Conspecific leaf litter and root competition inhibits shrub emergence in the Patagonian steppe. Plant Ecol 220, 985–993 (2019). https://doi.org/10.1007/s11258-019-00968-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-019-00968-3