Abstract
We investigated flowering phenology, pollinator visitation and visitor community composition in communities of self-incompatible sympatric Primula species in a high-elevation Himalayan ecosystem. Within the tight constraints imposed by short growing seasons in such ecosystems, interactions among co-occurring plants for pollinators may vary from competition to facilitation, depending on the specifics of the system. We found that pollinator community composition changed with elevation in this system: lepidopterans were the dominant visitors at lower elevations (2200–3000 masl), bees (other than bumblebees) dominated at mid-elevations (3000–3800 masl) and bumblebees dominated at higher elevations (3800–4600 masl). However, within an elevation zone, there were no significant differences in pollinators amongst co-occurring Primula species. At a focal study site where multiple Primula species co-occurred, our results showed that even while the overall flowering periods of these species broadly overlapped, the peak flowering periods of different Primula species were temporally segregated. Upon further inferring the nature of interaction amongst co-flowering Primula species, we found that plots with higher Primula diversity (≥ 2 species) and density (80–100 individuals) experienced significantly higher pollinator visitation, compared with plots with single species and low flower densities (40–50 individuals). Our results suggest that in this community of sympatric, self-incompatible Primula species, a broadly aggregated, synchronous floral display of multiple species results in pollinator facilitation by attracting a greater number of pollinator visitors. Within this broadly synchronous display, the temporal segregation of peak flowering period of individual species may reduce competition for pollinators and limit heterospecific pollen transfer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many self-incompatible flowering plants depend on insects for cross-pollination (Kearns et al. 1998; Klein et al. 2007), and competition among plants for pollinator services can drive plant community structure via differentiation of flowering phenologies (Campbell 1985; Stone et al. 1998). The effective pollination and reproductive success of an individual plant is therefore closely linked to its phenology (Primack 1985; English-Loeb and Karban 1992; Ollerton et al. 2011; Sproull et al. 2015). Flowering phenology may be especially critical to successful pollination strategies in regions where multiple self-incompatible plant species co-flower and share pollinators. Co-flowering species may either experience competition for shared pollinators (Campbell 1985; Stone et al. 1998) or pollinator facilitation if co-flowering attracts a significantly higher number and diversity of pollinators (Petanidou et al. 1995; Johnson et al. 2003). Depending on the relative strengths of these processes, co-flowering plant species that share pollinators may experience either increased or decreased reproductive success (Ghazoul 2006; Yang et al. 2007; Grab et al. 2017).
Facilitation amongst synchronously flowering sympatric species occurs if the presence of one species increases the pollinator visitation of another species (Rathcke 1984; Ghazoul 2006; Grab et al. 2017). For example, individuals of self-incompatible and less-attractive Raphanus raphanistrum received more pollinator visits when they flowered in combination with attractive co-flowering species (Ghazoul 2006). On the other hand, synchronous flowering may also generally increase heterospecific neighbour density. Where pollinators are shared, this may result in higher rates of heterospecific pollen transfer and reduced seed sets (Campbell 1985; Bell et al. 2005; Flanagan et al. 2010; Morales and Traveset 2008). For instance, Bell et al. (2005) reported that when Mimulus ringens and Lobelia siphilitica are grown in sympatry, the outcrossing rate of M. ringens decreased by 20% and seed set reduced by 37%.
Flowering time divergence may be an important mechanism that can ensure reproductive isolation between sympatric species (Elzinga et al. 2007). By staggering their flowering phenology in time, sympatric species may both avoid competition for pollinator services and reduce the costs of heterospecific pollen deposition, gamete wastage or hybridization (Borchsenius 2002; Aizen and Rovere 2010). Depending on the relative costs and benefits of pollinator sharing, such asynchronous flowering may range from complete asynchrony such that flowering occurs earlier or later than sympatric species, or partial asynchrony such that only peak flowering times of co-occurring species are segregated (Stone et al. 1998; Raine et al. 2007; Baldock 2011).
Here we examine flowering phenology and pollinator visitation in sympatric species of the herbaceous, alpine flowering plant genus Primula. Primula species are self-incompatible, high-elevation specialists with a high diversity of co-occurring species in the high elevations of the Himalayas. We first examine flowering phenology and pollinator visitation of six sympatric Primula species along their elevational distributional ranges. Secondly, we investigate changes in pollinator visitation with respect to diversity and the density of co-flowering species. We hypothesized that due to the short growing seasons at high elevations (Rawat and Pangtey 1987; Kudo 1991), sympatric Primula species may be constrained to overlap in their flowering phenology such that they experience competition for pollinator services. Alternatively, instead of competition, these species may experience facilitation if synchronous flowering attracts a large number and/or diversity of potential pollinators, provided they do not experience large costs in the form of heterospecific pollen transfer.
Methods
Study system
The genus Primula L. in the family Primulaceae is a genus of herbaceous flowering plants with an estimated global diversity of 430–500 species (Hu and Kelso 1996; Richards 2003). Primula species are restricted in their distribution, occurring in the cool and moist regions of the northern hemisphere (Richards 2003), including North America, Europe, China and the Himalaya, with the majority of species occurring in China and the Himalayan regions (Richards 1993). Our study was conducted in the Eastern Himalayan state of Sikkim (270°20′N, 88°40′E), India along the Lachen and Lachung valley. There are 58 Primula species found in the Sikkim Himalaya (Ghosh 1981), making it among the most species-rich sub-regions for this genus (Ghosh 1981; Smith and Fletcher 1948). In the Sikkim Himalaya, Primula species are distributed from the low-elevation temperate broad-leaved forest (1700–2700 meters above sea level (masl) to high-elevation alpine pastures above 4000 masl (Champion and Seth 1968; Polunin and Stainton 1984), with the highest diversity of co-occurring species at the higher elevation. The genus is typically associated with a wide range of insect pollinators including bees, butterflies, and moths (Heslop-Harrison et al. 1981; Kurian and Richards 1997; Richards 2003). Primula provides pollen and nectar rewards for the floral visitors (Richards 2003). While there have been extensive studies on the breeding system and phylogeny of the genus Primula L. (Bateson and Gregory 1905; Miller et al. 1994; Mast et al. 2006; Jacquemyn et al. 2009; Schmidt-Lebuhn et al. 2012; Zhang et al. 2013) the ecology of Primula communities in the wild remains largely understudied (with the exception of a few studies e.g., Richards 1993).
Population survey
Field surveys were carried out during the growing season from March to September during two consecutive years (2012–2013). In an initial reconnaissance survey, we sampled transects from 1500 to 5000 masl, which represent the lowest and the highest elevation limits of Primula species in this region (Hooker 1854; Polunin and Stainton 1984). Based on these surveys, Primulas were found to be absent below 2200 masl, therefore, transects below 2200 m were excluded.
We sampled 14 elevation bands between 2200 and 5000 masl in each valley (Lachen and Lachung). Every 200 m we laid three 100 m vertical transects that were 100 m apart from each other. Along each vertical transect, we laid 1 m2 quadrats at 1, 25, 50, 75, and 100 m, for a total of 15 quadrats within each 100-m wide elevation band. In each quadrat, the presence or absence of Primula species and numbers of individuals of each species was recorded. Transect numbers varied between the two valleys because of differences in habitat conditions and terrain. In total, we sampled 96 transects (Lachen = 54 and Lachung = 42) and 480 quadrats. We chose six Primula species which varied in their distribution along elevation gradients in the two valleys. Three species are widely distributed along the elevation gradient (Primula denticulata 2223–4400 masl, Primula sikkimensis 3500–4632 masl, Primula capitata 2966–4432 masl), while the three remaining species occurred at elevations greater than 3500 masl (Primula dickieana 3500–4010 masl, Primula glomerata 3800–4400 masl, Primula primulina 4320–4667 masl). All six species were observed to co-occur between 3400 and 4600 masl.
Flowering phenological patterns
Within each 1 m2 quadrat of the population survey from Lachen valley, we randomly selected four individuals of each Primula species and marked them to study flowering phenology along the elevation gradient. All marked individuals were observed every 6–8 days throughout the flowering period (March–September). The phenological phases of marked individuals were determined by counting the number of open flowers per inflorescence. In addition, we noted the onset of flowers, flowering duration and flowering peak date where each individual was in full/maximum bloom. For each individual, we calculated the flowering synchrony value. The flowering synchrony for individual i (Xi) is a measure of the amount of overlap of individual’s flowering days with those of other individuals in the population (here population refers to the elevation bands). Thus, for an individual, the flowering synchrony is a composite measure of the amount of overlap of a given individual’s flowering period with those of all individuals in the population (Augspurger 1983). Perfect synchrony is represented by X = 1, and no-synchrony or asynchrony by X = 0. Synchrony values below 0.5 are considered to be low, between 0.5 and 0.85 are considered medium and above 0.85 are considered high (Augspurger 1981, 1983). Flowering synchrony is calculated as
where X indicates the degree of synchrony, i and j represent individuals of same species within a population, ej is the number of days during which both individuals i and j flower synchronously, fi is the number of days individual i is in flower and n is the number of individuals in the population.
The amount of overlap of all individuals with each other in the population defines the population synchrony (Z), which is equal to the mean of the values of individual synchrony (Augspurger 1983). Population synchrony is determined by
Population synchrony (Z) of focal Primula species along the elevation gradient was compared with two-way ANOVA (Fujikoshi 1993). All data analyses were performed using the software R 3.1.0 (R Development Core Team 2014).
Flower visitors
During the peak blooming period (April–August), the composition and abundance of potential pollinators (hereafter flower visitors) were assessed by documenting insects visiting focal Primula species. We sampled twelve elevation bands between 2200 and 4600 masl in each valley (Lachen and Lachung). Every 200 m we laid three 2 m2 plots, giving a total of 72 plots (36 plots per valley). We counted the number of open flowers per focal Primula species and recorded the pollinator visitation rates for 3 h of observation/day/plot in 10-min intervals, giving a total of 74 h of observations. The time period 07:00–13:00 h was selected because preliminary observations indicated that flower visitors before and after this period were uncommon. All observations were made under favourable weather conditions (sunny days or days with no/little cloud cover), and three observers recorded all flower visitors in each 2 m2 vegetation plot. We categorized flower visitors into five categories: lepidopterans, bumblebees, other bees, flies, and others (wasps, beetles etc.).
The floral visitors along the elevation gradient as well as among Primula species were expected to be distinct. We calculated Shannon–Wiener diversity indices for visitor communities along the three elevation zones (i.e., 2200–3000 masl, 3000–3800 masl, and 3800–4600 masl) and compared these using Kruskal–Wallis H tests (Kruskal and Wallis 2012). We also used non-metric multidimensional scaling, implemented in the vegan package in R (O’Hara et al. 2013) to compare Bray–Curtis dissimilarities (Bray and Curtis 1957) of the community compositions of flower visitors across elevation zones and across focal Primula species.
Floral diversity and density
The objective of this observational study was to determine whether pollinator visitation increased with respect to diversity of co-flowering species. The study was carried out along the elevation gradient of Lachung valley and all the plots were within an elevation band of 3400–4600 masl where most of the focal species co-occurred. Nine 2 m2 plots were selected for this study, three plots with single focal species, three with two and the remaining three with three co-flowering focal species (Table 1). We used ANOVA to compare the average number of flower visitors visiting the plots with single, two co-occurring and three co-occurring species in 10-min time intervals.
In the second observational study, run simultaneously with the above study, the objective was to determine whether pollinator visitation rates increased with increasing density of co-flowering species. Eighteen 2 m2 plots were selected, nine with low flower density (40–50 flowering individuals) and nine with high flower density (80–100 flowering individuals). For each plot, 3 h of observations were recorded in 10-min time intervals on a single day, between 7:00 and 13:00 h. We used ANOVA to compare the average number of flower-visiting in plots with low and high flower densities.
Results
Flowering phenology: onset of flowers, flowering duration, flowering peak and synchrony
We observed a gradual delay in onset of the flowering of focal Primula species with increasing elevation along their elevational distribution ranges. The flowering of focal Primula species along the elevation gradient began in early March and ended by September. The timing of flowering differed among species at different elevations (Fig. 1a). The flowering began earlier at lower elevations (the first week of March) than at higher elevations (the second week of June). The average flowering duration of plants was 2–3 weeks at each elevation band.
The onset of flowers, flowering duration and peak flowering of focal Primula species. a Elevational differences in flowering date of focal Primula species during spring–summer seasons (2012–2013). The horizontal bars refer to the duration of flowering in each elevation band. b Segregated peak flowering of six focal Primula species within an elevation zone of 3400–4600 masl, where they co-occurred
Given that most of the Primula species in this system occurred at high elevation with short flowering periods, we observed overlap in the flowering period among co-occurring species (3400–4600 masl, Fig. 1a), with at least two species flowering at the same time. However, temporal segregation or phenological displacement of peak flowering period was observed among co-flowering species (Fig. 1b). Data on peak flowering times of the Primula species over two valleys for the year 2013 are shown in Fig. 1b; we observed temporal separation in peak flowering times where peak flowering of P. denticulata was the third and the fourth weeks of April; followed by P. dickieana—the third and the fourth weeks of May; P. sikkimensis—the first and the second week of June; P. glomerata—the forthth week of June and the first week of July; P. primulina—the third and the fourth weeks of July and P. capitata—the fourth week of July and the first week of August (Fig. 1b).
The average values of individual flowering synchrony for all the focal species were low in most of the elevation bands (< 0.5) with few exceptions (medium synchrony between 0.5 and 0.63). Population flowering synchrony (Z, which was the mean of values of individual synchrony) ranged from low to medium 0.3–0.63 (Fig. 2).
Visitation patterns: elevation gradient, floral diversity, and density
Flowers of Primula species attracted numerous insect visitors. A total of 2682 visitors were observed on 33,990 flowering individuals. Lepidopterans, bumblebees, other bees, and flies were the primary visitors of focal Primula species across the elevation gradient. Flower visitor abundance varied across elevation, with greater abundance at higher elevation zones (3000–3800 and 3800–4600 masl) compared to lower-elevation zones at 2200–2800 masl (Fig. 3). We also observed that Lepidopterans were the dominant visitors at the lower-elevation zones, replaced by bees (other than bumblebees) at mid-elevation and bumblebees at higher elevation zones (Fig. 3). The Shannon-Weiner diversity index of flower visitors in high-elevation zones was also more diverse than at lower-elevation zones (Fig. 4, Kruskal–Wallis H test: H = 15.23 (2, N = 36), p < 0.05). Community composition of flower visitors also differed significantly among elevation zones (Fig. 5a; PERMANOVA: F = 180.94, df = 2, p = 0.001), where the lower -levation zone with negative NMDS axis one score was correlated with lepidopterans, while mid-elevation with positive NMDS axis one score corresponded with bees and other insects and the higher elevation with positive NMDS axis one score corresponded to bumblebees and flies (Fig. 5a). However, no significant differences in community composition of flower visitors (Fig. 5b; PERMANOVA: F = 141.68, df = 5, p = 0.169) were observed among focal Primula species in the zone where they co-occurred.
Relative flower visitors’ abundance in focal Primula species along an elevation gradient. Average floral visitor abundance observed per 3-h sampling period in six Primula species from three elevation zones (2200–3000, 3000–3800 and 3800–4600 masl) were grouped into five categories: lepidopterans, bumblebees, other bees, flies, and others (wasps, beetles etc.)
Shannon–Wiener diversity of flower visitors of Primula species. Shannon–Wiener diversity of flower visitors for three elevation zones are based on Shannon–Wiener diversity measures for plots in each elevation zones. Boxes represent the interquartile range (25th–75th percentile). The line represents the median and whiskers are the furthest data points within 1.5 times the interquartile range
Non-metric multidimensional scaling using Bray–Curtis dissimilarity distances. A distance matrix of flower visitor community composition using the Bray–Curtis index was used to form a non-metric multidimensional scaling plot. Plots that are closer in ordination space have more similar flower visitor community composition. a Community composition among elevation zones, where ellipses represent groupings by elevation zones using 0.95 confidence interval. b Community composition among focal Primula species, where ellipses represent grouping by species using 0.95 confidence interval
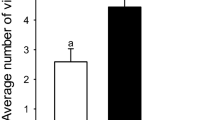
Plots with higher species diversity (≥ 2 species) showed a significantly higher average number of floral visitors compared to single species plots (Fig. 6a; ANOVA: F (2, 6) = 74.209, p = 0.001). Post hoc comparisons using the Tukey HSD test (Tukey 1949) indicated that the mean scores for the plots with different Primula species diversity were significantly different (single species: M = 23.33, SD = 2.52; two co-occurring species: M = 56, SD = 6.56; three co-occurring species: M = 81.67, SD = 7.37). We also observed a significantly higher average number of flower visitors in plots with higher flower density compared with plots with low flower density (Fig. 6b; ANOVA: F (1, 56) = 25.44, p = 0.001). Insect visitation rates were more than three times greater in high-density plots versus low-density plots. At relatively low flower density (40–50 flowering individuals), we observed less than 0.1 average number of flower visitors per open flower during 10 min of observation. However, the average number of flower visitors per open flower in higher density plots (80–100 flowering individuals) was observed to be greater than 0.3.
An average number of flower visitors per flower per 10-min interval. a Flower visitors number in single species, two co-occurring species, and three co-occurring species plots. The boxes in the graph with different alphabets A, B, and C represent significant differences. b Flower visitors number in low flower density and high flower density plots
Discussion
Sympatric flowering plants can respond to competition for shared pollinators by adjusting their flowering phenology (Campbell 1985; Stone et al. 1998). Our study suggests that pollinator-mediated facilitation may occur when sympatric Primula species with substantially overlapping flowering phenologies attract a greater number and/or diversity of potential pollinators. However, a potential cost of simultaneous flowering in sympatric species is the potential for the increased heterospecific pollen transfer. The temporal staggering of peak flowering period, as seen in this study, may limit heterospecific pollen transfer among sympatric species and thus act as an effective reproductive isolation mechanism (Grant 1992, 1994a, b; Hodges and Arnold 1994; Husband and Sabara 2004).
The sequential flowering of sympatric plant species is a commonly observed phenomenon and allows sympatric species to temporally segregate their use of pollinators and thereby reduce competition for a shared resource (Listabarth 1996; Henderson et al. 2000a, b; Borchsenius 2002; Barfod et al. 2011; Giorgis et al. 2015). The observed staggering of peak flowering periods of sympatric Primula species in this study (Fig. 1b) may likewise serve to increase the likelihood of successful pollination by reducing competition for pollinator visitation. Flowers in peak flowering periods tend to have greater possibilities of being fertilized (Trammell and Peterson 1978; Rodríguez-Pérez and Traveset 2016), and the observed differences in peak flowering periods of focal species may be especially significant for reproductive isolation.
Plots with higher plant diversity (≥ 2 co-occurring species) experienced significantly increased flower visitation compared to plots with single focal species (Fig. 6a). These data suggest a facilitative interaction among co-occurring Primula species where plots with greater floral diversity experienced greater numbers of insect visitors. Further, floral densities were also important, and plots with higher densities, irrespective of species composition, experienced higher visitation rates. Thus, in addition to higher floral diversity, higher floral densities were also important for attracting more floral visitors. Higher pollinator visitation rates may generally be associated with higher seed sets (Vidal et al. 2010; Osada et al. 2004), although we do not have the data to test this in our study. Most previous studies that provide evidence for facilitative interactions among congeners do so for those with similar floral structures (Moeller 2004; Gross et al. 2000) where it is not possible to determine whether pollinators failed to distinguish between species. However, a study on Raphanus raphanistrum demonstrated an increase in pollinator visitation rates when it occurred with one or more species with different floral forms and colours (Ghazoul 2006). Similarly, our study provides evidence for facilitative interactions among sympatric focal Primula species with similar as well as different floral structures and colours.
Our results suggest that the pollinator-mediated facilitation amongst sympatric Primula species may occur through several mechanisms. First, facilitation may occur when the aggregated floral displays in plots of higher density and species diversity attract greater numbers of floral visitors compared to plots with single focal species and lower densities. Second, facilitation may indirectly occur if staggered peak flowering reduces competition for pollinator services, and flowers thereby experience higher rates of pollen deposition during these peaks. These patterns suggest that sympatric Primula species in this high-elevation ecosystem may benefit each other by providing a broadly continuous and aggregated flower display over the growing season that collectively attracts more potential pollinators, even while the segregation in peak flowering times may allow them to temporally partition pollinators for short periods. Such a strategy may be optimal in the stressful abiotic environments with compressed growing seasons that are characteristic of high-elevation ecosystems (Bertness and Callaway 1994; Callaway and Walker 1997).
In conclusion, our results clearly indicate that the timing of flowering in sympatric Primula species has consequences for the pollinator services in these self-incompatible plants. While the broad temporal convergence of flowering times across species enabled higher visitation rates overall, the finer-scale temporal segregation of species’ flowering peaks likely limited losses from heterospecific pollen deposition. All of these responses suggest a fine-tuning of flowering phenology in these communities with the tightly constrained growing season in this high-altitude system. With high-altitude, alpine ecosystem being susceptible to potentially rapid and significant climate shifts; it is likely that such finely tuned plant–pollinator changes may be strongly affected. Indeed, our results provide further evidence that critical plant–pollinator interactions are particularly vulnerable to disruption in high-elevation mountain communities due to the significant impacts of climate change in these ecosystems (Beniston 2005; IPCC 2007).
References
Aizen Marcelo A, Rovere Adriana E (2010) Reproductive interactions mediated by flowering overlap in a temperate hummingbird–plant assemblage. Oikos 119:696–706
Augspurger CK (1981) Reproductive synchrony of a tropical shrub—experimental studies on effects of pollinators and seed predators on Hybanthus prunifolius (Violaceae). Ecology 62:775–788
Augspurger CK (1983) Phenology, flowering synchrony, and fruit set of six neotropical shrubs. Biotropica 15:257–267
Baldock KCR (2011) Daily temporal structure in African savanna flower visitation networks and consequences for network sampling. Ecology 92:687–698
Barfod AS et al (2011) Twenty-five years of progress in understanding pollination mechanisms in palms (Arecaceae). Ann Bot 108:1503–1516. https://doi.org/10.1093/aob/mcr192
Bateson W, Gregory RP (1905) On the inheritance of heterostylism in Primula. Proc R Soc Lond 76:581–586
Bell JM et al (2005) Interspecific competition for pollination lowers seed production and outcrossing in Mimulus ringens. Ecol Soc Am 86:762–771
Beniston M (2005) The risks associated with climatic change in mountain regions. In: Huber U, Bugmann H, Reasoner M (eds) Global change and mountain regions: an overview of current knowledge. Springer, Dordrecht, pp 511–520
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193
Borchsenius F (2002) Staggered flowering in four sympatric varieties of Geonoma cuneata (Palmae). Biotropica 34:603–606
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Callaway RM, Walker LR (1997) Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology 78:1958–1965
Campbell DR (1985) Pollinator sharing and seed set of Stellaria pubera: competition for pollination. Ecology 66:544–553
Champion HG, Seth SK (1968) A revised survey of forest types of India. Manager of Publications, India
Elzinga JA et al (2007) Time after time: flowering phenology and biotic interactions. Trends Ecol Evol 22:432–439
English-Loeb GM, Karban R (1992) Consequences of variation in flowering phenology for seed head herbivory and reproductive success in Erigeron glaucus (Compositae). Oecologia 89:588–595
Flanagan RJ et al (2010) Increased relative abundance of an invasive competitor for pollination, Lythrum salicaria, reduces seed number in Mimulus ringens. Oecologia 164:445–454
Fujikoshi Y (1993) Two-way ANOVA models with unbalanced data. Discret Math 116(1–3):315–334
Ghazoul J (2006) Floral diversity and the facilitation of pollination. J Ecol 94:295–304
Ghosh RB (1981) An analysis on the distribution of the Indian taxa of the genus Primula Linn. In the Eastern Himalaya with remarks on the species of Assam. Anales De La Estacion Experimental De Aula 15:219–228
Giorgis MA et al (2015) Flowering phenology, fruit set and seed mass and number of five coexisting Gymnocalycium (Cactaceae) species from Córdoba mountain, Argentina. J Torrey Bot Soc 142:220–230. https://doi.org/10.3159/TORREY-D-14-00017.1
Grab H et al (2017) Temporally dependent pollinator competition and facilitation with mass flowering crops affects yield in co-blooming crops. Sci Rep 7:1–9. https://doi.org/10.1038/srep45296
Grant V (1992) Floral isolation between ornithophilous and sphingophilous species of Ipomopsis and Aquilegia. Proc Natl Acad Sci USA 89:11828–11831
Grant V (1994a) Mechanical and ethological isolation between Pedicularis groenlandica and P. attllens (Scrophulariaceae). Biologisches Zentralblatt 113:43–51
Grant V (1994b) Modes and origins of mechanical and ethological isolation in angiosperms. Proc Natl Acad Sci USA 91:3–10
Gross CL et al (2000) Aggregated flowering phenologies among three sympatric legumes: the degree of non-randomness and the effect of overlap on fruit set. Plant Ecol 148:13–21
Henderson A et al (2000a) Flowering phenology of a palm community in a central Amazon forest. Brittonia 52:149–159
Henderson A et al (2000b) Pollination of Bactris (Palmae) in an Amazon forest. Brittonia 52:160–171
Heslop-Harrison J et al (1981) Heterostyly in Primula. 2. Sites of pollen inhibition, and effects of pollen constituents on compatible and incompatible pollen-tube growth. Protoplasma 107:319–337
Hodges SA, Arnold ML (1994) Floral and ecological isolation between Aquilegia formosa and Aquilegia pubescens. Proc Natl Acad Sci USA 91:2493–2496
Hooker JD (1854) Himalayan journals or notes of a naturalist. John Murray, London
Hu Q, Kelso S (1996) Primulaceae. In: Wu ZY, Raven PH (eds) Flora of China, vol 15. Science Press, Veijing, pp 99–185
Husband BC, Sabara HA (2004) Reproductive isolation between autotetraploids and their diploid progenitors in fireweed, Chamerion angustifolium (Onagraceae). New Phytol 161:703–713
IPCC (2007) Climate Change 2007: impacts, adaptation and vulnerability. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE (eds) Contribution of working group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Jacquemyn H et al (2009) Rapid loss of genetic variation in a founding population of Primula elatior (Primulaceae) after colonization. Ann Bot 103:777–783
Johnson SD et al (2003) Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology 84:2919–2927
Kearns CA et al (1998) Endangered mutualisms: the conservation of plant–pollinator interactions. Annu Rev Ecol Evol Syst 29:83–112
Klein AM et al (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc Lond B 274:303–313
Kruskal WH, Wallis WA (2012) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47(260):583–621. https://doi.org/10.1080/01621459.1952.10483441
Kudo G (1991) Effects of snow-free period on the phenology of alpine plants inhabiting snow patches. Arct Alp Res 23:436–443
Kurian V, Richards AJ (1997) A new recombinant in the heteromorphy ‘S’ supergene in Primula. Heredity 78:383–390
Listabarth C (1996) Pollination of Bactris by Phyllotrox and Epurea. Implications of the palm breeding beetles on pollination at the communitylevel. Biotropica 28:69–81
Mast AR et al (2006) Are any primroses (Primula) primitively monomorphic? New Phytol 171:605–616
Miller J et al (1994) Comparative reproductive biology of two alpine primrose species. Arct Alp Res 3:297–303
Moeller DA (2004) Facilitative interactions among plants via shared pollinators. Ecology 85:3289–3301
Morales CL, Traveset A (2008) Interspecific pollen transfer: magnitude, prevalence and consequences for plant fitness. Crit Rev Plant Sci 27:221–238
O’Hara RB et al (2013) vegan: Community Ecology Package. R Package Version 2.0–10. http://cran.r-project.org/package=vegan
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326
Osada N et al (2004) Community-level flowering phenology and fruit set: comparative study of 25 woody species in a secondary forest in Japan. Ecol Res 18:711–723
Petanidou T, den Nijs JCM, Oostermeijer JGB, EllisAdam AC (1995) Pollination ecology and patch-dependent reproductive success of the rare perennial Gentiana pneumonanthe L. New Phytol 129:155–163
Polunin O, Stainton A (1984) Flowers of the Himalaya. Oxford University Press, Delhi
Primack RB (1985) Patterns of flowering phenology on communities, populations, individuals, and single flowers. In: White J (ed) The population structure of vegetation. Dr W. Junk Puplishers, Dordrecht, pp 571–593
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raine NE et al (2007) Plant–pollinator interactions in a Mexican Acacia community. Arthropod Plant Interact 1:101–117. https://doi.org/10.1007/s11829-007-9010-7
Rathcke B (1984) Competition and facilitation among plants for pollination. In: Real L (ed) Pollination biology. Academic Press, New York, pp 305–338
Rawat GS, Pangtey YPS (1987) Floristic structure of snowline vegetation in central Himalaya, India. Arct Alp Res 19:195–201
Richards AJ (1993) Primula. Timer Press, Portland
Richards AJ (2003) Primula, revised edn. Timber Press, London
Rodríguez-Pérez J, Traveset A (2016) Effects of flowering phenology and synchrony on the reproductive success of a long-flowering shrub. AoB Plants 8:plw007. https://doi.org/10.1093/aobpla/plw007
Schmidt-Lebuhn AN et al (2012) Phylogenetic analysis of Primula section Primula reveals rampant non-monophyly among morphologically distinct species. Mol Phylogenet Evol 65:23–34
Smith WW, Fletcher HR (1948) The genus Primula: sections Cuneifolia, Floribundae, Parryi and Auricula. Transactions of the Royal Society of Edinburgh, London
Sproull GJ et al (2015) Long-term changes in composition, diversity and distribution patterns in four herbaceous plant communities along an elevational gradient. J Veg Sci 26:552–563
Stone GN et al (1998) Partitioning of pollinators during flowering in an African Acacia community. Ecology 79:2808–2827
Trammell KW, Peterson CE (1978) Relationships of blooming rate and duration of pollination to seed yield in onions (Allium cepa L.). HortScience 8:270
Tukey JW (1949) Comparing individual means in the analysis of variance. Biometrics 5:99–114
Vidal MG et al (2010) Pollination and fruit set in pumpkin (Cucurbita pepo) by honey bees. Rev Bras Bot 33:107–113
Yang CF, Gituru RW, Guo YH (2007) Reproductive isolation of two sympatric louseworts, Pedicularis rhinanthoides and Pedicularis longiflora (Orobanchaceae): how does the same pollinator type avoid interspecific pollen transfer? Biol J Lin Soc 90:37–48
Zhang L et al (2013) Comparative transcriptome analysis and marker development of two closely related Primrose species (Primula poissonii and Primula wilsonii). BMC Genomics 14:329–341
Acknowledgements
The research was supported by the Department of Biotechnology, Government of India under the project “Technological Innovations and Ecological Research for the Sustainable use of Bioresources in the Sikkim Himalaya”. The authors thank Mahesh Sankaran and Krishnapriya Tamma for helpful comments. The authors express their thanks to the Forests, Environment and Wildlife Management Department, Government of Sikkim; the Department of Home, Government of Sikkim; the Army and Police department of Sikkim for the research and road permits in the Kanchenjunga Biosphere Reserve and Shingba Rhododendron Sanctuary, North-Sikkim, India. The authors also thank the field assistants and the people of Lachen and Lachung valley, North-Sikkim for assisting in the field and logistics support. We offer our thanks to the Entomology Department, Gandhi Krishi Vignan Kendra, Bangalore, for insect identification.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William E. Rogers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gurung, P.D., Ratnam, J. & Ramakrishnan, U. Facilitative interactions among co-flowering Primula species mediated by pollinator sharing. Plant Ecol 219, 1159–1168 (2018). https://doi.org/10.1007/s11258-018-0868-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-018-0868-5