Abstract
Co-flowering congeneric plant species may either experience competition for the services of shared pollinators or facilitation when together, they attract a higher number and diversity of pollinators. In this study, we evaluate whether temporal segregation in flowering time and temporal partition of shared pollinators operate among sympatric Anthurium species as mechanisms to reduce competition to attract potential pollinators. We investigated flowering phenology, the intra-e interspecific synchrony, and the composition of the flower visitor community of seven coexisting Anthurium species biweekly for a whole year in Native and Pine forests. We also analyzed the structure of Anthurium -flower visitor networks and the functional role of species. Flowering was continuous thorough the year for most Anthurium species, but their flowering peaks were segregated significantly in time. Although the flowering periods of these species overlapped, flower visitor communities were very dissimilar among Anthurium species, sharing only a tiny fraction of insects that function as connectors among species in the network. The partition of potential pollinators in a fine temporal scale occurred through the rewiring of shared flower visitors to the most abundant flowering Anthurium species. On the other hand, a high number of inflorescences attracted larger abundance and richness of insect visitors. Facilitation occurred almost throughout the year, while competition occurred during the flowering peak, where a particular species was the best competitor increasing the constancy of pollinators. This study highlights the role of facilitation and competition as mechanisms that together shape the use of potential pollinator resources between sympatric congeneric plant species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reproductive success in plants with biotic pollination depends not only on floral traits but on reproductive investment, floral longevity, and the timing of flowering, all of which determine the level of synchrony with conspecific and heterospecific individuals (Conner & Rush 1996; Cardona et al. 2020; Moreno-Betancur & Cuartas-Hernández 2022). It also depends on pollinator density, activity, and foraging behavior (Rymer et al. 2005; Mu et al. 2018). These pollinators visit multiple plant species; so, co-flowering plants compete for the services of shared pollinators (Willmer 2011; Johnson et al. 2022). The competition may occur not only through reduced visitation of flowers by pollinators but also through changes in the amount and quality of pollen dispersed (Mitchell et al. 2009). These factors can have effects on the fitness of plant species and lead to imbalances that favor one competitor over others, reducing plant coexistence (Johnson et al. 2022).
An expected evolutionary consequence of competition is the partitioning of shared resources. This partitioning reduces the negative impacts of the competition between sympatric plant species (Pianka 1973; Schoener 1983) and can be achieved: (a) through segregation of flowering over the year or growing season (Elzinga et al. 2007; Giorgis et al. 2015; Gurung et al. 2018), (b) through the partition shared pollinators’ activity into finer daily timescales (Raine et al. 2007), and (c) through interspecific differences in pollinator guilds, the recruitment of exclusive pollinators, or the deposit of pollen on different parts of a shared pollinator’s body (Armbruster et al. 1994; Ruchisansakun et al. 2016).
Regarding segregation of flowering time, this not only allows for the temporal partitioning of pollinators, but also reduces the costs of heterospecific pollen deposition or hybridization (Baack et al. 2015; Esposito et al. 2018), and functions as a mechanism of reproductive isolation between sympatric and congeneric species (i.e., phenological isolation) (Elzinga et al. 2007; Baack et al. 2015; Ma et al. 2016). The timing of flowering may also play a critical role in plant reproduction since this should coincide with the emergence of pollinators; otherwise, seed production could be pollen-limited (Elzinga et al. 2007; Bennet et al. 2018).
On the other hand, the coexisting plant species might be constrained to co-flower when they show similar responses to environmental signals (e.g., water availability, temperature, etc.) (Bartomeus et al. 2011; Cardoso et al. 2020), or when they are closely related and show limited divergence from ancestral flowering patterns (Wright & Calderon 1995; Mesquita-Neto et al. 2015). As a consequence of flowering periods overlap in sympatric species (i.e., interspecific synchronous flowering), the heterospecific flower neighbor density increases. This causes the co-flowering species to either experience competition by shared pollinators (Campbell 1985; Stone et al. 1998) or pollinator facilitation, if they attract a significantly higher number and diversity of pollinators (Johnson et al. 2003), leading to a reduction or increment of reproductive success, respectively (Yang et al. 2007; Grab et al. 2017; Gurung et al. 2018).
In mountain forests in the state of Antioquia, Colombia, several species of Anthurium genus grow sympatrically, showing high variation in population abundance (Gómez-Murillo & Cuartas-Hernández 2016; Benavides et al. 2015). The genus Anthurium Schott is the largest genera within the Araceae family and contributes significantly to herbaceous/epiphytic biodiversity and biomass in the neotropics (Croat 2015). Anthurium species display an enormous variation in growth habit, color, and size of inflorescences (Mayo et al. 1997). Particularly, in the Parque Arví Reserve, ten Anthurium species grow in the understory. The Reserve is in the peri-urban area of Medellin, a city which has been transformed by human activity, and has experienced forest fragmentation, pine plantations, and urbanization (Colorado-Zuluaga et al. 2017). There, native forests and pine plantations present differences in vegetation structure and composition, and in abiotic conditions. The latter might influence not only the patterns of flowering of understory herbs but also the composition of insect flower visitor assemblages and their functional roles (Olesen et al. 2007). In fact, multispecies Anthurium assemblages commonly co-flower in mountain tropical forests and share some flower visitors (Gómez-Murillo & Cuartas-Hernández 2016). However, little is known about the pollination systems, the reproductive biology, and the spatial and temporal scale at which abiotic and biotic factors operate on these interactions and on the structure of Anthurium—flower visitor networks (Hartley & Gibernau 2019).
If co-flowering Anthurium species do compete for shared pollinators, we thus hypothesize that (a) temporal segregation in flowering time among species (i.e., peaks of flowering to be significantly spaced across species through the year) (Pleasants 1980; Minckley et al. 1994; Waser 1983), and (b) temporal partitioning of shared pollinators (Armbruster et al. 1994; Raine et al. 2007) will be mechanisms operating to reduce competition between these congeneric plant species.
To analyze the relationship between co-flowering sympatric Anthurium species and their flower insect visitor assemblages through time in the Parque Arví, we examined the annual patterns of flowering and tested for differences in the level of flowering synchrony, and the spatial and temporal variation in the composition of insect flower visitor assemblages in seven Anthurium species, based on records of visits every two weeks in Native and Pine forests. We also evaluated the potential for self-fertilization of each Anthurium species to determine the level of dependence on pollinators for seed production and the temporal reproductive isolation among co-flowering species. In addition, we described the structure of the Anthurium—flower visitor network in each forest type and the plant and insect functional roles within the network. In doing this, we addressed the following questions: (1) Do temporal flowering patterns overlap among Anthurium species? (2) What is the level of intra/interspecific flowering synchrony? (3) Is there a facilitative interaction between Anthurium species that increases floral visitors (i.e., potential pollinators)? (4) Does the functional role of Anthurium and flower visitor species within the network interaction change spatially (i.e. among forest types)? We considered that such local-scale detailed studies would help to better understand the nature of plant–flower visitor interactions and their role in the coexistence mechanisms of closely related species in ecologically realistic scenarios.
Materials and methods
Study site
This study was performed in Parque Arví, an area within the Río Nare Reserve, in the Central Mountain Range of Santa Elena, in Medellín, Colombia (6° 15′ 56″ N, 75° 29′ 49″ W). This Reserve comprises an area of 1.761 hectares and has been transformed by anthropogenic activities. The Reserve covers an elevation gradient from 2400 to 2600 m.a.s.l. and has a mean annual temperature of 14 °C (range = 5–28.5 °C). Precipitation exhibits a bimodal pattern with two rainy periods (March–May and June–August) and two dry periods (Corantioquia 1999). The area presents a mosaic of native forests, pine plantations, orchards, and urban development (Colorado-Zuluaga et al. 2017). The prevailing forest covers in the area are native mountain forests dominated by Quercus humboldtii with several vegetation strata, and pine plantations dominated by Pinus patula, with two vegetation strata: Pine forming the dosel; and herbaceous plants, in which Anthurium is dominant, forming the understory. Both forest types are different in the abiotic environment (i.e., light intensity), which might influence the flowering patterns of the understory plants.
For the purpose of this study, we established a sampling site in each vegetation cover type (Native and Pine forests). Each site included six Anthurium species. These sites were selected based on (a) high abundance of Anthurium which allowed to observe insect visits to inflorescences, (b) the fact that the area was free from tourist activities, which meant there was no damage to the studied plants, and, (c) easy access. The sites were 1 km apart from each other.
Study system
The genus Anthurium belongs to the Araceae family, is strictly neotropical, and is distributed from southern Mexico to northern Argentina. It is the most species-rich genus of all aroids (ca. 1690 species) and the most conspicuous genus in the understory of montane and lowland rain forests (Croat 2015; Mayo et al. 1997). It displays an enormous variation in leaf morphology, growth habit, leaf venation pattern, and size and color of inflorescences, which consist of one cylindrical spadix with protogynous bisexual flowers organized in spirals and a subtended bract called the spathe (Mayo et al. 1997). Besides, it presents a rewarding mutualism with its pollinators (Chartier et al. 2014). These rewards are varied and can be spadix exudates, pollen, or fragrant oils. Despite the immense diversity and the ecological importance that it has in Neotropical forests, the pollination of the genus Anthurium remains largely understudied (Hartley & Gibernau 2019).
In the Parque Arví Reserve, where this study was conducted, there are 10 Anthurium species (Benavides et al. 2015). The species chosen for the study belong to different sections within the Anthurium genus: A. bogotense, A. caramantae (Cardiolonchium section), A. caucanum (Tetraspermium section), A. cupreum, A. longegeniculatum, A. microspadix (Xyalophyllum section), and A. yarumalense (Calomystrium section) (Croat & Scheffer 1983). These species differ in plant size, and in inflorescence and infructescence size, color, and aroma (Supplemental Table S1).
Sampling of plant–flower visitor interactions
To carry out the sampling of interactions among inflorescences and insects, one transect of 100 × 4 m was drawn in Native and Pine forests. Each transect was divided into ten quadrats of 10 × 4 m, which were randomly sampled in the morning and the afternoon. The sampling of insects visiting inflorescences was performed in each forest from 10:00 to 16:00 h. This period of observation was defined because preliminary records indicated that flower visitors were uncommon before and after those hours. Once determined, the period of observation was divided into 20-min intervals, for a total of 18 intervals per day. The sampling was performed for two consecutive days, one day on each site (Native and Pine forests), and two people per quadrat recorded the interactions. Samplings of open inflorescences of each Anthurium species and the insects visiting those inflorescences were performed once every two weeks from August 2020 to August 2021 in each forest type. In total, 864 samples of 20-min interval/quadrat (18 observation periods × 2 days × 24 samplings) and 288 h of observation were recorded. The complete sampling period included rainy and dry months, which allowed us to evaluate the effect of precipitation on the composition of the flowering Anthurium and flower visitor assemblages.
Interactions were thought to take place when an insect (hereafter flower visitor) visiting an inflorescence contacted the reproductive structures of flowers, regardless of its efficiency as a pollen vector. Most insects were too minute to be identified at a glance, thus, they were collected and deposited in Eppendorf tubes with alcohol 70% for later identification. The insects were identified at the family level, and when it was possible, they were identified to genera. Insects were assigned to morphospecies for the subsequent analyses. Vouchers were deposited at the Entomological Collection of the Universidad de Antioquia (CEUA).
Pooled samples from each sampling and for each quadrat of flowering Anthurium and visiting insect species were used as samples of local diversity per forest type. The abundance and richness S (i.e., the total number of species in the sample) of visiting insect species were calculated for each Anthurium species, sampling, and forest type.
The differences in insect flower visitor richness among Anthurium species were evaluated based on the abundance records of 24 samplings performed during the sampling year using the rarefaction/extrapolation analysis method of the iNEXT package called by R (Hsieh et al. 2016). This method permits to compare the richness of insect flower visitors of different assemblages (i.e., Anthurium species) when sample sizes of the assemblages are different. It estimates confidence intervals (95%) for richness estimates for different sample sizes; thus, two assemblages are considered to have significantly different richness when their confidence intervals do not overlap (Chao et al. 2014). Although in our study the sampling effort was equal for all Anthurium species in terms of sampled area and time, the number of inflorescences from which insects were sampled was variable probably because Anthurium species have different densities of individuals as a result of their different competitive abilities in each forest type.
Flowering phenology and intra/interspecific synchrony
In each transect, every individual Anthurium was marked, and open inflorescences were counted at each visit. Each individual of these species produces several inflorescences during the flowering period; however, only one inflorescence is open at one time. Also, each individual plant of each Anthurium species was censused within each sampling quadrat to estimate population size. Given that flowering in mountain forests does not present a discrete seasonal flowering period and is almost continuous for most Anthurium species throughout the year, it was not possible to identify the onset or duration of flowering. Nonetheless, it was possible to determine the peak of flowering (Giorgis et al. 2015).
The intraspecific synchrony was calculated as the percentage of individuals of the total population that was flowering on the same sampling date in each quadrat. The event was considered asynchronous when 20% or fewer individuals bloomed at the same time. It was considered to have a low synchrony when between 21 and 60% of the population flowered simultaneously, and it was considered to have a high synchrony when more than 60% of the individuals flowered on a specific sampling date (Benckey & Morellato 2002). In addition, the strength of temporal reproductive isolation due to flowering phenology (i.e., asynchrony) between each pair of Anthurium species for each forest type was estimated (Martin & Willis 2007).
Given that the variation in the vegetation cover could potentially result in microclimatic differences among sampling sites, which might influence flowering timing in Anthurium, precipitation and light intensity were measured in each forest type. The amount of precipitation was recorded once every two weeks and the mean light intensity was obtained based on the illuminance measures in each of the ten quadrats on each site, assuming there were low changes in the vegetation cover through time due to the protected status of the Reserve.
Finally, the potential of self-pollination in 10 inflorescences of each Anthurium species on each site was evaluated. The inflorescences were covered with a fine mesh bag prior to the spathe opening to exclude pollinators. They were monitored until they formed infructescences or until they were rotten. A species was considered to be potentially self-pollinating if at least one inflorescence had set fruit (Chouteau et al. 2008).
Similarity between insect flower visitor communities
To assess the dissimilarity of insect visitor species composition between each pair of Anthurium species, samplings, and forest types, the Bray–Curtis dissimilarity measure (×100) was estimated on abundance matrices. This dissimilarity is bound between zero and one and reaches its maximum value when there are no shared species between two compared communities. Also, a SIMPER (Similarity Percentage) analysis which allows the detection of shared and exclusive insect visitors to each Anthurium species in each sampling was performed using PAST Software (Paleontological Statistic software v. 2.12) (Hammer 2011). Finally, a dendrogram was built based on the similarity in flower visitor composition between Anthurium species.
Network analysis and species functional roles
To conduct a network analysis, a cumulative interaction Anthurium—flower visitor matrix was built for the Native and Pine forests pooling interactions recorded in 24 samplings. Then, the modularity and nestedness of each network were estimated (Felix et al. 2022). Also, the degree, interaction strength, specialization (d´) (Bluethgen et al. 2006), and functional roles of each species within the network were evaluated (Olesen et al. 2007). In modular networks, this functional role is defined by the values of two parameters, c (i.e., among-module connectivity: a measure of how connected a species is to all modules in the network) and z (i.e., within-module degree: the number of links of a species to other species within a module). These parameters group species into peripherals, connectors, module hubs, and network hubs (Olesen et al. 2007). We used the Bipartite package (Dormann et al. 2009) called by R to perform the network analysis and the Circlize package (Gu et al. 2014) called by R to draw the chord diagrams of the network.
Data analysis
The goodness of fit to a normal distribution was evaluated for each variable using a Shapiro–Wilk test. The Anthurium and insect species abundance and richness were related to the precipitation in each sampling date and the mean illuminance per site using linear regressions. A t test was also performed to investigate whether precipitation and illuminance differed between sites.
To evaluate the interspecific synchrony among Anthurium species, the counts of inflorescences per species in the ten quadrats on each forest/sampling were added, and the whole sampling period (one year) was transformed into a Julian date format. To test whether the time (Julian days) when the flowering peak of each Anthurium species (i.e., the highest number of open inflorescences of each species in a sampling) was significantly different among species, a Kruskal–Wallis test was used, since variables did not fulfill the normality or homoscedasticity criterion. In addition, an HSD Tukey test was used to evaluate whether the time when flowering peak occurred was different among pairs of Anthurium species in the community (Giorgis et al. 2015).
To evaluate whether flower visitor abundance and richness differed among low, medium, and high abundance of Anthurium open inflorescences, a Tukey test was used (Zar 1999). Considering that the number of open inflorescences was very different among forest types, abundance categories were defined based on the difference between the maximum and minimum value (i.e., range) observed in every sampling and the abundance range was divided into three categories that represent the flowering dynamics in each forest type (Native forest: low 0–6, Medium 7–12, High ≥ 13 and Pine forest: low 0–14, Medium 15–27, High ≥ 27). Also, an HSD Tukey test was used to evaluate differences in abundance and richness of flower visitors across flowering abundance categories (Zar 1999). Finally, the relationship between the degree, interaction strength, specialization (d’), and the functional role of each species estimated by c and z values with its abundance in each forest type was evaluated using linear regressions. All analyses were performed using R software (R Development Core Team 2018). The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Anthurium communities
Anthurium communities showed great differences in species abundance and composition among forest types. First, in the Native forest, the total abundance of Anthurium individuals was six times lower (N = 156) than in the Pine forest (N = 941), and the total number of flowering individuals was four times lower (Native N = 1166, Pine N = 4597). Also, in the Native forest, seven species of Anthurium were recorded, while in the Pine forest, A. microspadix was not present. Second, A. cupreum was the dominant species in both sites followed by A. microspadix and A. longegeniculatum in the Native forest, and by A. caucanum and A. bogotense in the Pine forest (Table 1).
With respect to the environmental variables, the accumulated precipitation every two weeks was three times higher in the Native forest than in the Pine forest (t = 2.215, P = 0.037). However, average illuminance showed the opposite trend (t = 4.679, P = 0.0001). In the Pine forest, a carpet of Anthurium dominated the understory, while in the Native forest there were other herbs or shrubs belonging to Rubiaceae and Melastomataceae, two typical plant families of tropical mountain forest (Idarraga-Piedrahita et al. 2011) which presented a very sporadic flowering in low abundance.
Flowering phenology and intra/interspecific synchrony
In both study sites, a constant and simultaneous flowering was evidenced and the flowering peak of each Anthurium species was significantly temporarily segregated, as indicated by the results of the Tukey test (Fig. 1; Table 2). The average values of the intraspecific flowering synchrony of each Anthurium species showed that in general, the species had asynchronous flowering events (less than 20%), regardless of their population abundance, with few exceptions of low synchrony, as is the case of populations of A. yarumalense (30%) and A. microspadix (34%) in the Native forest (Supplemental Table S2). Moreover, reproductive isolation values (RI) were higher than 70% in most of the comparisons between most pairs of Anthurium species which suggests that flowering phenology may function as a reproductive isolation barrier among the Anthurium species studied. In the case of A. caramantae, the estimated reproductive isolation with all other Anthurium species was low (33%) in the Native forest; however, due to the low number of individuals of this species, this result should be interpreted prudently (Supplemental Table S3).
Flowering phenology of seven Anthurium species in Parque Arví (Antioquia, Colombia) in Native and Pine forest. a Population size and total number of flowering individuals of each Anthurium species during the whole sampling period in Native forest, and b in Pine forest. Note that the Y-axes have different scales. c Number of flowering individuals in each of the 25 samplings performed every two weeks from August 2020 to August 2021 in the Native forest and d in the Pine forest. Due to the great differences in the number of flowering individuals between Anthurium species in the Pine forest, a second Y-axis in panel d was added, and it represents the number of flowering individuals for A. cupreum and A. caucanum
The total number of flowering Anthurium individuals per sampling in the Native forest did not show any relationship with precipitation levels (R2 = 0.02, β = − 0.02, F = 0.47, P = 0.4900). In contrast, in the Pine forest, the number of flowering individuals increased with precipitation (R2 = 0.20, β = 0.95, F = 5.85, P = 0.0200). Besides, the total number of flowering individuals per quadrat did not show a relationship with illuminance in any site. In general, these trends were observed when analyzing individual species, with some exceptions (Supplemental Table S4). Similarly, the abundance and richness of visiting insects in each sampling or quadrat did not depend on the two-week period average rainfall in either Native or Pine forests, or on the average illuminance of each transect (Supplemental Table S4). Regarding the bagged inflorescences of each Anthurium species, none of these produced infructescence, which indicates that seed production depends on pollinators.
Insect flower visitor communities
A total of 2059 individual insects of 123 morphospecies visiting the inflorescences of Anthurium were recorded. The most abundant orders were Thysanoptera (63%), with Frankliniella as the only genus of this order; Hymenoptera (11%), with Formicidae and Platygastridae as the most abundant families; Coleoptera (9%) with Curculionidae as the most abundant family; and Diptera (5%), with Drosophilidae as the most abundant family. The remaining 12% of the insect morphospecies was composed of individuals of other insect orders.
The abundance and composition of insect visitor assemblages differed among forest types (Pine forest: N = 1471, Native forest: N = 659, Bray–Curtis dissimilarity = 0.49) and among samplings in both study sites (~40%), with some exceptions of higher values (Supplemental Table S5). It also differed among Anthurium species. In both Native and Pine forest, A. yarumalense and A. cupreum had a higher abundance of insects visiting their inflorescences: 328 and 1257 insect individuals, respectively. Based on the rarefaction/extrapolation analysis, in the Native forest, A. caramantae showed a significantly lower insect richness compared to the other Anthurium species. In the Pine forest, A. cupreum had a higher richness than the other Anthurium species only when insect abundance was higher than 400 individuals (Supplemental Fig. S1, Table S6).
The composition of flower visitor assemblages showed high levels of dissimilarity (> 70%) among Anthurium species for most paired comparisons (Fig. 2; Supplemental Table S5, Table S6). However, three groups of Anthurium species which were related to spadix color and size were obtained: dark and medium size spadix (A. bogotense, A. caucanum and A. caramantae), green and small spadix (A. longegeniculatum and A. microspadix), and reddish/green and large spadix (A. cupreum and A. yarumalense) (Supplemental Fig. S2).
The shared fraction of insect visitors was variable among pairs of Anthurium species (0–0.48%) and was composed mainly of the most abundant morphospecies which visited between four and six species of Anthurium in each forest type (Fig. 2). In the Native forest, those species were Cyclanthura sp1, Frankliniella, and Myrmelachista sp.1. In the Pine forest, they were Cyclanthura sp.1, Frankliniella, and Phyllotrox sp.2. Some of these common insect morphospecies were observed shifting to the most abundant Anthurium flowering species in consecutive samplings (Supplemental Fig. S3).
Relationship between number of flowering individuals and insect visitor abundance and richness
In the Native forest, there was an increase in the abundance of flower visitors at medium floral abundance, and an increase in richness of visitors at medium and high floral abundance (Abundance floral visitor: χ2 = 17.69, P = 0.0001; Richness floral visitor: χ2 = 14.27, P = 0.0007, Supplemental Table S7). Contrastingly, in the Pine forest, the increase in both the abundance and richness of flower visitors occurred at medium or high floral abundance (Abundance floral visitor: χ2 = 15.34, P = 0.0004; Richness floral visitor: χ2 = 14.05, P = 0.0008) (Fig. 3; Supplemental Table S7).
Abundance of floral visitor assemblages to low, medium, and high abundance categories of flowering Anthurium individuals a in Native forest and b in Pine forest. Richness of flower visitor assemblages to low, medium, and high abundance categories of flowering Anthurium individuals c in Native forest and d Pine forest. Dotted lines in each panel represent the average abundance and richness of flower visitors in each forest type, and asterisks represent significant statistical differences among flowering abundance categories
Network structure and functional role of species
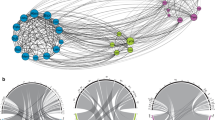
A significant modular structure was detected in Anthurium—flower visitor networks in both forests. One or two Anthurium species formed a module, which suggests that certain insect morphospecies visit predominantly one Anthurium species and few insects function as connectors among species (modules). The species degree and interaction strength increased with species abundance in the Pine forest but not in the Native forest. The species connectivity within and between modules (z and c values) was positively related to species abundance, except the z value in the Pine forest (Supplemental Table S8). The connector species were Frankliniella, in the Native forest; and Cyclanthura sp.1, in the Pine forest (Fig. 4; Supplemental Table S8). These species functioned as non-hubs connectors (z < 2.5) with links across several modules (c > 0.62). The rest of species were peripheral (c ≤ 0·62), with most links inside their own module (Olesen et al. 2007).
Discussion
Results from the study of Native and Pine forests showed differences in composition and abundance of Anthurium species, which reflected the contrasting environmental conditions and successional patterns in both forest types. As a consequence of these contrasting conditions, insect flower visitor communities also showed differences. However, a high resemblance in processes such as Anthurium flowering patterns and the temporal organization of interactions with insects visiting inflorescences was observed in both forest types.
Another important finding was that low precipitation and high illuminance promoted flowering in most Anthurium species, and therefore, the intensity of flowering was higher in the Pine forest than in the Native forest. However, the flowering in A. longegeniculatum, A. yarumalense, and A. microspadix was high in the Native forest where the illuminance intensity was low. These differences in flowering intensities suggest that signals that trigger flowering differ among species within the same genus and that the species flowering patterns obey to specific environmental cues, probably due to differences in evolutionary history, life strategies, and ecological adaptations (Kochmer & Handel 1986).
The results also revealed the occurrence of temporal segregation of flowering peaks and the high dissimilarity in the assemblage of flower visitors among Anthurium species in both forest types which might function to reduce competition. Moreover, they indicate that the continuous and overlapping flowering pattern of most Anthurium species forms a more attractive pool of flower resources for insects visiting flowers all year, which might function a facilitative mechanism of pollination through overlapping flowering. Finally, they evidenced that the differential use of shared flower visitors on fine time scales reduces competition in coexisting Anthurium species. Together, these strategies ensure the permanence of the flower visitor populations and the reproductive isolation among the Anthurium species.
In general, the study established that the flowering of Anthurium species can be categorized as continual according to Newstrom et al.’s (1994) classification, which means that there is a continuous flowering throughout the year, interrupted only by a few short breaks. This continuous flowering of several Anthurium species leads to overlapping flowering phenologies, with low levels of intraspecific synchrony and significant temporal segregation of the flowering peaks.
The above-mentioned phenomenon, called partial asynchrony, allows temporary segregation in the use of a shared resource such as pollinators, and reduces competition (Henderson et al. 2000; Husband & Sabara 2004; Gurung et al. 2018). The temporal segregation of flowering peaks may also limit the transfer of heterospecific pollen and act as an effective reproductive isolation mechanism between pairs of Anthurium species (Martin & Willis 2007). Besides, each Anthurium species may become a stronger competitor during its flowering peak, increasing the probability of massive and successful pollination due to a visitor moving sequentially and reliably among conspecific inflorescences (Willmer 2011; Gurung et al. 2018). Furthermore, continuous and asynchronous flowering promotes outcrossing in plant species with temporarily separated sexual phases (i.e., dichogamy) and self-incompatibility, such as Anthurium. This is due to the fact that it prevents all individuals within the population from being in the same sexual phase simultaneously and increases the probability of outcrossing by forcing pollinators to disperse pollen between plants (Rathcke & Loncey 1985; Bronstein et al. 1990).
At the same time, intraspecific asynchronous flowering events result in low flower density, making it hard to maintain pollinator assemblages and lowering the chances of reproductive success, as flower visitors leave patches when resources are scarce (Augspurger 1981). In this community, this might be prevented due to the co-flowering of several Anthurium species over time, which guarantees permanent resources for the maintenance of potential pollinators that depend on the flowering of Anthurium for the permanence of their populations and ecological functions. This co-flowering of Anthurium species may be related to a facilitation mechanism evidenced by the significant increase in the abundance and richness of insect visitors when the density of open inflorescences was high. Thus, a continuous and aggregated floral display in a specific time attracted more potential pollinators from more different taxa, which might improve the reproductive success of the coexisting Anthurium species. These mechanisms allowed the species with low population density (i.e., rare) and low investment in flower production to be visited by pollinators and reproduce (i.e., increase in fitness) on account of other flowering species (Valladares et al. 2015). Each Anthurium species contributed throughout the year to the maintenance of the floral display in higher or lower proportion. For example, A. cupreum invested in more and larger inflorescences compared to the other Anthurium species in the community which present medium to small inflorescences in lower numbers. In consequence, the fitness gain could be asymmetric among Anthurium species since some species obtain higher benefits (i.e., insect visits), which mitigates the adverse effects of low density for pollination (Moeller 2004). Evidence of this is, A. caramantae, a species with high ornamental value which has been extracted from the forest, leading to a very small population size, small number of inflorescences, and low probability of insect visits. However, it received few visits when co-flowering with other Anthurium species. Such great differences in Anthurium species densities have also been recorded in other mountain forests in the state of Antioquia, where A. cupreum was also dominant (Gómez-Murillo & Cuartas-Hernández 2016).
We hypothesized that facilitation for pollination services could be a mechanism to ensure seed production in Anthurium species, which in general, present low reproductive success, related to an inefficient pollination (Uemura et al. 1993; Moreno-Betancur & Cuartas-Hernández 2022), or short viability of pollen grains (Albre & Gibernau 2008). Although we did not examine fruit or seed production, which limits our conclusions about the facilitation as a mechanism operating among the studied Anthurium species, the shown increase in abundance and richness of flower visitors when there were more available flower resources is a first insight for this assumption. The reproductive success of Anthurium species is a matter to research in the future. Most studies describing the mechanism of facilitation for pollination in congeneric species have not associated such mechanism with fitness estimates (Gross et al. 2000; Gurung et al. 2018; Bergamo et al. 2020). In studies on communities of congeneric species of Clarkia and grassland plant species, for example, facilitation has been described based on the increase on pollen deposition and number of pollen tubes as estimates of fitness related to the presence of multiple congeners or to flowering synchrony and floral trait similarity, respectively (Moeller et al. 2004, Bergamo et al. 2020). The positive effect on the abundance and diversity of flowers on pollinator visitation in congeneric species of Primula in the Himalayas was considered an argument for pollination facilitation, as in our case (Gurung et al. 2018). Facilitation has also been described in studies on species from different genus with different floral morphologies (Gross et al. 2000, Ghazoul et al. 2006, Bergamo et al. 2020).
Other relevant finding of the study was that more than half of the interactions between plants and insect occurred once or twice, both in the Pine forest (79%) and the Native forest (79%), reflecting the erratic use of flower resources. However, a small fraction of interactions (12% for both forest types) were frequent and performed by four insect morphospecies (Frankliniella, Cyclanthura sp.1, Myrmelachista sp.1, Phyllotrox sp.2) that were the most abundant and that visited five or six Anthurium species throughout the year, functioning as connector species that link modules together and are thus important to network coherence. Although networks of both forest types presented modular structures, the functional role of species changed spatially. Frankliniella functioned as a connector species in the Native forest (c = 0.72, z = 1.93), while this role was played by Cyclanthura sp.1 in the Pine forest (c = 0.67, z = 1.5).
On the other hand, the interactions changed over time due to the rewiring of shared and frequent insect taxa (i.e., connectors) to the most abundant flower resource in a specific sampling date, due not only to the higher availability but to more intense attraction signals of inflorescences (i.e., aroma, color) (Levin & Anderson 1970; Bergamo et al. 2020).
Another interesting result finding is that the studied Anthurium species could be grouped into a generalist pollination syndrome (Willmer 2011) with all Anthurium species being visited by a diverse array of small insects representing four to five orders (Bawa et al. 1985). The generalist pollination syndrome can be related to the open morphology of inflorescences which do not limit the spectra of visitors, which has also been described for several species within the genus Anthurium (Díaz Jiménez et al. 2019). Although specialized relationship of some Anthurium species with Euglossine bees has been described in other ecosystems (Hentrich et al. 2010), we did not observe bees visiting inflorescences of the studied Anthurium species. Nevertheless, the differences in the composition of flower visitors among the three groups of Anthurium species obtained seem to be related to differences in spadix color and size. In grassland communities in Brazil, co-flowering plants possessing similar floral color flowered synchronously, and also shared pollinators. In addition, those co-flowering plants had higher fitness via joint pollinator attraction, suggesting that facilitative mechanisms act favoring flowering synchrony and trait similarity (Bergamo et al. 2020).
On the other hand, there were also certain tighter interactions between A. cupreum – Frankliniella, and A. bogotense—Cyclanthura sp.1 which were constant during the year and in both forest types, highlighting the importance of small insects of Thysanoptera and Coleoptera orders as potential pollinators in Anthurium (Wardhaugh 2015; Sayers et al. 2019).
This study contributes to understanding the mechanisms underlying the coexistence of sympatric Anthurium species (Vogt 2009). Facilitation and competition seem to shape the use of floral visitor resources and operate at different temporal scales (Valladares et al. 2015). Facilitation occurs through almost the whole year, while competition occurs in a temporal recruitment window in which exogenous temporal variability (e.g., precipitation and illuminance intensity) determines which species is the best competitor (i.e., has more intense flowering) in each habitat type during specific periods, limiting the use of the resource by other species (Barot 2004). Faegri & van der Pijl (1966) called this the Arnell´s dominating flower phenomenon.
Thus, facilitation might occur through pollination service sharing in Anthurium species, which might be an advantageous strategy in the cold environment of the mountain forest, where plant reproduction can be limited (Valladares et al. 2015) due to low insect abundance (Blionis & Vokou 2001; Barrios et al. 2010; Cuartas-Hernández & Gómez-Murillo 2015).
Shifts from competition to facilitation have been demonstrated in stressful and milder environments highlighting the importance of positive interactions on species coexistence and maintenance of diversity in plant communities (Holmgren & Scheffer 2010; Gross et al. 2013).
Lastly, differences in insect composition in both forest types highlight the relevance of continuing the conservation efforts of several vegetation cover types resulting from different successional processes to maintain the insect diversity and ensure the pollination services in the understory plants in the Parque Arví Reserve.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Albre J, Gibernau M (2008) Reproductive biology of Arum italicum (Araceae) in the south of France. Bot J Linnean Soc 156(1):43–49. https://doi.org/10.1111/j.1095-8339.2007.00737.x
Armbruster WS, Edwards ME, Debevec EM (1994) Floral character displacement generates assemblage structure of western Australian triggerplants (Stylidium). Ecology 75(2):315–329. https://doi.org/10.2307/1939537
Augspurger CK (1981) Reproductive synchrony of a tropical shrub: experimental studies on effects of pollinators and seed predators in Hybanthus prunifolius (Violaceae). Ecology 62(3):775–788. https://doi.org/10.2307/1937745
Baack E, Melo MC, Rieseberg LH, Ortiz-Barrientos D (2015) The origins of reproductive isolation in plants. New Phytol 207(4):968–984. https://doi.org/10.1111/nph.13424
Barot S (2004) Mechanisms promoting plant coexistence: can all the proposed processes be reconciled? Forum Oikos 106(1):185–192
Barrios Y, Ramírez N, Ramírez E, Sánchez E, Del Castill R (2010) Importancia de los polinizadores en la reproducción de seis especies de subpáramo del pico Naiguatá (Parque Nacional El ÁvilaVenezuela). Acta Botanica Venezuelica 33(2):213–231
Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, Kornbluth S, Winfree R (2011) Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc Natl Acad Sci 108(51):20645–20649. https://doi.org/10.1073/pnas.1115559108
Bawa KS, Bullock SH, Perry DR, Coville RE, Grayum, MH (1985) Reproductive biology of tropical lowland rain forest trees. II. Pollination systems. Am J Bot 72(3):346–356. https://doi.org/10.1002/j.1537-2197.1985.tb05358.x
Benavides AM, Morales PA, Cardona FA, Aguirre G, Molina DM, Hernández D, Valencia Y, Gutiérrez P, Vanegas C (2015) Tercer monitoreo de la comunidad de especies indicadoras y la medición de variables físicas del suelo en los senderos del parque Arví. Alcaldía de Medellín, Secretaría de Medio Ambiente.
Bencke CSC, Morellato LPC (2002) Estudo comparativo da fenologia de nove espécies arbóreas em três tipos de florsta atlântica no sudeste do Brasil. Revista Brasileira De Botânica 25:237–248. https://doi.org/10.1590/S0100-84042002000200012
Bennett JM, Thompson A, Goia I, Feldmann R, Ştefan V, Bogdan A, Rakosy D, Beloiu M, Biro I, Bluemel S, Filip M, Madaj AM, Martin A, Passonneau S, Kalisch DP, Scherer G, Knight T (2018) A review of European studies on pollination networks and pollen limitation, and a case study designed to fill in a gap. AoB PLANTS 10(6):ply068. https://doi.org/10.1093/aobpla/ply068
Bergamo PJ, Streher NS, Wolowski M, Sazima M (2020) Pollinator-mediated facilitation is associated with floral abundance, trait similarity and enhanced community-level fitness. J Ecol 108:1334–1346. https://doi.org/10.1111/1365-2745.13348
Blionis GJ, Vokou D (2001) Pollination ecology of Campanula species on Mt Olympos. Greece Ecography 24(3):287–297. https://doi.org/10.1111/j.1600-0587.2001.tb00201.x
Blüthgen N, Menzel F, Blüthgen N (2006) Measuring specialization in species interaction networks. BMC Ecol 6(1):1–12. https://doi.org/10.1186/1472-6785-6-9
Bronstein JL, Gouyon PH, Gliddon C, Kjellberg F, Michaloud G (1990) The ecological consequences of flowering asynchrony in monoecious figs: a simulation study. Ecology 71(6):2145–2156. https://doi.org/10.2307/1938628
Campbell DR (1985) Pollinator sharing and seed set of Stellaria pubera: competition for pollination. Ecology 66(2):544–553. https://doi.org/10.2307/1940403
Cardona J, Lara C, Ornelas JF (2020) Pollinator divergence and pollination isolation between hybrids with different floral color and morphology in two sympatric Penstemon species. Sci Rep 10(1):8126. https://doi.org/10.1038/s41598-020-64964-8
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67. https://doi.org/10.1890/13-0133.1
Chartier M, Gibernau M, Renner SS (2014) The evolution of pollinator-plant interaction types in the Araceae. Evolution 68(5):1533–1543. https://doi.org/10.1111/evo.12318
Chouteau M, Gibernau M, Barabé D (2008) Relationship between floral characters, pollination mechanisms, life forms, and habitats in Araceae. Biol J Lin Soc 156(1):29–42. https://doi.org/10.1111/j.1095-8339.2007.00753.x
Colorado Zuluaga GJ, Vásquez Muñoz JL, Mazo Zuluaga IN (2017) Modelo de conectividad ecológica de fragmentos de bosque andino en Santa Elena (Medellín, Colombia). Acta Biológica Colombiana 22(3):379–393. https://doi.org/10.15446/abc.v22n3.63013
Conner JK, Rush S (1996) Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum. Oecologia 105:509–516. https://doi.org/10.1007/BF00330014
Corantioquia (1999) (Ministerio del Medio Ambiente), Convenio BID CORANTIOQUIA. Plan de ordenamiento y manejo del Parque Regional Arví. Medellín.
Croat TB (2015) A review of studies on neotropical Araceae. Aroideana 38(1):44–54
Croat TB, Sheffer D (1983) The Sectional Groupings of Anthurium (Araceae). Aroideana 6(3):85–123
Cuartas-Hernández S, Gómez-Murillo L (2015) Effect of biotic and abiotic factors on diversity patterns of anthophyllous insect communities in a tropical mountain forest. Neotrop Entomol 44:214–223. https://doi.org/10.1007/s13744-014-0265-2
Díaz Jiménez P, Hentrich H, Aguilar-Rodríguez PA, Krömer T, Chartier M, MacSwiney MCG, Gibernau M (2019) A review on the pollination of aroids with bisexual flowers. Ann Mo Bot Gard 104:83–104
Dormann CF, Fründ J, Blüthgen N, Gruber B (2009) Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol J 2:7–24
Elzinga JA, Atlan A, Biere A, Gigord L, Weis AE, Bernasconi G (2007) Time after time: flowering phenology and biotic interactions. Trends Ecol Evol 22(8):432–439. https://doi.org/10.1016/j.tree.2007.05.006
Esposito F, Vereecken NJ, Gammella M, Rinaldi R, Laurent P, Tyteca D (2018) Characterization of sympatric Platanthera bifolia and Platanthera chlorantha (Orchidaceae) populations with intermediate plants. PeerJ 6:e4256. https://doi.org/10.7717/peerj.4256
Faegri K, van der Pijl L (1966) The principles of pollination ecology. Pergamon, New York, p 248
Felix GM, Pinheiro RBP, Jorge LR, Lewinsohn TM (2022) A framework for hierarchical compound topologies in species interaction networks. Oikos 2022(12):e09538. https://doi.org/10.1111/oik.09538
Ghazoul J (2006) Floral diversity and the facilitation of pollination. J Ecol 94:295–304
Giorgis MA, Cingolani AM, Gurvich DE (2015) Flowering phenology, fruit-set and seed mass and number of five coexistent Gymnocalycium (Cactaceae) species from Cordoba Mountain, Argentina. J Torrey Bot Soc 142:220–230. https://doi.org/10.3159/TORREY-d-14-00017.1
Gómez-Murillo L, Cuartas-Hernández SE (2016) Patterns of diversity of flower-visitor assemblages to the understory Araceae in a tropical mountain forest in Colombia. J Insect Conserv 20:1069–1085. https://doi.org/10.1007/s10841-016-9945-z
Grab H, Blitzer EJ, Danforth B, Loeb G, Poveda K (2017) Temporally dependent pollinator competition and facilitation with mass flowering crops affects yield in co-blooming crops. Sci Rep 7(1):45296. https://doi.org/10.1038/srep45296
Gross CL, Mackay DA, Whalen MA (2000) Aggregated flowering phenologies among three sympatric legumes–the degree of nonrandomness and the effect of overlap on fruit set. Plant Ecol 148:13–21
Gross N, Börger L, Soriano-Morales SI, LeBagousse-Pinguet Y, Quero JL, García-Gómez M, Valencia-Gómez M, Maestre FT (2013) Uncovering multiscale effects of aridity and biotic interactions on the functional structure of Mediterranean shrublands. J Ecol 101(3):637–649. https://doi.org/10.1111/1365-2745.12063
Gu Z, Gu L, Eils R, Schlesner M, Brors B (2014) Circlize Implements and enhances circular visualization in R. Bioinformatics. https://doi.org/10.1093/bioinformatics/btu393
Gurung PD, Ratnam J, Ramakrishnan U (2018) Facilitative interactions among co-flowering Primula species mediated by pollinator sharing. Plant Ecol 219:1159–1168. https://doi.org/10.1007/s11258-018-0868-5
Hammer Ø (2011) PAleontological STatistics PAST. Version 2.12. Natural History Museum. University of Oslo, Norway.
Hartley N, Gibernau M (2019) High Diversity of Biotic Interactions in the Megagenus Anthurium Schott (Araceae). Aroideana 42:139–249
Henderson A, Fischer B, Scariot A, Pacheco MAW, Pardini R (2000) Flowering phenology of a palm community in a central Amazon forest. Brittonia 52:149–159. https://doi.org/10.2307/2666506
Hentrich H, Kaiser R, Gottsberger G (2010) Floral biology and reproductive isolation by floral scent in three sympatric aroid species in French Guiana. Plant Biol 12:587–596
Holmgren M, Scheffer M (2010) Strong facilitation in mild environments: the stress gradient hypothesis revisited. J Ecol 98(6):1269–1275. https://doi.org/10.1111/j.1365-2745.2010.01709.x
Hsieh TC, Ma KH, Chao A (2016) APPLICATION iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. https://doi.org/10.1111/2041-210X.12613
Husband BC, Sabara HA (2004) Reproductive isolation between autotetraploids and their diploid progenitors in fireweed, Chamerion angustifolium (Onagraceae). New Phytol 161(3):703–713
Idarraga-Piedrahita A, del Carmen Ortiz R, Callejas-Posada R, Merello M (2011) Flora de Antioquia. Catálogo de las plantas vasculares. Volumen II. Listado de las plantas vasculares del departamento de Antioquia. Universidad de Antioquia, Medellín, Colombia.
Johnson SD, Peter IC, Nilsson A, Agren J (2003) Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology 84(11):2919–2927. https://doi.org/10.1890/02-0471
Johnson CA, Dutt P, Levine JM (2022) Competition for pollinators destabilizes plant coexistence. Nature 607(7920):721–725. https://doi.org/10.1038/s41586-022-04973-x
Kochmer JP, Handel SN (1986) Constraints and competition in the evolution of flowering phenology. Ecol Monographs 56(4):303–325. https://doi.org/10.2307/1942549
Levin DA, Anderson WW (1970) Competition for pollinators between simultaneously flowering species. Am Nat 104(939):455–467
Ma YP, Xie WJ, Sun WB, Marczewski T (2016) Strong reproductive isolation despite occasional hybridization between a widely distributed and a narrow endemic Rhododendron species. Sci Rep 6(1):19146. https://doi.org/10.1038/srep19146
Martin NN, Willis JH (2007) Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61(1):68–82. https://doi.org/10.1111/j.1558-5646.2007.00006.x
Mayo S, Bogner J, Boyce P (1997) The Genera of Araceae. Kew Bull 53:505–507
Mesquita-Neto JF, Silva-Neto CM, Franceschinelli EV (2015) Theoretical predictions of plant-pollinator interactions in sympatric species of Psychotria (Rubiaceae) in Cerrado of Brazil. Plant Ecol Evol 148(2):229–236
Minckley RL, Wcislo WT, Yanega D, Buchmann SL (1994) Behavior and phenology of a specialist bee (Dieunomia) and sunflower (Helianthus) pollen availability. Ecology 75(5):1406–1419. https://doi.org/10.2307/1937464
Mitchell RJ, Irwin RE, Flanagan RJ, Karron JD (2009) Ecology and evolution of plant–pollinator interactions. Ann Bot 103:1355–1363. https://doi.org/10.1093/aob/mcp122
Moeller DA (2004) Facilitative interactions among plants via shared pollinators. Ecology 85(12):3289–3301. https://doi.org/10.1890/03-0810
Moreno-Betancur DJ, Cuartas-Hernández SE (2022) Divergencia en la estrategia reproducti-va de dos especies simpátricas de Anthurium (Araceae) en un bosque andino tropical. Caldasia 44(1):54–68. https://doi.org/10.15446/caldasia.v44n1.89347
Mu J, Wu Q, Yang Y, Huang M, Grozinger CM (2018) Plant reproductive strategies vary under low and high pollinator densities. Oikos 127:1081–1094. https://doi.org/10.1111/oik.04711
Newstrom LE, Frankie GW, Baker HG (1994) A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La Selva, Costa Rica. Biotropica 26:141–159
Olesen JM, Bascompte J, Dupont YL, Jordano P (2007) The modularity of pollination networks. Proc Natl Acad Sci 104(50):19891–19896. https://doi.org/10.1073/pnas.070637510
Pianka ER (1973) The structure of lizard communities. Annu Rev Ecol Syst 4(1):53–74. https://doi.org/10.1146/annurev.es.04.110173.000413
Pleasants JM (1980) Competition for bumblebee pollinators in rocky mountain plant communities. Ecology 61(6):1446–1459. https://doi.org/10.2307/1939053
R Core Team (2018) The R project for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org.
Raine NE, Pierson AS, Stone GN (2007) Plant–pollinator interactions in a Mexican Acacia community. Arthropod-Plant Interactions 1:101–117. https://doi.org/10.1007/s11829-007-9010-7
Rathcke B, Lacey EP (1985) Phenological patterns of terrestrial plants. Annu Rev Ecol Syst 16(1):179–214. https://doi.org/10.1146/annurev.es.16.110185.001143
Ruchisansakun S, Tangtorwongsakul P, Cozien RJ, Smets EF, van der Niet T (2016) Floral specialization for different pollinators and divergent use of the same pollinator among co-occurring Impatiens species (Balsaminaceae) from Southeast Asia. Bot J Linn Soc 181(4):651–666. https://doi.org/10.1111/boj.12427
Rymer PD, Whelan RJ, Ayre DJ, Weston PH, Russell KG (2005) Reproductive success and pollinator effectiveness differ in common and rare Persoonia species (Proteaceae). Biol Cons 123:521–532
Sayers TDJ, Steinbauer MJ, Miller RE (2019) Visitor or vector? The extent of rove beetles (Coleoptera; Staphylinidae) pollination and floral interactions. Arthropod-Plant Interactions 13:685–701. https://doi.org/10.1007/s11829-019-09698-9
Schoener TW (1983) Field experiments on interspecific competition. Am Nat 122(2):240–285
Stone GN, Willmer P, Rowe JA (1998) Partitioning of pollinators during flowering in an African Acacia community. Ecology 79(8):2808–2827. https://doi.org/10.1890/0012-9658(1998)079[2808:POPDFI]2.0.CO;2
Uemura S, Ohkawara K, Kudo G, Wada N, Higashi S (1993) Heat-production and cross-pollination of the Asian skunk cabbage Symplocarpus renifolius (Araceae). Am J Bot 80(6):635–640. https://doi.org/10.1002/j.1537-2197.1993.tb15233.x
Valladares F, Bastias CC, Godoy O, Granda E, Escudero A (2015) Species coexistence in a changing world. Front Plant Sci 6:866
Vogt DR (2009) Spatial mechanisms promoting plant coexistence: the role of dispersal and competition. Phd thesis. Universität Basel.
Wardhaugh CW (2015) How many species of arthropods visit flowers? Arthropod-Plant Interactions 9(6):547–565. https://doi.org/10.1007/s11829-015-9398-4
Waser NM (1983) Competition for pollination and floral character differences among sympatric plant species: a review of evidence. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand Reinhold, New York, pp 277–293
Willmer P (2011) Pollination and floral ecology. Princeton University Press. https://doi.org/10.1515/9781400838943
Wright SJ, Calderon O (1995) Phylogenetic patterns among tropical flowering phenologies. J Ecol 83(6):937–948
Yang CF, Gituru RW, Guo YH (2007) Reproductive isolation of two sympatric louseworts, Pedicularis rhinanthoides and Pedicularis longiflora (Orobanchaceae): how does the same pollinator type avoid interspecific pollen transfer? Biol J Lin Soc 90(1):37–48. https://doi.org/10.1111/j.1095-8312.2007.00709.x
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Englewood Cliffs, p 663
Acknowledgements
Thanks to Corporación Parque Arví for the logistic support. We are greatly indebted to Augusto León Montoya for the identification of insects of Syrphidae family, Herbario Universidad de Antioquia for assistance with identification of plant species, and Deisy Vasquez, Claudia Vanegas and Kevin Jaramillo for their assistance in the fieldwork. This work was supported by Convenio de Cooperación N° 21460001-0274-2020 Universidad de Antioquia- Corporación Parque Arví to develop the project entitled: “Ensamblaje de visitantes florales en cinco especies de Anthurium del sotobosque en el Parque Arví (Antioquia, Colombia)” 2020-36171. Collection license: Permiso marco de recolección de especímenes de la Universidad de Antioquia, Resolución 0524 de 2014.
Funding
Open Access funding provided by Colombia Consortium. Funding was supported by Corporación Parque Arví, 21460001-0274-2020.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by MFBC. The analyses were performed by both authors. The manuscript was written, read and approved by both authors.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Handling Editor: Ingeborg Menzler-Hokkanen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cano, M.F.B., Hernández, S.E.C. Flowering phenology patterns promotes pollination facilitation in coexisting Anthurium species from a mountain forest in Colombia. Arthropod-Plant Interactions (2024). https://doi.org/10.1007/s11829-024-10096-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11829-024-10096-z