Abstract
There is increasing evidence that parasitism represents an unpredictable dimension of the ecological impacts of biological invasions. In addition to the risk of exotic pathogen transmission, other mechanisms such as parasite-release, could contribute to shaping the relationship between introduced species and native communities. In this study, we used the Eurasian round goby (Neogobius menalostomus) in the Great Lakes-St. Lawrence River ecosystem to further explore these ideas. As predicted by the parasite-release hypothesis, recently established populations of round goby were parasitized by a depauperate community of generalist helminths (8 taxa), all commonly found in the St. Lawrence River. In comparison, two native species, the logperch (Percina caprodes) and spottail shiner (Notropis hudsonius), were the hosts of 25 and 24 taxa respectively. Round gobies from each of 3 sampled localities were also less heavily infected than both indigenous species. This is in contrast to what is observed in round goby’s native range where the species is often the most parasitized among gobid competitors. This relative difference in parasite pressure could enhance its competitiveness in the introduced range. However, our study of an older population of round goby in Lake St. Clair suggests that this advantage over native species could be of short duration. Within 15 years, the parasite abundance and richness in the round goby has more than doubled whereas the number of parasite species per fish has increased to levels of those typical of fish indigenous to the St. Lawrence-Great Lakes watershed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research on the impact of invasive species on native communities has primarily focused on the ways invaders affect resident species directly through predation and/or competition for resources and space. In the last decade however, scientists have become increasingly aware of the critical role of parasites in the structuring of ecological communities (Marcogliese 2004; Horwitz and Wilcox 2005; Hudson et al. 2006) and the numerous ramifications of their influence in the context of biological invasions (Cunningham et al. 2003; Telfer et al. 2005; Torchin and Lafferty 2009; Dunn 2009; Tompkins et al. 2010).
It has long been known that introduced species can act as vectors of exotic parasites with potential devastating effects for native hosts which did not co-evolve with these newcomers (Anderson and May 1986; Cunningham et al. 2003; Taraschewski 2006; Marcogliese 2008). Aside from the risk of introducing pathogens, there are several other mechanisms by which parasites could contribute to shaping the relationships between introduced species and native communities (Torchin and Mitchell 2004; Telfer et al. 2005; Kopp and Jokela 2007; Kelly et al. 2009). An increasing number of empirical studies notably have revealed that introduced populations tend to have lower prevalence of infection by fewer parasite taxa in an invaded range compared to their counterparts in their original range (Torchin et al. 2003; Torchin and Mitchell 2004; Marr et al. 2008; Blakeslee et al. 2009; Ross et al. 2010). Following the enemy-release hypothesis, this escape from parasites and other natural enemies could confer a competitive advantage to certain non-native species, contribute to their demographic expansion and enhance their invasion success (Torchin et al. 2003; Torchin and Mitchell 2004; Torchin and Lafferty 2009).
The phenomenon of parasite-escape has many interlinked explanations (Torchin and Mitchell 2004; MacLeod et al. 2010). Firstly, a large part of the original parasite fauna of an intruder is usually left behind in the process of invasion. Actually, the colonization of new habitats is often realized by just a handful of colonists, sometimes in the form of early life-history stages (eggs, larvae). As parasites are generally aggregated among hosts and tend to accumulate in larger/older individuals, the likelihood of bringing in infected individuals is therefore reduced (Shaw and Dobson 1995; Sasal et al. 1997). The founders who survive the stress of translocation might also be less parasitized and/or more resistant to infection and disease than others. Even the parasites that accompany their hosts may not establish due to ecological constraints they encounter in the new habitats. This is particularly the case for metazoan parasites, many having complex life cycles requiring several hosts, which may be lacking in the invaded region. Directly transmitted parasites that are dependent on host density for their transmission might also fail to establish when introduced populations arise from a limited number of founders.
A number of authors have expressed divergent views in regards to the real implications of the parasite escape phenomenon (Colautti et al. 2004; Krakau et al. 2006; Liu and Stiling 2006). Most of the controversy surrounding the enemy release hypothesis originates from the difficulty in demonstrating that escaping their native parasites truly translates into measurable increase in the invader’s fitness. Except for a few cases such as the release of the introduced green crab populations from the demographic constraint imposed by parasitic castrators in their native range (Torchin et al. 2001), the advantage conferred by a translocation-induced parasite release has more often been assumed than concretely addressed (but see Roche et al. 2010). Moreover, in most studies, the level of parasite escape has been essentially quantified by comparing the level of parasitism of the species between areas of the introduced and original ranges (e.g. Torchin et al. 2001; Kvach and Stepien 2008; Blakeslee et al. 2009), which would vary considerably depending on local habitat characteristics, community structure as well as a number of other environmental factors (Colautti et al. 2004). Less is known about the relative difference in parasite load between the invasive species and its local competitors in the invaded and native environments (but see Lymbery et al. 2010; Roche et al. 2010). Another consideration that would influence the level of impact of the parasite escape phenomenon is its actual duration. New host-parasite associations are known to form over time in the invaded range leading to parasites being progressively recruited by introduced hosts (Cornell and Hawkins 1993; Krakau et al. 2006). Hence, the release from parasites could be a transitory situation. In an extensive review of published studies across diverse animal and plant taxa, Torchin and Mitchell (2004) found that introduced species had indeed accumulated parasites from the invaded communities but that the number of taxa recruited within the time frame observed was less than half the number they had escaped in their native range. How long this gap would last however, and more importantly, to which extent the recruited parasites could eventually offset the advantage (if any) induced by the loss of the original parasite fauna remain to be investigated.
Using the round goby (Neogobius melanostomus) as a model species in the St. Lawrence River-Great Lakes basin, we tested (1) whether the introduced populations of gobies were less parasitized than fish competitors native to the invaded range, and (2) whether this low level of parasitism was limited to the early phase of the invasion or maintained through time. For this purpose, parasitism of the round goby was compared with that of two native co-existing fish, the logperch (Percina caprodes) and the spottail shiner (Notropis hudsonius), in recently invaded areas of the St. Lawrence River (Quebec, Canada). The colonization of the introduced round goby by native parasites over time was assessed in the Great Lakes, where the fish is established for a longer time period, taking advantage of a historical portrait of the parasite fauna published soon after the species was originally introduced in North America (Muzzall et al. 1995).
Native to the Ponto Caspian region in Eastern Europe, the round goby was most likely transported to North America in the ballast waters of transoceanic freighters (Charlebois et al. 1997). Within a decade of its first sighting in St. Clair River in 1990, it had dispersed to most of the Laurentian Great Lakes where it is believed to threaten native benthic fish community through competition for resources and habitats (Dubs and Corkum 1996; Balshine et al. 2005; Bergstrom and Mensinger 2009). Coincidently with the expansion phase of its populations, declines of sculpins and native darters, such as the logperch, have been documented (Janssen and Jude 2001; Charlebois et al. 1997; Lauer et al. 2004). In the St. Lawrence River, which connects the Great Lakes to the Gulf of St. Lawrence and the Atlantic Ocean, the round goby was first reported in 1997 in the Quebec City region, where it seems to have been restricted for a period of time. It is only several years later (by 2004) that it invaded the upstream reach of the river where it is now widespread and abundant. A recent evaluation of the diet of predatory fish in the St. Lawrence River indicates that the round goby is progressively becoming a significant component of the local food web (Reyjola et al. 2010).
Materials and methods
Study area and fish sampling
Comparison with indigenous species
For this part of the study, we selected three sites along the St. Lawrence River recently invaded by the round goby (Fig. 1). In order to capture the recent invasion, sampling was performed within 2 years after first sighting of the species locally, that is in June of 2007 at Îlet Vert (45°42.430′N; 73°26.960′W) and Sorel (46°02.588′N; 73°08.824′ W), and in June of 2009 at Îles de la Paix (45°20.574′ N 73°50.963′W). A sample was also collected in June 2006 at Îlet Vert as part of a preliminary survey. In addition to the round goby, two co-occurring native fish species were sampled at each site. The fish community of the Great Lakes-St. Lawrence River system lacks representatives of the highly diversified gobiid family that inhabit the waters of the Ponto-Caspian region of Eurasia. The typical ecological niche of gobies is predominantly occupied in the St. Lawrence River by benthic percids (darters) and cottids (sculpins). One of the two indigenous species selected for comparison in this study, the logperch (Percina caprodes), is a common percid belonging to the darter group, that has seen its populations decline sharply in the Great Lakes since the expansion of the round goby, presumably due to competition for food and habitat (Charlebois et al. 1997). The parasite fauna of the round goby was also compared with that of the spottail shiner (Notropis hudsonius). Although more distant ecologically and phylogenetically to the invader than the logperch, this cyprinid fish is representative of small-sized/forage fish occupying the middle levels of the food web and is widespread and abundant in the shallow shorelines sampled in this study. Moreover, the parasite fauna of the spottail shiner in the St. Lawrence River has been extensively studied over the last 10 years (Marcogliese et al. 2006). All specimens were collected using a beach seine (22.6 m × 1.15 m; 3 mm mesh) held by hand or partially deployed from a boat. Approximately 30 fish per species per site ranging from 40 to 80 mm (standard length) were euthanized using a 50 mg/L Eugenol (clove oil) solution, individually bagged and frozen for subsequent parasitological examination. An additional sample of 5–10 fish per species/site was brought alive to the laboratory and kept in aerated tanks where they were maintained for less than 1 week until processed. These fish were used as a source of voucher specimens.
Study area in the St. Lawrence River. Shaded areas indicate reported occurrences of round gobies (Neogobius melanostomus) since the species was first discovered in 1997 near Quebec City. Fish icons illustrate locations where round gobies, logperch and spottail shiners were sampled: 1 = Sorel; 2 = Îlet Vert; 3 = Îles de la Paix
Temporal change in parasite fauna
To study the colonization of round goby by indigenous parasites over time, fish were collected in 2009 from the northwestern part of Lake St. Clair (Michigan), an area of the Great Lakes where the species has established since 1990. Every attempt was made to mirror the sampling design used in 1994 in the first study to report on parasites of the round goby in the Great Lakes (Muzzall et al. 1995). Specimens collected in 2009 were within the same size range (60–117 mm total length) and were captured at the same locations within Lake St. Clair (Fig. 2) by the same crew at the same time of year (end of August, beginning of September) using the same gear (mainly bottom trawl) as in 1994. Fish were captured at station 6413 (42°37.537′N, 82°43.816′W), 6414 (42° 38.029′N, 82° 43.036′W), 6415 (42° 38.077′N, 82° 43.036′W) and Dock (42° 35.594′N, 82° 46.348′W). All trawling was conducted from the Michigan Department of Natural Resource (MDNR) RV Channel Cat in water depths exceeding 2 m during daylight hours. Trawling gear consisted of a 10.7-m headrope otter trawl towed with single warp and a 45.7-m bridle. The trawl was constructed of 76, 38, and 32 mm graded stretched measure mesh from gape to cod end with a 9 mm stretched mesh liner in the cod end. Whenever possible, tows were made with the trawl on the lake bottom for 10 min at approximately 2.0 knots. Occasionally, tow duration was shortened to avoid heavy plant growth or other physical obstructions. A total of 42 round gobies collected following the above-mentioned procedure was frozen for subsequent parasitological examination.
Sampling locations in Lake St. Clair in August and September 2009. Round gobies (Neogobius melanostomus) were collected at four stations previously sampled in 1994 (see Muzzall et al. 1995)
Parasitological examination
Prior to dissection, standard and total lengths were measured to the nearest millimeter and each fish was weighed to the nearest 0.1 g and sexed. Fish were screened externally for parasites before being dissected. All organ/tissue were thoroughly examined for helminth parasites using a dissecting microscope and following routine parasitological techniques. Parasites were excysted, cleaned, enumerated and fixed. Cysts were either torn open mechanically or chemically dissolved by exposing them briefly to a diluted hypochlorite solution similar to the one used to excyst nauplii of Artemia sp. (Campton and Busack 1989). This technique is efficient in removing thick gelatinous multi-layer cysts of digenean metacerariae (e.g. Apatemon spp.) and of tiny nematode larvae, without damaging diagnostic structures. Unknown nematodes were cleared in glycerol whereas other helminths were stained in acetocarmine and whole mounts were prepared on slides for identification using the keys in Arai (1989), Moravec (1994), Gibson (1996), Hoffman (1999), Amin (2002) as well as Amin and Muzzall (2009). Parasites obtained from round goby in Lake St. Clair in 1994 and deposited by Muzzall et al. (1995) in the US National Parasite Collection (New York, USA) were examined and compared with specimens collected in 2009. Voucher specimens from the present study have been deposited in the Royal Ontario Museum (Toronto, Canada): Allopodocotyle sp. (ROMIZ C554), Azygia sp. (ROMIZ C555, ROMIZ C565), Bucephalidae gen. sp. (ROMIZ C567), Cosmocephalus obvelatus (ROMIZ F326), Crepidostomum sp. (ROMIZ C556), Cryptogonimus sp. (ROMIZ C557), Cucullanus sp. (ROMIZ F328), Diplostomum sp. (ROMIZ C558), Dichelyne sp. (ROMIZ F327), Ichthyocotylurus sp. (ROMIZ C559), Neochasmus sp. (ROMIZ C560, ROMIZ C566), Neoechinorhynchus tenellus (ROMIZ F320, ROMIZ F320), Neoechinorhynchus rutili (ROMIZ F321, ROMIZ F321), Ornithodiplostomum sp. (ROMIZ C561), Paracuaria adunca (ROMIZ F329), Paraquimperia tenerrima (ROMIZ F330), Plagioporus sinitsini (ROMIZ C562), Phyllodistomum sp. (ROMIZ C563), Tetrameres sp. (ROMIZ F325), Tylodelphys scheuringi (ROMIZ C564).
Diet analysis
We compared gut contents to estimate diet overlap as a measure of potential competition between the invasive round goby and the native logperch. After being screened for parasites, digestive tract contents of specimens collected in the St. Lawrence River in 2007 were preserved in 70% ethyl alcohol and sent to a specialized laboratory for identification of food items (Laboratoire SAB, Longueuil). Order was the targeted taxonomical level but organisms found were identified down to the family level whenever possible. Counts were provided for each identified taxon. However, due to prior manipulations of digestive tract contents to extract parasites potentially leading to loss or destruction of food organisms, these numbers are considered conservative.
Calculations and statistical analyses
Parasitological descriptors referred to below are as defined in Bush et al. (1997). Prevalence is the percentage of fish host infected with a particular parasite in a given sample. Mean abundance is the mean number of parasites of a given taxon per host in a sample, including uninfected hosts. Mean intensity is defined as the mean number of individuals of a given parasite taxon per infected host in a sample. Parasite community descriptors presented herein include component community richness and mean infracommunity richness. Infracommunity refers to the community of parasites in a host individual whereas component community encompasses the assemblage of parasites recovered from the entire sample of a host species (Bush et al. 1997). Fulton’s condition factor (K) was calculated as (W/L3) × 105 where W is the weight of the fish in gm and L is the standard length in mm (Ricker 1975). Diet results are presented as frequency of occurrence, corresponding to the percent fish in a sample having a given food organism in their digestive tracts.
The between-year comparison of the parasite fauna of round gobies from Lake St. Clair implied revisiting research results published 15 years ago (Muzzall et al. 1995). As the original data were not available (Patrick Muzzall, pers. comm.) we worked from summary data presented in the paper (Table 1) to derive missing parasitological descriptors (Table 1, Muzzall et al. 1995). Using mean intensity of infection by individual parasites, associated standard deviation and minimum and maximum intensity values when provided, a range of possible values for mean abundance and associated standard errors were generated. Similarly, possible values for infracommunity richness were calculated from prevalence of individual parasite species. When exact values could not be derived mathematically, maximal possible values (either mean or standard error) were reported. This was done in order to avoid overestimating the between-year difference. As an example, the standard error reported for the mean infracommunity richness corresponds to the scenario of maximum inter-specific aggregation of parasites among hosts.
Data were analysed using SAS® 9.1.3 (SAS Institute Inc., Cary, NC, USA). The combined effect of fish host and locality on mean abundance and mean infracommunity richness was first examined through two-way analyses of variance (ANOVA). Afterwards, the difference between the round goby and indigenous fish hosts in the St. Lawrence River was tested, for each locality, through one-way ANOVA followed by Tukey–Kramer multiple comparison of means. As parasitological data are typically aggregated and rarely meet normality assumptions of parametric analyses, ANOVAs were performed on rank-transformed data, using a normal score transformation instead of standard ranks (Conover 1999). The infection levels between two consecutive years at one locality of the St. Lawrence River were also compared as described above using two-way ANOVA (effect: year, species). The relationship between Fulton’s condition factor (K) and parasite abundance was tested using nonparametric correlation analyses for each host species. Between-year differences in mean abundance and infracommunity richness in Lake St. Clair were assessed using standard t tests. It should be noted however that, because of the unavailability of raw values, it was not possible to perform these tests on rank-transformed data.
Results
Gobies versus native fish in the St. Lawrence River
A total of 267 fish belonging to three species (round goby, logperch, spottail shiner) collected from three sites (Sorel, Îlet Vert, Îles de la Paix) in the St. Lawrence River were examined for helminth parasites. Data on fish length, weight and condition along with sample size for each species/site are presented in Table 1. At a given site, mean standard length of the fish retained for parasitological examination (ranging from 40 to 80 mm) were similar across species, except for spottail shiners from Îlet Vert that were significantly smaller than the other two species (Table 1). However, round gobies were significantly heavier than both native species at similar lengths, the difference being more pronounced in comparison with the logperch. Examination of the digestive contents confirmed significant overlap in the diet composition between gobies and logperch in the St. Lawrence River within the size range examined. The three dominant prey items in the diet of the logperch, with frequency of occurrence exceeding 30% (Amphipoda, Chironomidae and other Diptera), were among the most frequently consumed by round gobies. The non-native fish also relied heavily on Ostracoda, a prey that was virtually absent from the diet of the logperch (Fig. 3).
Of the 90 round gobies examined, around half were infected by helminth parasites. Prevalence of infection in the introduced species varied according to site of capture from 47% at Îles de la Paix and Sorel to 73% at Îlet Vert but was clearly lower than in native species whereas nearly all logperch (Îles de la Paix = 96%; Sorel = 100%; Îlet Vert = 100%) and spottail shiners (Îles de la Paix = 97%; Sorel = 97%; Îlet Vert = 100%) were infected by at least one parasite. All of the 8 helminth taxa found in the round goby were larval forms using the fish as an intermediate or transport (paratenic) host whereas both indigenous logperch and spottail shiner were the hosts of respectively 25 and 24 species, of which 7 and 5 were adult helminths (Table 2). The eyeflukes Diplostomum spp. were the predominant parasites of the round goby at these three sites, representing 57% of all parasites. The second most abundant parasite was an acanthocephalan, Neoechinorhynchus tenellus (23%), followed by larval nematodes (14%) such as Cosmocephalus obvelatus, Paracuaria adunca and Tetrameres sp. Other parasites included a few larval digeneans and cestodes that were only infrequently encountered (Table 2). No monogenean was found on the gills, fins or skin of round gobies from the St. Lawrence River. In contrast, the logperch and the spottail shiner were the hosts of a diversified helminth fauna largely dominated by digeneans but which also included species of acanthocephalans, nematodes, cestodes, and monogeneans (Table 2).
The mean infracommunity parasite species richness was significantly different among hosts and sites (two-way ANOVA; host: P < 0.0001, site: P < 0.0001, host*site: P = 0.1489). Round gobies were parasitized by significantly fewer taxa (P < 0.0001) than either indigenous fish species at each site (one-way ANOVA, Tukey post hoc comparison of the means; Fig. 4A). The total number of helminth species found at a given site (component community) in the round goby varied between 4 and 7, and was never more than half that of the logperch (17–20 taxa) or the spottail shiner (14–18 taxa).
Infection of round gobies (Neogobius melanostomus), logperch (Percina caprodes) and spottail shiners (Notropis hudsonius) by helminth parasites at three localities of the St. Lawrence River. Samples were collected within two years of first sighting of the round goby, that is in June 2007 at Îlet Vert and Sorel and in June 2009 at Îles de la Paix. a Mean infracommunity richness: mean number of helminth species per fish ± SEM. Numbers within histograms indicate the total number of helminth species in a given species/locality, corresponding to the component community richness. b Mean abundance: mean number of helminths per fish ± SEM. Significant differences among species within each locality are indicated by different letters above histograms
The mean abundance of all parasites followed the same trend as the infracommunity richness (two-way ANOVA; host: P < 0.0001, site: P < 0.0001). Round gobies were infected by consistently and significantly lower numbers of helminths than either the logperch or the spottail shiner, at each site (one-way ANOVA; P < 0.0001, Tukey post hoc multiple comparison of the means; Fig. 4B). There was considerable variation in mean abundance of parasites across sites for native hosts but not for the round goby where mean abundance was never more than 2 (site*host interaction: P < 0.009). The difference in parasite load between native and non-native fish was the greatest at Îlet Vert and Sorel in 2007 where the logperch was up to 18 times more parasitized than the round goby. In contrast, the level of infection was low in both the logperch and the spottail shiners at Îles de la Paix in 2009, but still about 7 times that of the round goby. Overall, digeneans contributed the most to the difference in helminth abundance between the round goby and native species, more specifically Neochasmus sp., Apatemon sp. and bucephalids in the logperch, and Diplostomum spp. and Plagioporus sinitsini in the spottail shiner.
Notwithstanding capture site, fish condition factor tended to decrease slightly with parasite abundance in both native fish but these correlations were not significant (spottail shiners: ρ = −0.11, P = 0.2891; logperch: ρ = −0.15, P = 0.1741). A few significant associations were detected between individual parasite taxa and fish condition. In the logperch, the number of bucephalid metacercariae (digenean) was negatively correlated with Fulton’s condition factor (ρ = −0.31, P = 0.0036) when fish were pooled over sites but the relationship was weaker and not significant when each site was considered separately. However, that same parasite was positively correlated with fish condition in the spottail shiner (ρ = 0.44, P = <0.0001). In this fish, the condition factor significantly decreased as the abundance of adult Neoechinorhynchus rutili (acanthocephalan) in the intestine increases (ρ = −0.50, P < 0.0001). No negative trend between body condition and parasite abundance of all parasites pooled (ρ = 0.10, P = 0.3476) or of individual taxa was observed in the round goby. All relationships tested were not significant. However, parasite abundance was very low in this species and a significant proportion of the fish were uninfected.
The level of parasite infection was also compared between two consecutive years (2006 and 2007) at Îlet Vert (Fig. 5). At that site, the mean infracommunity richness, component community richness and mean abundance in both the introduced round goby and the indigenous logperch were not found to fluctuate significantly between years.
Between-year change in parasite infection level in round gobies (Neogobius melanostomus) and logperch (Percina caprodes) at Îlet Vert. Mean abundance is expressed as the mean number of helminths per fish ± SEM. Mean infracommunity richness is the mean number of helminth species per fish ± SEM. Results of two-way ANOVA for each of these two variables are presented below the left and right panel, respectively
Temporal comparison in Lake St. Clair
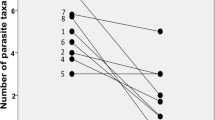
Table 3 compares the parasite fauna of the round goby from Lake St. Clair (Anchor Bay) in 2009 with published data on its parasites from the same locations in 1994. In 2009, the 42 round gobies were infected by a total of 15 helminth taxa whereas in 1994, 6 helminth taxa were found in the 62 specimens examined for parasites. This is paralleled by a change in the mean infracommunity species richness that significantly increased from 1 helminth per host in 1994 up to 3, in 2009 (t test; P < 0.0001). The eyeflukes Diplostomum spp. were the predominant parasites in 1994 and remained so in 2009, although four more digenean species have since colonized the round goby. More species of nematodes and acanthocephalans were also observed in 2009 and one cestode species was found whereas these parasites were absent from the 1994 sample. Among those taxa, one nematode species, Raphidascaris acus, was reported by Pronin et al. (1997) from a sample of 6 round gobies collected in Lake St. Clair south of Anchor Bay.
In addition to the increase in richness, the mean number of helminths per fish (mean abundance) almost doubled between 1994 and 2009 (Fig. 6). While digeneans were slightly more abundant in 2009 than in 1994 (but not significantly), nematodes and acanthocephalans showed the largest between-year difference in abundance, respectively increasing by a factor of 5 and 10 over that time period. This increase was mostly driven by five species including Neoechinorhynchus tenellus, Cucullanus sp., Paraquimperia tenerrima and Raphidascaris acus.
Mean abundance of helminths in round gobies (Neogobius melanostomus) from Lake St. Clair between 1994 and 2009. Error bars refers to SEM; Max maximum SEM among possible values as derived mathematically from Muzzall et al. (1995), all other SEM and means being exact values; All all helminth taxa together; Significant differences in mean abundance between 1994 and 2009, as revealed by a t test, are indicated by * (P < 0.05) or **** (P < 0.0001) above histograms; NS not significantly different
Discussion
This study of the parasite fauna of the introduced round goby provides additional empirical evidence in support of the enemy escape hypothesis, but at the same time suggests that the reduced parasitism experienced by an introduced species may be of short duration. Our results actually revealed that a significant rise in parasite infection occurred in less than two decades in the Great Lakes, where the round goby was colonized by an increasing number of indigenous parasites over that time.
To date, studies reporting on the parasite fauna of the invasive round goby have compared populations in invaded habitats with those in its native range (Pronin et al. 1997; Camp et al. 1999; Kvach and Stepien 2008; Ondračkova et al. 2005; Kvach and Skóra 2006; Rolbiecki 2006). To the best of our knowledge, the current research is the first to specifically test the parasite release hypothesis by comparing the parasite infection level between the round goby and co-existing competitors in invaded habitats. Our results indicate that in the early phase of its establishment in the St. Lawrence River, the round goby was significantly less infected by helminth parasites than two indigenous fish species from the same habitats.
None of the round gobies examined herein was found to host exotic parasites. Although many of the helminths observed have circumpolar distributions encompassing the goby’s native range, it is assumed that these parasites have been acquired post-invasion. Similar conclusions have been reached by Muzzall et al. (1995), Camp et al. (1999), and Kvach and Stepien (2008) for the Great Lakes populations. Until now, the only presumed parasite introduction by the round goby in North America is that of a myxozoan species (Sphaeromyxa sevastopoli) discovered in the gall bladder of a few individuals in St. Clair River and Lake St. Clair (Pronin et al. 1997). Despite systematic examination of gall bladder smears, we found no trace of this species or other myxozoans (David Cone, unpublished data) in the fish examined to date.
Introduced round gobies not only lost their native parasites, but their colonization by parasites native to the St. Lawrence River was also limited. While almost all the native logperch and shiners were infected by at least one helminth parasite, half of the round gobies collected at three sites in the St. Lawrence River were uninfected. Moreover, the parasitized gobies were intermediate or transport hosts for a depauperate community of helminths composed of eight taxa which were mainly larval generalist parasites commonly found in the St. Lawrence River/Great Lakes watershed. Predominance of widespread generalist parasites was also reported for other parts of the invaded range of the round goby including the Great Lakes and the Baltic Sea (Muzzall et al. 1995; Camp et al. 1999; Kvach and Stepien 2008). The parasite fauna of round gobies collected in the St. Lawrence River was dominated by eyeflukes (metacercariae of Diplostomum spp.), ubiquitous digeneans (Marcogliese and Compagna 1999; Marcogliese et al. 2006; Krause et al. 2010) which represented around half of the parasite load, followed by cystacanths of the acanthocephalan Neoechninorhynchus tenellus. Other representatives of Neoechinorhynchus spp. were previously reported elsewhere in round goby’s native and invaded range (e.g. N. rutili: Özer 2007; N. tumidus: Kvach and Stepien 2008) but N. tenellus, which typically uses percids and centrarchids as transport or definitive hosts, is a new record for the round goby (host extension).
In contrast to gobies, logperch and spottail shiners hosted a rich parasite community composed of 25 and 24 helminth taxa respectively which included mature worms using the fish as definitive hosts. This was paralleled by a consistently and significantly higher infracommunity richness in these two indigenous fish compared to the round goby, notwithstanding capture site. The parasite fauna of both the logperch and the spottail shiner was largely dominated by digeneans (15 and 14 taxa respectively) but it also comprised all other helminth groups including monogeneans which have not yet been found in the exotic round goby in North America (this study; Muzzall et al. 1995; Pronin et al. 1997; Camp et al. 1999; Kvach and Stepien 2008). However, monogeneans are typically considered fairly host specific (Whittington et al. 2000).
The potential advantage of being infected by a reduced number of parasite taxa could be offset if individual taxa responded by increasing their abundance as a consequence of density-dependent effects (Dezfuli et al. 2002; Fredensborg and Poulin 2005). We found no evidence thereof in our data. To the contrary, the difference in the mean abundance of helminths between the round goby and the indigenous hosts was even greater than that found in terms of infracommunity richness. Whereas the parasite load of native hosts varied across sites, it was constantly low, never exceeding 2 parasites per fish in the introduced goby. Much higher values of parasite abundance are commonly reported in goby populations in its native range (Kvach 2002, 2005; Özer 2007). For instance, in a study comparing the parasite fauna of the round goby with that of three other gobiid species in the Hryhoryivsky Estuary, Black Sea region, Kvach (2002) recorded an overall mean abundance of 704 helminths per fish for the round goby. The helminth fauna there was mainly composed of metacercariae of digeneans but also included adult and larval stages of nematodes and acanthocephalans. Two pathogenic species of the genus Cryptocotyle (C. lingua and C. concavum), which cause blackspot disease when in high numbers, were especially abundant reaching maximum values of 2,500 worms per host. In contrast, round gobies collected in the St. Lawrence River were free of infection by Apophallus spp., closely-related species also causing blackspot that commonly infects a large array of freshwater fish in North America, including Perciforms (Muzzall and Whelan 2011). But most importantly, round gobies in the Hryhoryivsky Estuary were more parasitized both in terms of overall abundance and richness than all other gobid fish competitors examined in that study. Other surveys of the parasite fauna of the round goby from various areas of the Black Sea such as the Gulf of Odessa, the Dniester Estuary, the Tuzzly’s Lagoon (Kvach 2005) and the coast of Sinop (Özer 2007), also reported mean number of parasites per fish in the hundreds. Altogether, these data demonstrate that the round goby can accumulate parasites in large numbers. Considerable variations exist among populations and across habitats in its native range but the extremely low levels of parasitism documented in the St. Lawrence River in comparison to native competitors are unusual, and cannot be attributed to an innate ability to resist parasites.
Hence, as opposed to its native habitats, the round goby inhabiting the St. Lawrence River appears to coexist and compete for resources and space with more heavily parasitized species. According to the enemy-release hypothesis, this reduced parasite pressure could provide a competitive advantage to the introduced species and contribute to its ability to displace native ones. There are several reports suggesting that the round goby has negatively affected the benthic fish communities throughout the Great Lakes. Since its introduction, it has been implicated in the decline and local extirpation of small bottom-dwelling species like the logperch, the johnny darter (Etheostoma nigrum) and the mottled sculpin (Cottus bairdi), all showing substantial dietary overlap with young round gobies (Janssen and Jude 2001; Charlebois et al. 1997; Lauer et al. 2004). In the St. Lawrence River, impact of gobies on native populations has not yet been empirically documented. However, further dispersion of the species in the tributaries of the St. Lawrence is of particular concern, notably in the Richelieu River, where this non-native species could compromise the survival of local threatened benthic fish species such as the channel darter (Percina copelandi) and the sand darter (Ammocrypta pellucida) (Lapointe 1997; Gaudreau 2005). Recent laboratory experiments have demonstrated that in situations of limited food supply, the round goby clearly dominates its competitors, gaining weight in the presence of native species which themselves experienced a weight loss (Bergstrom and Mensinger 2009). Considering the costs of parasitism, the lower levels of parasite infection observed in the introduced round goby could potentiate its ability to displace indigenous fish, especially species like the logperch which rely on the same prey.
The extent to which parasite release actually translates into a competitive advantage remains difficult to demonstrate and quantify as it involves natural enemies whose impacts are, in contrast to predators or highly virulent pathogens, subtle, complex and hard to measure (Marcogliese and Pietrock 2011). As a general rule, the magnitude of damage or cost associated with helminth parasitism is a function of the intensity of infection that is the actual number of worms within a host (see for e.g. Brassard et al. 1982). However, the threshold at which significant impacts actually occur is unknown for a large number of parasites and would also depend on the size and composition of the remaining infracommunity of parasites inhabiting the host. For these reasons, it remains difficult to establish clear relationship between indicators of health or fitness and intensity of parasitism, particularly when studying natural populations as these associations can be blurred by the multiple influences of uncontrolled stressors and environmental factors (see for instance Ondrackova et al. 2010). However, in a recent study comparing the parasite community of the introduced Nile tilapia (Oreochromis niloticus) and a native cichlid fish competitor in the Panama Canal watershed, Roche et al. (2010) did find a marginally significant negative association between total parasite abundance and host condition for the native fish, but no such association for the invader. This finding suggests that the level of infection reached in the invasive fish was below the threshold at which an energetic cost could be detected (in terms of that particular metric), a level that was nearly attained in the native species examined. Here we found similar negative trend with a few parasite taxa for both native hosts but not for the introduced round goby. However, the associations were weak when significant.
Following the colonization time hypothesis, introduced species are expected to accumulate indigenous parasites over time: the longer an invader is established, the more native parasites it should have acquired (Blaustein et al. 1983; Guégan and Kennedy 1993; Torchin and Lafferty 2009). In support of this hypothesis, Guégan and Kennedy (1993) and Torchin et al. (2001) found that the time elapsed since invasion was a good predictor of parasite richness, which tends to increase in older introductions. However, available information suggests that parasite colonization of exotic species is a slow process. For instance, Torchin et al. (2001) reported an accumulation rate of 1 parasite taxa every hundred years in introduced green crab populations. Data presented herein suggest that substantial parasite acquisition can occur within a much shorter time period. After 15 years of residence in Lake St. Clair (between 1994 and 2009), we found that round gobies were more heavily parasitized than they were shortly after their arrival. Within that time period, the parasite richness has more than doubled whereas the number of parasite species per fish has increased, almost reaching the levels of infracommunity richness typical of fish species indigenous to the St. Lawrence-Great Lakes system (this study; Marcogliese et al. 2006; Krause et al. 2010). Not all helminth species found by Muzzall et al. (1995) in 1994 were recovered in 2009 and one of the novel species observed in this study has already been reported in gobies from Lake St. Clair by Pronin et al. (1997) suggesting variation in species composition over time. However, the detection of rare parasites being particularly sensitive to sampling effort (Walther et al. 1995), it would be hazardous to interpret the presence or absence of a particular parasite species across sampling years as a gain or loss of that species with time. Notwithstanding the apparent fluctuation in species composition, the parasite diversity observed after examining a comparable number of fish hosts, has nevertheless increased significantly between 1994 and 2009. These findings are in contrast with results reported by Kvach and Stepien (2008). These authors found no trend toward increasing round goby parasitism in the Great Lakes over years, from which they concluded that period of low parasitism may extend for decades after an overseas introduction. In particular, the round gobies they collected from Lake St. Clair in 2006 were infected by a comparable number of helminth species (5 species) as recorded in 1994 (6 species; Muzzall et al. 1995). Nonetheless, their evaluation was based on the examination of a limited number of fish (only 15 specimens) compared to the number examined by previous workers (62 specimens; Muzzall et al. 1995). Because the detection of rare parasites increases as more hosts are examined (Walther et al. 1995), the small sample size in Kvach and Stepien’s study has probably resulted in an underestimate of the parasite richness. Moreover, other factors known to affect parasite infection such as host size, locality and season of sampling (Poulin 2001) were uncontrolled or differed from prior work, thus opening the door for confounding effects.
In the present study, every effort was made to replicate the methodology described by Muzzall et al. (1995) precisely to maximize the comparability of results. However, the strict demonstration of a time-driven accumulation of parasites would have required following parasite infections over years in a native fish sharing the same ecological niche and collected coincidently with the round goby. This interspecific comparison would have allowed us to determine whether the large increase in parasite abundance and richness that we observed in round gobies from Lake St. Clair between 1994 and 2009 was paralleled by similar increase in the native fish community, and whether it was partly or entirely due to factors other than time. Unfortunately, such data do not exist. The expansion of the parasite fauna could reflect an increase in the availability of parasites due to environmental changes that took place in Lake St. Clair over that time period. For instance, water levels in the Lake have declined considerably since 1994 and have remained low during years since 2000 (www.glerl.noaa.gov/data/now/wlevels/lowlevels/plot/St.Clair.gif). However, levels of parasites that should increase in conditions of low water levels and reduced flow rates, including Diplostomum spp. and Rhabdochona sp. (Voth et al. 1974; Stables and Chappell 1986; Janovy et al. 1997; Marcogliese 2001) have stayed the same or declined in gobies. On the other hand, the introduction and proliferation of the invasive zebra mussel have profoundly disrupted the ecology of the Great Lakes including Lake St. Clair, notably by inducing a dramatic shift in productivity from the pelagic to the littoral/benthic zone (Ricciardi et al. 1998). This phenomenon could have resulted in increasing the abundance of invertebrate intermediate hosts and subsequent parasite transmission to fish. However, studies indicate that after increasing sharply (Griffiths 1993; Dermott et al. 1993; Haynes 1997), benthic macroinvertebrates biomass has since declined to pre-invasion levels (Haynes et al. 2005). Altogether, these observations suggest that neither the hydrological changes nor the dreissenid-related effect on macroinvertebrates are likely explanations of the recent expansion in the invader’s parasite fauna.
In conclusion, we found that round gobies recently established in the St. Lawrence River have left behind their original parasite fauna, being infected by only a few generalist and widespread parasites recruited from the invaded habitats. More importantly, the introduced species was considerably less parasitized than coexisting indigenous fish competitors, in contrast to what is occurring in its native range where the goby is often one of the most parasitized among its competitors. However, our study suggests that the initial parasite escape could be of shorter duration than generally expected. This striking observation contributes to the ongoing discussion around the parasite release hypothesis and its ecological implications. It also calls for more research on the factors governing parasite recruitment in freshwater ecosystems. A better understanding of the conditions that influence the rate of parasite colonization of non-native fish hosts may help identify the means of reducing the advantage that parasite release presumably confers to exotic species.
References
Amin OM (2002) Revision of Neoechinorhynchus Stiles and Hassall, 1905 (Acanthocephala: Neoechinorhychidae) with keys to 88 species in two subgenera. Syst Parasitol 53:1–18
Amin OM, Muzzall PM (2009) Redescription of Neoechinorhynchus tenellus (Acanthocephala: Neoechinorhynchidae) from Esox lucius (Esocidae) and Sander vitreus (Percidae), among other Percid and Centrarchid fish, in Michigan, U.S.A. Comp Parasitol 76(1):44–50
Anderson RM, May RM (1986) The invasion, persistence and spread of infectious diseases with animal and plant communities. Phil Trans R Soc Lond B Biol Sci 314:533–570
Arai HP (1989) Acanthocephala. In: Margolis L, Kabata Z (ed) Guide to the parasites of fishes of Canada. Part III. Can Spec Publ Fish Aquat Sci 107:1–90
Balshine S, Verma A, Chant V, Theysmeyer T (2005) Competitive interactions between round gobies and logperch. J Great Lakes Res 31:68–77
Bergstrom MA, Mensinger AF (2009) Interspecific resource competition between the invasive round goby and three native species: logperch, slimy sculpin, and spoonhead sculpin. Trans Am Fish Soc 138:1009–1017
Blakeslee AMH, Keogh CL, Byers JE, Kuris AM, Lafferty KD, Torkin ME (2009) Differential escape from parasites by two competing introduced crabs. Marine Ecol Prog Ser 393:83–96
Blaustein AR, Kuris AM, Alio JJ (1983) Pest and parasite species richness problems. Am Nat 122:556–566
Brassard P, Rau ME, Curtis MA (1982) Infection dynamics of Diplostomum spathaceum cercariae and parasite-induced mortality of fish hosts. Parasitology 85:489–493
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83(4):575–583
Camp JW, Blaney LM, Barnes DK (1999) Helminths of the round goby, Neogobius melanostomus (Perciformes: Gobiidae), from Southern Lake Michigan, Indiana. J Helminthol Soc Wash 66(1):70–72
Campton DE, Busack CA (1989) Simple procedure for decapsulating and hatching cysts of brine shrimp (Artemia spp.). Prog Fish-Cult 51:176–179
Charlebois PM, Marsden JE, Goettel RG, Wolfe RK, Jude DJ, Rudnika S (1997) The round goby, Neogobius melanostomus (Pallas), a review of European and North American literature. Illinois-Indiana Sea Grant Program and Illinois Natural History Survey, Illinois
Colautti R, Ricciardi A, Grigorovitch IA (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Conover W (1999) Practical non-parametric statistics, 3rd edn. Wiley, New York
Cornell HV, Hawkins BA (1993) Accumulation of native parasitoid species on introduced herbivores: a comparison of “hosts-as-natives” and “hosts-as-invaders”. American Naturalist 141:847–865
Cunningham AA, Daszak P, Rodriguez JP (2003) Pathogen pollution: defining a parasitological threat to biodiversity conservation. J Parasitol 89:S78–S83
Dermott R, Mitchell J, Murray I, Fera E (1993) Biomass and production of zebra mussels (Dreissena polymorpha) in shallow waters of northeastern Lake Erie. In: Nalepa TF, Schloesser DW (eds) Zebra mussels: biology, impacts and control. Lewis Publishers, Boca Raton, FL, pp 399–413
Dezfuli BS, Volponi S, Beltrami I, Poulin R (2002) Intra- and interspecific density-dependent effects on growth in helminth parasites of the cormorant, Phalacrocorax carbo sinensis. Parasitology 124(5):537–544
Dubs DOL, Corkum LD (1996) Behavioral interactions between round gobies (Neogobius melanostomus) and mottled sculpins (Cottus bairdi). J Great Lakes Res 22(4):838–844
Dunn AM (2009) Parasites and biological invasions. Adv Parasitol 68:161–184
Fredensborg BL, Poulin R (2005) Larval helminths in intermediate hosts: does competition early in life determine the fitness of adult parasites? Int J Parasitol 35(10):1061–1070
Gaudreau N (2005) Rapport sur la situation du dard de sable (Ammocrypta pellucida) au Québec. Ministère des Ressources naturelles et de la Faune du Québec, Secteur Faune Québec
Gibson DI (1996) Trematoda. In: Margolis L, Kabata Z (ed) Guide to the parasites of fishes of Canada. Part IV. Can Spec Publ Fish Aquat Sci 124:1–373
Griffiths RW (1993) Effects of zebra mussels (Dreissena polymorpha) on the benthic fauna of Lake St. Clair. In: Nalepa TF, Schloesser DW (eds) Zebra mussels: biology, impacts and control. Lewis Publishers, Boca Raton, pp 415–437
Guégan JF, Kennedy CR (1993) Maximum local helminth parasite community richness in British freshwater fish: a test of the colonization time hypothesis. Parasitology 106:91–100
Haynes JM (1997) Zebra mussels and benthic macroinvertebrate communities of southwestern Lake Ontario and selected tributaries: unexpected results? Great Lakes Res Rev 3(1):9–15
Haynes JM, Tisch NA, Mayer CM, Rhyne RS (2005) Benthic macroinvertebrate communities in southwester Lake Ontario following invasion of Dreissena, Echinogammarus : 1983 to 2000. J N Am Benthol Soc 24(10):148–167
Hoffman GL (1999) Parasites of North American freshwater fishes, 2nd edn. Comstock Publishing Associates, Ithaca
Horwitz P, Wilcox BA (2005) Parasites, ecosystems and sustainability: an ecological and complex systems perspective. Int J Parasitol 35(7):725–732
Hudson P, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol 21(7):381–385
Janovy J Jr, Snyder SD, Clopton RE (1997) Evolutionary constraints on population structure: the parasites of Fundulus zebrinus (Pisces: Cyprinodontidae) in the South Platte River of Nebraska. J Parasitol 83:584–592
Janssen J, Jude DJ (2001) Recruitment failure of mottled sculpin Cottus bairdi in Calumet Harbor, southern Lake Michigan, induced by the newly introduced round goby Neogobius melanostomus. J Great Lakes Res 27(3):319–328
Kelly DW, Paterson RA, Townsend CR, Poulin R, Tompkins DM (2009) Parasite spillback: A neglected concept in invasion ecology? Ecology 90(8):2047–2056
Kopp K, Jokela J (2007) Resistant invaders can convey benefits to native species. Oikos 116:295–301
Krakau M, Thieltges DW, Reise K (2006) Native parasites adopt introduced bivalves of the North Sea. Biol Invasions 8:919–925
Krause RJ, McLaughlin D, Marcogliese DJ (2010) Parasite fauna of Etheostoma nigrum (Percidae: Etheostomatinae) in localities of varying pollution stress in the St. Lawrence River, Quebec, Canada. Parasitol Res 107:285–294
Kvach Y (2002) Helminths of goby fish of the Hryhoryivsky estuary (Black sea, Ukraine). Vestnik Zoologii 36(3):71–76
Kvach Y (2005) A comparative analysis of helminth faunas and infection parameters of ten species of gobiid fishes (Actinopterygii: Gobiidae) from the north-western Black Sea. Acta Ichthyol Pisc 35(2):102–110
Kvach Y, Skóra KE (2006) Metazoan parasites of the invasive round goby Apollonia melanostoma (Neogobius melanostomus) (Pallas) (Gobiidae: Osteichthyes) in the Gulf of Gdansk, Blatic Sea, Poland: a comparison with the Black Sea. Parasitol Res 100:767–774
Kvach Y, Stepien CA (2008) Metazoan parasites of introduced round and tubenose gobies in the Great Lakes: support for the “enemy release hypothesis”. J Great Lakes Res 34:23–35
Lapointe M (1997) Rapport sur la situation du fouille-roche gris (Percina copelandi) au Québec. Ministère de l’Environnement et de la Faune, Direction de la faune et des habitats Québec
Lauer TE, Allen PJ, McComish TS (2004) Changes in mottled sculpin and johnny darter trawl catches after the appearance of round gobies in the Indiana waters of Lake Michigan. Trans Am Fish Soc 133:185–189
Liu H, Stiling P (2006) Testing the enemy release hypothesis: a review and meta-analysis. Biol Invasions 8:1535–1545
Lymbery AJ, Hassan M, Morgan DL, Beatty SJ, Doupé RG (2010) Parasites of native and exotic freshwater fishes in south-western Australia. J Fish Biol 76:1770–1785
MacLeod C, Paterson AM, Tompkins DM, Duncan RP (2010) Parasites lost–do invaders miss the boat or drown on arrival? Ecol Lett 13(4):516–527
Marcogliese DJ (2001) Implications of climate change for parasitism of animals in the aquatic environment. Can J Zool 79:1331–1352
Marcogliese DJ (2004) Parasites: small players with crucial roles in the ecological theatre. EcoHealth 1:151–164
Marcogliese DJ (2008) First report of the Asian fish tapeworm in the Great Lakes. J Great Lakes Res 34:566–569
Marcogliese DJ, Compagna S (1999) Diplostomatid eye flukes in young-of-the-year and forage fishes in the St. Lawrence River, Quebec. J Aquat Anim Health 11:275–282
Marcogliese DJ, Pietrock M (2011) Combined effects of parasites and contaminants on animal health: parasites do matter. Trends Parasitol 27:123–130
Marcogliese DJ, Gendron AD, Plante C, Fournier M, Cyr D (2006) Parasites of spottail shiners (Notropis hudsonius) in the St. Lawrence River: effects of municipal effluents and habitat. Can J Zool 84:1461–1481
Marr S, Mautz WJ, Hara AH (2008) Parasite loss and introduced species: a comparison of the parasites of the Puerto Rican tree frog (Eleutherodactylus coqui), in its native and introduced ranges. Biol Invasions 10:1289–1298
Moravec F (1994) Parasitic nematodes of freshwater fishes of Europe. Kluwer Academic Publishers, Dordrecht
Muzzall PM, Whelan G (2011) Parasites of fish from the Great Lakes: a synopsis and review of the literature, 1871–2010. Great Lakes Fish Comm Misc Publ 2011-01
Muzzall PM, Peebles CR, Thomas MV (1995) Parasites of the round goby, Neogobius melanostomus, and tubenose goby, Proterorhinus marmoratus (Perciformes: Gobiidae), from the St. Clair River and Lake St. Clair, Michigan. J Helminthol Soc Wash 62(2):226–228
Ondračkova M, Dávidová M, Pečinková M, Blažek R, Glnar M, Vlaová Z, Černý J (2005) Metazoan parasites of Neogobius fishes in the Slovak section of the River Danube. J Appl Ichthyol 21:345–349
Ondračkova M, Francova K, Dávidová M, Dávidová M, Polačik M, Jurajda P (2010) Condition status and parasite infection of Neogobius kessleri and N. melanostomus (Gobiidae) in their native and non-native area of distribution of the Danube River. Ecol Res 25:857–866
Özer A (2007) Metazoan parasite fauna of the round goby Neogobius melanostomus Pallas, 1811 (Perciformes: Gobiidae) collected from the Black Sea coast at Sinop, Turkey. J Nat Hist 41(9–12):483–492
Poulin R (2001) Another look at the richness of helminth communities in tropical freshwater fish. J Biogeogr 28(6):737–743
Pronin NM, Fleisher GW, Baldanova DR, Pronina SV (1997) Parasites of the recently established round goby (Neogobius melanostomus) and tubenose goby (Proterorhinus marmoratus) (Cottidae) from the St. Clair River and Lake St. Clair, Michigan, USA. Folia Parasitol 44:1–6
Reyjola Y, Brodeur P, Mailhot Y, Mingelbier M, Dumont P (2010) Do native predators feed on non-native prey? The case of round goby in a fluvial piscivorous fish assemblage. J Great Lakes Res 36(4):618–624
Ricciardi A, Neves RJ, Rasmussen JB (1998) Impending extinctions of North American freshwater mussels (Unionoida) following the zebra mussel (Dreissena polymorpha) invasion. J Anim Ecol 67:613–619
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bull Fish Res Board Can 191:1–382
Roche DG, Leung B, Mendoza Franco EF, Torchin ME (2010) Higher parasite richness, abundance and impact in native versus introduced cichlid fishes. Int J Parasitol 40:1525–1530
Rolbiecki L (2006) Parasites of the round goby, Neogobius melanostomus (Pallas, 1811), an invasive species in the Polish fauna of the Vistula Lagoon ecosystem. Oceanologia 48(4):545–561
Ross JL, Ivanova ES, Severns PM, Wilson MJ (2010) The role of parasite release in invasion of the USA by European slugs. Biol Invasions 12:603–610
Sasal P, Morand S, Guégan JF (1997) Determinants of parasite species richness in Mediterranean marine fishes. Mar Ecol Prog Ser 149:61–71
Shaw DJ, Dobson AP (1995) Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology 111:S111–S133
Stables LH, Chappell JN (1986) Diplostomum spathaceum (Rud. 1819) effect of physical factors on the infection of rainbow trout (Salmo gairdneri) by cercariae. Parasitology 93:71–79
Taraschewski H (2006) Hosts and parasites as aliens. J Helminthol 80:99–128
Telfer S, Bown KJ, Seules R, Begon M, Hayden T, Birtles R (2005) Disruption of a host-parasite system following the introduction of an exotic host species. Parasitology 130:661–668
Tompkins DM, Dunn AM, Smith MJ, Telfer S (2010) Wildlife diseases: from individuals to ecosystems. J Anim Ecol 80(1):19–38
Torchin ME, Lafferty KD (2009) Escape from parasites. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems. Ecological studies 204. Springer, Berlin, pp 203–214
Torchin ME, Mitchell CE (2004) Parasites, pathogens, and invasions by plants and animals. Front Ecol Environ 2:183–190
Torchin ME, Lafferty KD, Kuris AM (2001) Release from parasites as natural enemies: increased performance of a globally introduced marine crab. Biol Invasions 3:333–345
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421:628–630
Voth DR, Anderson LF, Kleinschuster SJ (1974) The influence of waterflow on brown trout parasites. Prog Fish-Cult 36:212
Walther BA, Cotgreave P, Price RD, Gregory RD, Clayton DH (1995) Sampling effort and parasite species richness. Parasitol Today 11(8):306–310
Whittington ID, Cribb BW, Hamwood TE, Halliday JA (2000) Host-specificity of monogenean (platyhelminth) parasites: a role for anterior adhesive areas? Int J Parasitol 30(3):305–320
Acknowledgments
We would like to thank Germain Brault, Michel Arsenault, Claude Lessard, Sophie Trépanier, Marie-Pier Ricard, Jacinthe Gosselin, Eugénie Schaff, Pierre-Olivier Benoit, Maxime Guérard, Jean-François Lafond, Maude Lachapelle, Hubert Désilets, Lila Gagnon-Brambilla, Roxane Pétel and Maxime Thibault for their help in the collection of gobies from the St. Lawrence River and/or examination of fish for parasites. Round gobies from Lake St. Clair were collected by staff from the Michigan Department of Natural Resources’ Lake St. Clair Fisheries Research Station during scheduled surveys conducted under Federal Aid Study F-81-R-9 Study 230488. We are thankful to Christopher Blanar for his contribution to parasite identification. Pierre Gagnon is acknowledged for mathematical and statistical advice. The insightful suggestions and comments of two anonymous referees significantly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gendron, A.D., Marcogliese, D.J. & Thomas, M. Invasive species are less parasitized than native competitors, but for how long? The case of the round goby in the Great Lakes-St. Lawrence Basin. Biol Invasions 14, 367–384 (2012). https://doi.org/10.1007/s10530-011-0083-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0083-y