Abstract
Five species of stoloniferous plants originating from the same field site (Galeobdolon montanum, Glechoma hederacea, Potentilla anserina, Ranunculus repens and Trifolium repens) were studied with respect to their interaction with arbuscular mycorrhizal (AM) fungi. More specifically, the question was addressed whether mycorrhizal growth response of host plant species could be related to their vegetative mobility. The roots of all the species examined were colonised with AM fungi in the field, with the percentage of colonisation varying among species from approximately 40% to 90%. In a subsequent pot experiment, plants of all the species were either left non-inoculated or were inoculated with a mixture of three native AM fungi isolated from the site of plant origin (Glomus mosseae, G. intraradices and G. microaggregatum). AM fungi increased phosphorus uptake in all the plant species; however, plant growth response to inoculation varied widely from negative to positive. In addition to the biomass response, AM inoculation led to a change in clonal growth traits such as stolon number and length or ramet number in some species. Possible causes of the observed differences in mycorrhizal growth response of various stoloniferous plants are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most widespread type of mycorrhizal association is arbuscular mycorrhizal (AM) symbiosis, which is formed by most vascular plants. The importance of AM fungi for plant growth and nutrition, particularly the uptake of poorly mobile phosphorus, is widely acknowledged (Smith and Read 2008), together with their potential to influence plant community structure (van der Heijden et al. 1998). Nevertheless, considerable variability in the effectiveness of the symbiosis with respect to plant growth promotion and nutrient uptake has been documented for different plant species (Hetrick et al. 1992; Scheublin et al 2007; Klironomos 2003) and cultivars or ecotypes (Sylvia et al. 2003; Jurkiewicz et al. 2004) as well as for different fungal species (Jakobsen et al. 1992; Streitwolf-Engel et al. 1997; van der Heijden et al. 1998, 2003) and isolates (Munkvold et al. 2004; Koch et al. 2006).
Among others, AM association predominates in temperate grasslands (Smith and Read 2008) where high incidence of clonal plant species is, as a rule, observed (Klimeš et al. 1997). In addition to grasses, stoloniferous forbs can contribute substantially to the biomass production of these plant communities, especially in more open parts of the habitats. Stoloniferous herbs spread laterally by means of aboveground plagiotropic stems that bear potentially autonomous ramets at their nodes, thus forming a network of offsprings that are temporarily physically interconnected and physiologically integrated (Stuefer et al. 2004). Although many stoloniferous plants are known to have roots colonised by mycorrhizal fungi in nature (Harley and Harley 1987; Wang and Qiu 2006), information about the impact of native AM fungi on the performance and resource acquisition of these mobile species is rather scarce.
A hypothesis about the trade-off between plant vegetative mobility and mycorrhizal association was formulated by Onipchenko and Zobel (2000), suggesting that plants preferably invest in only one of these carbon-costly strategies to attain resources, i.e. form stolons (or rhizomes) or establish mycorrhizal symbiosis. This theory was supported in their study of alpine grasslands, in which the authors showed that plants with an annual mobility higher than 2 cm had a lower rate of root colonisation than sessile species. However, experimental studies demonstrated significant effects of AM fungi on biomass production and clonal growth traits of stoloniferous species as well as their phosphorus uptake. Plant growth promotion in response to AM inoculation was reported for Prunella grandiflora and P. vulgaris (Streitwolf-Engel et al. 1997, 2001), Trifolium repens (Zhu et al. 2007), Teucrium scorodonia or Lysimachia nummularia (Helgason et al. 2002). In contrast, inoculation with native AM fungi had no effects on the growth of Glechoma hederacea and Ajuga reptans (Helgason et al. 2002), and pronounced growth depression was recently observed in two highly mobile stoloniferous species with stolons reaching dozens of centimetres from a parent ramet: Fragaria moschata and Potentilla reptans (Sudová and Vosátka 2008). Based on these results, a hypothesis was proposed that mycorrhizal growth response of stoloniferous species could be related to their vegetative mobility.
This study addressed the following questions: (i) to what extent are the selected stoloniferous species naturally colonised by AM fungi, (ii) whether colonisation with native AM fungi influences clonal growth traits of these stoloniferous plants and (iii) whether there is a relationship between AM growth response and vegetative mobility of the host plant species. To enable comparison with the above-mentioned study (Sudová and Vosátka 2008), five co-occurring stoloniferous species from the same field site and the same mixture of native AM fungi were chosen for the study.
Materials and methods
Field sampling
Five stoloniferous plant species were selected for the study, i.e. Galeobdolon montanum (Pers.) Rchb., Glechoma hederacea L. (both Lamiaceae), Potentilla anserina L. (Rosaceae), Ranunculus repens L. (Ranunculaceae) and Trifolium repens L. (Fabaceae). Thirty individuals per species were sampled from their natural populations at a mown mesotrophic meadow adjacent to a broad-leaved forest at Litožnice in September 2004 (Central Bohemia, Czech Republic; 50°04′10″ N, 14°36′30″ E, 230 m; sampling area approximately 8 × 20 m). Plant roots were washed to remove soil debris and stained for mycorrhizal colonisation with 0.05% trypan blue in lactoglycerol (Koske and Gemma 1989). The percentage of root colonisation was assessed using a modified intersections method of McGonigle et al. (1990). Root samples were spread out evenly on microscope slides and observed for the presence or absence of mycorrhizal structures (arbuscules, vesicles or hyphae) under a compound microscope at 100× magnification.
Pot experiment
Plants and AM fungi
Parental plants of each above-mentioned species were collected in the field in spring 2004. To ensure genetic uniformity of plant material entering into the subsequent experiment, several interconnected ramets belonging to the same genet were taken from each plant species due to tracking stolonal connections. The sampled plants were then planted in pots and their offspring ramets that did not come into contact with the substrate were separated as soon as new stolons started to grow out of the pots and were rooted in a γ-sterilised (25 kGy) 1:1 (v/v) mixture of sand and soil from the field site. Stock plants obtained in this way had roots free of AM fungi. These plants were then vegetatively multiplied in a greenhouse to obtain a sufficient amount of genetically uniform individuals for the experiment. Similarly sized ramets of the same developmental stage that were produced on primary stolons of the stock plants were then used for setting up the experiment. A mixture of three AM species isolated from the field site of plant origin served as the mycorrhizal inoculum: Glomus intraradices Schenck & Smith, G. mosseae (Nicolson & Gerd.) Gerd. & Trappe and G. microaggregatum Koske, Gemma & Olexia. These isolates are obviously generalists at the field site, as inferred from the fact that they were repeatedly isolated in several trap-culture attempts, both in pots established from root samples of target and neighbouring plant species as well as from rhizosphere soil. All the above-mentioned isolates are maintained in the culture collection of AM fungi at the Institute of Botany, Academy of Sciences of the Czech Republic.
Experimental design
Two inoculation treatments were established for all the plant species, with plants either left non-inoculated or inoculated with AM fungi. Each treatment included ten replicates. The plants were planted in plastic pots (12 × 12 × 10 cm) in a greenhouse under natural light conditions with supplementary 12-h illumination provided by metal halide lamps (400 W). The plants were grown in a γ-sterilised mixture of river sand and soil obtained from the field site (1:1, v/v). The resulting substrate had the following characteristics: pH (KCl) 5.6, organic carbon C 2.5%, N 0.2%, P (Olsen) 15.6 mg kg−1.
Plants in the mycorrhizal treatments were inoculated with 10 ml of a suspension mixture containing all AM isolates (each at one-third the volume). The inoculum of each isolate was prepared separately by wet sieving (Gerdemann and Nicolson 1963), using mature pure cultures with root colonisation exceeding 80%. It involved colonised root segments, extraradical mycelium (ERM) and spores. The non-inoculated plants received the same amount of autoclaved inoculum (30 min, 121°C). They were also treated with a filtrate from a non-sterile mycorrhizal inoculum (10 ml per pot) in an attempt to balance the composition of the microbial community. The filtrate was obtained by passing a 1:10 suspension of the soil inoculum through a filter paper (Whatman No. 1, UK) to remove AM propagules. Moreover, all the pots were treated with a filtrate of non-sterile soil from a field site (10 ml per pot) to mimic the natural composition of the microbial community (preparation of the filtrate as above). The plants were irrigated with distilled water as required and once a week, they were fertilised (50 ml per pot) with nutrient solution P2N3 (Gryndler et al. 1992). The plants were grown in the greenhouse from July to December 2005.
Harvest
After 24 weeks of growth, plant shoots were cut off, and the roots were gently washed to rinse away substrate particles. For each original ramet, the following clonal growth traits were recorded: numbers of newly produced stolons and ramets, and stolon length. Total leaf area was assessed using an area metre (LI-3100, LI-COR), and dry weights of the shoots and roots were recorded after drying at 65°C. To analyse for phosphorus concentrations, shoot biomass was ground and digested in HNO3 and H2O2. Phosphorus concentrations were assessed spectrophotometrically (Unicam UV4-100) at a wavelength of 630 nm (Olsen et al. 1954). Sub-samples were taken from each root system and processed as described above to assess the percentage of root length colonised with AM fungi. To evaluate the length of ERM in the substrate, small aliquots (3 g) of thoroughly homogenised substrate were taken from each pot, and the mycelium was extracted using a modified membrane filtration technique (Jakobsen et al. 1992). Total ERM length was assessed by the grid-line intersect method under a compound microscope, using an ocular grid at 100× magnification, and expressed as metres of hyphae in 1 g of air-dried substrate. The background length of hyphae found in non-inoculated treatments was subtracted from the values recorded for inoculated plants.
Statistical analysis
The data were analysed using Statistica 6.0 Software (StatSoft Inc., USA). Data on mycorrhizal colonisation and length of ERM were evaluated using one-way analysis of variance (ANOVA), with plant species as an independent variable (percentage of root colonisation was arcsine transformed prior to the analysis). Comparisons between means were carried out using the Tukey HSD test at a significance level of P < 0.05. The effects of AM inoculation on plant growth parameters were analysed by one-way ANOVA. Data were checked for normality and homogeneity of variances prior to ANOVA, and logarithmically transformed when necessary. Data on the numbers of stolon apices were analysed using a generalised linear model (S-Plus 2000, MathSoft Inc., USA) assuming Poisson distribution of the dependent variable.
Results
Field sampling
Roots of all the plants sampled in the field were colonised by AM fungi; however, differences in the level of mycorrhizal colonisation were observed among species (Table 1). Whereas the percentage of colonised roots exceeded 80% in T. repens, R. repens and P. anserina, both species from the family Lamiaceae showed significantly lower root colonisation (42% and 44% for G. montanum and G. hederacea, respectively). P. anserina, G. hederacea and T. repens formed typical Arum-type of mycorrhizal colonisation, with intercellular hyphae and terminal arbuscules on intracellular hyphal branches. In comparison, an intermediate morphology between Arum- and Paris-type was observed in the roots of R. repens and G. montanum (i.e., terminal arbuscules and arbusculate coils of variable abundance).
Pot experiment
Mycorrhizal parameters
No mycorrhizal structures were found in the roots of non-inoculated plants. In inoculated plants, a high percentage of mycorrhizal colonisation was observed in roots of all the plant species tested, with a little variation between samples (Table 1). All the species had colonisation higher than 90%, except for G. montanum with 82% of the root length colonised. Similarly, high densities of ERM were observed in the substrate. The most extraradical hyphae were associated with T. repens and P. anserina, while G. montanum plants showed the lowest density of ERM in the substrate (Table 1). Comparison of the mycorrhizal colonisation of the roots from the experiment and the field showed that field samples of G. hederacea and G. montanum showed a considerably lower percentage of root colonisation; no such marked difference was observed for the other plant species.
Plant growth parameters
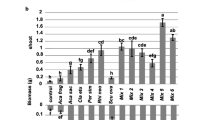
The species examined differed considerably in their growth response to AM inoculation (Table 2, Fig. 1). When measured as shoot biomass production, a significant benefit from AM inoculation was observed only in T. repens, the inoculated plants of which showed 42% higher shoot dry weight compared to their non-inoculated counterparts. This AM-induced increase in shoot biomass corresponded with about 50% longer total stolon length, higher ramet number and 25% larger leaf area. However, when clonal growth traits were related to biomass unit (to separate from AM effect on biomass production), they did not show any significant differences between inoculated and non-inoculated plants (data not shown). Hence, mycorrhization did not influence clonal architecture of this species independently of biomass response.
The effect of inoculation with arbuscular mycorrhizal (AM) fungi on shoot and root dry weight of five stoloniferous plant species (−AM non-inoculated plants, +AM inoculated plants). Columns represent means (±SE) of 10 replicates. Significant differences between non-inoculated and inoculated plants of the same species are marked with asterisks (according to one-way ANOVA at P < 0.05)
In contrast to T. repens, a significant reduction in shoot dry weight in response to AM inoculation was recorded for G. hederacea (Fig. 1). Clonal growth traits did not, however, differ significantly between inoculated and non-inoculated plants of this species (Table 2). If considered per biomass unit, significantly longer stolons and more ramets were produced by inoculated plants having lower biomass. No effect of AM inoculation on biomass production and clonal growth traits was observed for P. anserina and R. repens (with the exception of larger leaf area). In G. montanum, a positive effect of inoculation on stolon and ramet number as well as total leaf area was observed despite the lack of AM effect on total shoot dry weight. Inoculated plants of T. repens and G. hederacea showed a significantly reduced ratio of root-to-shoot dry biomass, while no effect of AM inoculation on biomass allocation between roots and shoots was observed in the remaining species (Table 2).
AM inoculation significantly increased phosphorus concentrations (Table 2) in the shoot biomass of all species examined, including G. hederacea, which demonstrated growth depression in response to inoculation. Phosphorus contents were also significantly higher in inoculated plants of all species but R. repens (Table 2).
Discussion
Both clonality and mycorrhizal symbiosis play a key role in plant adaptation to their environment. AM association provide the host plants with an array of benefits, particularly improved nutrient acquisition (Smith and Read 2008) and, at least in part, relief from different abiotic and biotic stresses such as drought (Augé 2001), high concentrations of heavy metals (Leyval et al. 1997) or pathogen attack (Selosse et al. 2004). Clonal growth enables plants to escape from adverse to more favourable conditions, helps young ramets to establish by supplying them with resources from parent ramets, maximises plant performance in spatially and temporally variable habitats due to labour division among different ramets and minimises the risks of lethal plant damage due to herbivory (Pitelka and Ashmun 1985). Nevertheless, all these benefits resulting from either mycorrhizal association or clonality are achieved at considerable carbon costs to the host or parent plants (Johnson et al. 1997; Onipchenko and Zobel 2000). To better understand the benefits and costs arising from the combination of AM association and clonal mobility, more information about their interaction in natural habitats as well as under experimental conditions is needed.
In this study, high rates of mycorrhizal colonisation were observed in field root samples of all the plant species and therefore, being colonised seems to be the norm for these stoloniferous plants. This is in contrast to the hypothesis proposed by Onipchenko and Zobel (2000) suggesting lower investment of mobile plant species into mycorrhiza. Five co-occurring stoloniferous species, however, responded differently to inoculation with a mixture of native AM isolates, regardless of higher shoot P concentrations consistently observed in inoculated plants of all the species. Variation in the response of stoloniferous plant species to AM fungi was also reported by Helgason et al. (2002) who observed a positive mycorrhizal growth effect for two plant species, while two others did not respond to AM inoculation. In this study, only T. repens profited from AM inoculation in terms of biomass production, which is in accordance with the results of previously reported experiments (Li et al. 1991; Jongen et al. 1996; Chen et al. 2007). Nevertheless, McGonigle and Fitter (1998) did not observe any growth benefit of T. repens from mycorrhizal association in their field study. In contrast to white clover-related studies, disproportionately less is known about AM interactions with the remaining stoloniferous species examined. Plant biomass of P. anserina, R. repens and G. montanum remained unaffected by mycorrhization and G. hederacea showed even growth depression in response to AM inoculation in this study experiment. In comparison, Helgason et al. (2002) observed lack of growth response of G. hederacea to inoculation with three different AM species, despite significantly increased P concentrations in tissues of inoculated plants. These results thus confirm that the outcome of AM association depends largely on the particular plant–fungus combination (Wilson and Hartnett 1998; Klironomos 2003), and that AM symbiosis is not always beneficial for plant hosts, but functions along the mutualism–parasitism continuum (Johnson et al. 1997).
In the case of G. montanum, positive mycorrhizal growth effects were manifested only on clonal growth characteristics and not shoot biomass. Stolon length and ramet number produced per biomass unit increased in response to AM inoculation also in G. hederacea, i.e. plants formed thinner stolons with more densely spaced ramets if mycorrhizal. Independent effects of AM fungi on plant biomass on the one hand and clonal reproduction and architecture on the other hand were first demonstrated for Prunella vulgaris (Streitwolf-Engel et al. 2001). Recently, Varga and Kytöviita (2008) also reported that formation of new ramets of Antennaria dioica was stimulated by AM inoculation more than biomass accumulation. Considering that clonal architecture reflects the capability of stoloniferous plants to colonise their environment and establish new ramets in favourable patches (de Kroon and Hutchings 1995), these results point to the need to record not only biomass production, but also clonal growth traits when describing the effects of mycorrhiza on clonal plant species.
The differences in AM effect on plant growth that were observed in this study experiment cannot be attributed to differences in the extent of root colonisation, because similar levels of colonised root length were observed for all the species. Neither the differences in root morphology sensu the Baylis’s (1975) theory provide sufficient explanation for distinct plant responses to AM inoculation. Contrary to the expectation, species having coarse, fleshy roots (R. repens) did not profit from mycorrhizal colonisation more than species with thin, fibrous roots, including T. repens which positively responded to AM inoculation. The differences among plant species tested, in terms of mycorrhizal growth effect, might, however, be related to their different vegetative mobility, as both plant species showing positive mycorrhizal growth response (i.e., T. repens and G. montanum) had considerably lower mean stolon length (17 cm on average) than the other species (55 cm on average). In line with the suggested hypothesis on a lower importance of AM association for highly mobile stoloniferous species, a growth depression was also observed in response to inoculation with the same mixture of AM isolates in two species with mean stolon length exceeding 100 cm (Sudová and Vosátka 2008).
The fact that all stoloniferous plants tested showed high mycorrhizal colonisation at the field site, but only two of them profited from experimental inoculation with ecologically relevant AM fungi in terms of growth promotion, raises the hypothesis that benefits other than improved growth are derived from their association with mycorrhizal fungi in natural ecosystems. In this respect, resistance to root pathogenic fungi (e.g. Newsham et al. 1994) or parasitic nematodes (e.g. Habte et al. 1999) can be suggested. Mycorrhizal fungi were also shown to suppress herbivore attack on host plants (e.g. Wooley and Paine 2007), which is interesting in the light of the results by Gómez et al. (2008) who observed the spread of systemic-induced resistance among ramets of clonal plants in response to herbivory. It can also be hypothesised that the profit of stoloniferous plants from being mycorrhizal is higher in patchy soil environment where some parts of the clonal network suffer from insufficient nutrient availability. Under such conditions, AM symbiosis could serve as an additional means of buffering environmental heterogeneity, supplementary to intraclonal physiological integration.
To conclude, this study demonstrated the ability of AM fungi to affect biomass production as well as clonal growth traits of fast-spreading stoloniferous species. A continuum of mycorrhizal growth responses from negative to positive was observed for different stoloniferous species when inoculated with the same mixture of naturally co-existing AM fungi, indicating the potential of mycorrhizal symbiosis to alter competitive interactions within plant communities. The hypothesis on the relationship between plant vegetative mobility and mycorrhizal growth response seems plausible in the light of the obtained results. However, considering that only a single genotype of each stoloniferous species was included in this study, further investigation using a more representative set of co-existing plant species and genotypes with different vegetative mobility is required to corroborate this hypothesis.
References
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42. doi:10.1007/s005720100097
Baylis GTS (1975) The magnolioid mycorrhiza and mycotrophy in root systems derived from it. In: Sanders FE, Mosse B, Tinker PB (eds) Endomycorrhizas. Academic Press, New York, pp 373–389
Chen BD, Zhu YG, Duan J, Xiao XY, Smith SE (2007) Effects of the arbuscular mycorrhizal fungus Glomus mosseae on growth and metal uptake by four plant species in copper mine tailings. Environ Pollut 147:374–380. doi:10.1016/j.envpol.2006.04.027
de Kroon H, Hutchings MJ (1995) Morphological plasticity in clonal plants—the foraging concept reconsidered. J Ecol 83:143–152. doi:10.2307/2261158
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet-sieving and decanting. Trans Br Mycol Soc 46:235–244
Gómez S, Onoda Y, Ossipov V, Stuefer JF (2008) Systemic induced resistance: a risk-spreading strategy in clonal plant networks? New Phytol 179:1142–1153. doi:10.1111/j.1469-8137.2008.02542.x
Gryndler M, Vejsadová H, Vančura V (1992) The effect of magnesium ions on the vesicular-arbuscular mycorrhizal infection of maize roots. New Phytol 122:455–460. doi:10.1111/j.1469-8137.1992.tb00073.x
Habte M, Zhang YC, Schmitt DP (1999) Effectiveness of Glomus species in protecting white clover against nematode damage. Can J Bot 77:135–139. doi:10.1139/cjb-77-1-135
Harley JL, Harley EL (1987) A check-list of mycorrhiza in the British flora. New Phytol 105(Suppl 1):1–102. doi:10.1111/j.1469-8137.1987.tb00674.x
Helgason T, Merryweather JW, Denison J, Wilson P, Young JPW, Fitter AH (2002) Selectivity and functional diversity in arbuscular mycorrhizas of co-occurring fungi and plants from a temperate deciduous woodland. J Ecol 90:371–384. doi:10.1046/j.1365-2745.2001.00674.x
Hetrick BAD, Wilson GWT, Todd TC (1992) Relationships of mycorrhizal symbiosis, rooting strategy, and phenology among tallgrass pairie forbs. Can J Bot 70:1521–1528. doi:10.1139/b92-253
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol 120:371–380. doi:10.1111/j.1469-8137.1992.tb01077.x
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–586. doi:10.1046/j.1469-8137.1997.00729.x
Jongen M, Fay P, Jones MB (1996) Effects of elevated carbon dioxide and arbuscular mycorrhizal infection on Trifolium repens. New Phytol 132:413–423. doi:10.1111/j.1469-8137.1996.tb01861.x
Jurkiewicz A, Orlowska E, Anielska T, Godzik B, Turnau K (2004) The influence of mycorrhiza and EDTA application on heavy metal uptake by different maize varieties. Acta Biol Cracov 46:7–18
Klimeš L, Klimešová J, Hendriks R, van Groenendael J (1997) Clonal plant architecture: a comparative analysis of form and function. In: de Kroon H, van Groenendael J (eds) The ecology and evolution of clonal plants. Backhuys Publishers, Leiden, pp 1–29
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301. doi:10.1890/02-0413
Koch AM, Croll D, Sanders IR (2006) Genetic variability in a population of arbuscular mycorrhizal fungi causes variation in plant growth. Ecol Lett 9:103–110. doi:10.1111/j.1461-0248.2005.00853.x
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res 92:486–505
Leyval C, Turnau K, Haselwandter K (1997) Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza 7:139–153. doi:10.1007/s005720050174
Li XL, George E, Marschner H (1991) Extension of the phosphorus depletion zone in VA-mycorrhizal white clover in a calcareous soil. Plant Soil 136:41–48. doi:10.1007/BF02465218
McGonigle TP, Fitter AH (1998) Growth and phosphorus inflows of Trifolium repens L. with a range of indigenous vesicular-arbuscular mycorrhizal infection levels under field conditions. New Phytol 108:59–65. doi:10.1111/j.1469-8137.1988.tb00204.x
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonisation of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. doi:10.1111/j.1469-8137.1990.tb00476.x
Munkvold L, Kjoller R, Vestberg M, Rosendahl S, Jakobsen I (2004) High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol 164:357–364. doi:10.1111/j.1469-8137.2004.01169.x
Newsham KK, Fitter AH, Watkinson AR (1994) Root pathogenic and arbuscular mycorrhizal fungi determine fecundity of asymptomatic plants in the field. J Ecol 82:805–814. doi:10.2307/2261445
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Dep Agric Circ 939:1–19
Onipchenko VG, Zobel M (2000) Mycorrhiza, vegetative mobility and responses to disturbance of alpine plants in the Northwestern Caucasus. Folia Geobot 35:1–11. doi:10.1007/BF02803083
Pitelka LF, Ashmun JW (1985) Physiology and integration of ramets in clonal plants. In: Jackson JBC, Buss LW, Cook RE (eds) Population biology and evolution of clonal organisms. Yale University Press, New Haven, pp 399–435
Scheublin TR, van Logtestijn RSP, van der Heijden MGA (2007) Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J Ecol 95:631–638. doi:10.1111/j.1365-2745.2007.01244.x
Selosse MA, Baudoin E, Vandenkoornhuyse P (2004) Symbiotic microorganisms, a key for ecological success and protection of plants. C R Biol 327:639–648. doi:10.1016/j.crvi.2003.12.008
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, London
Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1997) Clonal growth traits of two Prunella species are determined by co-occurring arbuscular mycorrhizal fungi from a calcareous grassland. J Ecol 85:181–191. doi:10.2307/2960650
Streitwolf-Engel R, van der Heijden MGA, Wiemken A, Sanders IR (2001) The ecological significance of arbuscular mycorrhizal fungal effects on clonal reproduction in plants. Ecology 82:2846–2859
Stuefer JF, Gómez S, van Mölken T (2004) Clonal integration beyond resource sharing: implications for defence signalling and disease transmission in clonal plant networks. Evol Ecol 18:647–667. doi:10.1007/s10682-004-5148-2
Sudová R, Vosátka M (2008) Effects of inoculation with native arbuscular mycorrhizal fungi on clonal growth of Potentilla reptans and Fragaria moschata (Rosaceae). Plant Soil 308:55–67. doi:10.1007/s11104-008-9605-5
Sylvia DM, Alagely AK, Kane ME, Philman NL (2003) Compatible host/mycorrhizal fungus combinations for micropropagated sea oats. I. Field sampling and greenhouse evaluations. Mycorrhiza 13:177–183. doi:10.1007/s00572-003-0232-y
van der Heijden MGA, Boller T, Wiemken A, Sanders IR (1998) Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79:2082–2091
van der Heijden MGA, Wiemken A, Sanders IR (2003) Different arbuscular mycorrhizal fungi alter coexistence and resource distribution between co-occurring plant. New Phytol 157:569–578. doi:10.1046/j.1469-8137.2003.00688.x
Varga S, Kytöviita MM (2008) Sex-specific responses to mycorrhiza in a dioecious species. Am J Bot 95:1225–1232. doi:10.3732/ajb.0800068
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363. doi:10.1007/s00572-005-0033-6
Wilson GWT, Hartnett DC (1998) Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am J Bot 85:1732–1738. doi:10.2307/2446507
Wooley SC, Paine TD (2007) Can intra-specific genetic variation in arbuscular mycorrhizal fungi (Glomus etunicatum) affect a mesophyll-feeding herbivore (Tupiocoris notatus Distant)? Ecol Entomol 32:428–434. doi:10.1111/j.1365-2311.2007.00883.x
Zhu HH, Yao Q, Sun XT, Hu YL (2007) Colonization, ALP activity and plant growth promotion of native and exotic arbuscular mycorrhizal fungi at low pH. Soil Biol Biochem 39:942–950. doi:10.1016/j.soilbio.2006.11.006
Acknowledgements
Financial supports by the Czech Science Foundation, project No. 526/05/P063, and the Grant Agency of the Academy of Sciences of the Czech Republic within the institutional project AV0Z60050516 are gratefully acknowledged. My sincere thanks go also to Marie Albrechtová for performing chemical analyses, and to J. Suda, Z. Sýkorová, J. Rydlová, M. Vosátka, and two anonymous reviewers for their comments on an earlier draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sudová, R. Different growth response of five co-existing stoloniferous plant species to inoculation with native arbuscular mycorrhizal fungi. Plant Ecol 204, 135–143 (2009). https://doi.org/10.1007/s11258-009-9576-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-009-9576-5




