Abstract
Inoculation of arbuscular mycorrhizal fungi (AMF) as plant growth promoters has mostly been conducted using single-species inoculum. In this study, we investigated whether co-inoculation of different native AMF species induced an improvement of plant growth in an ultramafic soil. We analyzed the effects of six species of AMF from a New Caledonian ultramafic soil on plant growth and nutrition, using mono-inoculations and mixtures comprising different numbers of AMF species, in a greenhouse experiment. The endemic Metrosideros laurifolia was used as a host plant. Our results suggest that, when the plant faced multiple abiotic stress factors (nutrient deficiencies and high concentrations of different heavy metals), co-inoculation of AMF belonging to different families was more efficient than mono-inoculation in improving biomass, mineral nutrition, Ca/Mg ratio, and tolerance to heavy metals of plants in ultramafic soil. This performance suggested functional complementarity between distantly related AMF. Our findings will have important implications for restoration ecology and mycorrhizal biotechnology applied to ultramafic soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal fungi (AMF) are obligate symbionts having a huge potential to produce biostimulants for plants (Berruti et al. 2016). AMF improve plant growth by different mechanisms, including increased mineral nutrition (Hawkins et al. 2000; Smith and Read 2008; Feddermann et al. 2010), enhanced defense against pathogens and insects (Pozo and Azcón-Aguilar 2007; St-Arnaud and Vujanovic 2007; Shrivastava et al. 2015), and increased abiotic stress tolerance (Ruiz-Lozano and Aroca 2010; Bárzana et al. 2012; Augé et al. 2015; Ferrol et al. 2016). However, the effects of AMF on plant growth are highly variable within fungal taxa, as well as plant taxa (Koch et al. 2017) and it is still unclear how variation in plant responsiveness to mycorrhizal colonization is regulated (van der Heijden et al. 2015). In natural environments, each single plant can host a large diversity of AMF species in its root system (Vandenkoornhuyse et al. 2007; Kiers et al. 2011). One of the main challenges in AMF research is to understand how taxonomic diversity of AMF is related to their efficiency to promote plant growth and ability to withstand stresses. The answer to this question is essential for effective inoculum production for large-scale use of AMF in agronomic and environmental contexts.
A recent meta-analysis review suggests that the effect of AMF taxa diversity on plant performance depends on taxonomic resolution (Yang et al. 2016). Plant performance was positively correlated with AMF family richness, but no significant correlation was found for fungal species richness. However, as stressed by these authors, very few studies compared the effects of monocultures and polycultures of AMF on the same plant species (Maherali and Klironomos 2007; Kiers et al. 2011; Thonar et al. 2014; Gosling et al. 2016). Maherali and Klironomos (2007), working on Plantago lanceolata, demonstrated that plant performance was increased by AMF family richness but not by species richness within a single family. To explain these findings, they hypothesized that competition and functional conservatism within AMF families limits the positive additive effects of the different closely related AMF species on plant performance. Otherwise, functional differentiation among AMF families allows complementarity of their effects on the plant. This hypothesis was supported by other studies showing that different families have different functional capacities (Thonar et al. 2014; Yang et al. 2016). Finally, Yang et al. (2016) concluded that conservation of AMF communities is essential to maintain a full complement of ecosystem functions and requires the presence of diverse families and not simply diverse species of the same family. This diversity may be of key importance for ecosystem health and productivity under various environmental perturbations to which AMF families may respond differently. Indeed, a study by Gosling et al. (2016) showed that co-inoculation of AMF species belonging to different families did not induce more beneficial effects on plant performance when the host plant was exposed to a single stress, because only some of these species are able to relieve this stress. Those authors concluded that the failure to show a benefit from high AMF diversity in their study and other studies may be the result of experimental conditions, with the benefits of AMF diversity only becoming apparent when the host plant faced multiple stress factors. All these studies taken together suggest that in environments facing different stress factors, inoculation with several species from different families of AMF may be essential, but the different AMF species may need to be compatible with each other and therefore native from the same ecosystem (Berruti et al. 2016; Gosling et al. 2016; Koch et al. 2017).
In New Caledonia, serpentine ecosystems which have developed over ultramafic rocks (peridotites, serpentinites) cover one third of the surface area of the main island called Grande Terre. The uniqueness of these ecosystems, where plant endemism reaches 82%, is not only due to their geographic isolation but also to the adaptation to strong edaphic constraints (Jaffré and L’Huillier 2010a) with multiple stress factors. The ultramafic soils present three main constraints to plant growth (Brooks 1987; Kazakou et al. 2008). The first major constraint is the limitation of nutrients in these soils, due to a low level of organic matter, but also to parent material often deficient in K and P inducing a very low growth rate of plants. A second constraint is the very low (< 1) Ca/Mg ratio, which can lead to Ca deficiency in plants. The last important constraint concerns high concentrations of the heavy metals Ni, Co, Cr, and Mn which can be phytotoxic (Brooks 1987; Jaffré and L’Huillier 2010b). To avoid metal toxicity in metal rich substrates, plants generally reduce their translocation to aerial parts (Baker 1987; Kazakou et al. 2008). This fact can be expressed in the “translocation factor” (Singh et al. 2010; Wang et al. 2014), which is the ratio of the aerial concentration to the root concentration of the metal. Finally, these multiple stress conditions may be a good model system to study synergistic effects of AMF diversity.
The stripping of large areas, for the purpose of nickel mining, endangers New Caledonian ultramafic ecosystems. Ecological restoration of degraded mine sites is therefore a major concern in New Caledonia. Improvement of currently used techniques for revegetation (Luçon et al. 1997) requires a good understanding of the role of mycorrhizal associations involved in ultramafic soils (Graham 2009; Amir et al. 2013). Previous studies on New Caledonian ultramafic maquis (shrublands) have highlighted that nearly all plant species are arbuscular mycorrhizal (Amir et al. 1997; Perrier et al. 2006; Amir and Ducousso 2010), including Ni-hyperaccumulating plants (Amir et al. 2008) and species of Cyperaceae, a family generally considered as non-mycorrhizal (Perrier et al. 2006; Lagrange et al. 2011; Lagrange et al. 2013). Arbuscular mycorrhizal fungi isolates from ultramafic soils have been found to be highly tolerant to Ni and clearly more tolerant to this metal than non-native isolates (Amir et al. 2008). In these conditions, native AMF can have great importance for plant nutrition and adaptation to ultramafic constraints (Orłowska et al. 2011; Amir et al. 2013; Doubková et al. 2013). Therefore, native AMF are potentially a good target to be used as plant growth promoters for ecological restoration of mine-degraded areas (Orłowska et al. 2005; Berruti et al. 2016).
Recently, six AMF isolates from New Caledonian ultramafic soil, including five new species, were described: Acaulospora saccata, A. fragilissima, Pervetustus simplex, Rhizophagus neocaledonicus, Scutellospora ovalis (Błaszkowski et al. 2017; Crossay et al. 2018), and Claroideoglomus etunicatum isolate nc (Crossay et al. 2017; Crossay 2018). In the present study, we tested the effect of different combined inoculations of six isolates of these native AMF species, belonging to five different families, on growth and adaptation of Metrosideros laurifolia, an endemic small tree (Myrtaceae) used in restoration programs. We aimed to investigate the following questions: (i) are combinations of different taxa more efficient than individual ones in improving plant adaptation to the three physico-chemical stress factors (low major nutrients, low Ca/Mg ratio, and high levels of heavy metals)? (ii) in the presence of AMF combinations, what synergistic effects or what complementation of capacities can explain the observed improvement of plant growth and adaptation to ultramafic soil?
Materials and methods
We tested the variations of plant growth and adaptation to ultramafic soils induced by different combinations of AMF taxa in a glasshouse using six AMF species and one host plant species. The experiment was conducted between January and September 2017.
AMF isolates
The six tested isolates belonging to six different species are affiliated to three orders (Diversisporales, Glomerales, and Paraglomerales), five families (Acaulosporaceae, Gigasporaceae, Glomeraceae, Claroideoglomeraceae, and Pervertustaceae), and five genera. Two species belong to the genus Acaulospora (A. saccata, A. fragilissima); the other species belong to the genera Scutellospora (S. ovalis), Rhizophagus (R. neocaledonicus), Claroideoglomus (C. etunicatum nc), and Pervetustus (Pervetustus simplex nc). The origin and taxonomic description and affiliation of each isolate have been described in detail (Błaszkowski et al. 2017; Crossay et al. 2017; Crossay et al. 2018; Crossay 2018); all these AMF were isolated from rhizospheric soil in tropical ultramafic maquis in New Caledonia (Plum area: 22° 16′ S, 166° 38′ E). Inoculum of each isolate was obtained from LIVE (Laboratoire Insulaire du Vivant et de l’Environnement) and was grown using the “cone-tainer technique” (Koske and Gemma 1997; https://invam.wvu.edu/methods/cultures/single-species-cultures) as described by Crossay et al. (2018). Inocula consisted of ultramafic soil of single-species cultures with Sorghum vulgare containing spores and root fragments. The substrate of each pure culture was verified for the presence of viable AMF spores of the correct morphotype.
Host plant
The host plant species was a woody Myrtaceae endemic to New Caledonia and frequently used in ecological restoration programs: Metrosideros laurifolia. The seeds of Metrosideros laurifolia collected from a few adjacent trees in an ultramafic area were provided by SIRAS Pacifique, Noumea. They were surface-disinfected in a 1.25% solution of sodium hypochlorite (12° chlorometric) for 15 min and then rinsed with distilled water in sterilized Petri dishes. The seeds were subsequently sown in sterilized vermiculite (autoclaved for 60 min; at 120 °C) before transfer to experimental pots.
Substrate
The soil used in greenhouse experiments is a colluvial lateritic soil (ferralsol) with the following characteristics: coarse sand, 39.4%; fine sand, 22.1%; silt-clay, 37.2%; pH H20, 5.9; pH KCl, 5.6; total C, 42.1 g kg−1; total N, 2.2 g kg−1; total P, 147 mg kg−1; available P (Mehlich), 3 mg kg−1; total Ca, 1.06 g kg−1; total Mg, 5.08 g kg−1; Ca/Mg, 0.207; total Ni, 4.78 g kg−1; DTPA extractable Ni, 91.4 mg kg−1; DTPA extractable Co, 71.0 mg kg−1; DTPA extractable Cr, 0.5 mg kg−1; DTPA extractable Mn, 864.8 mg kg−1. This soil was sampled in New Caledonian ultramafic maquis in Plum area (22° 16′ S, 166° 38′ E). As plant growth in this pure ultramafic soil was very slow, a mixture of 80% ultramafic soil and 20% commercial compost (v/v) was used. The composition of the commercial compost was as follows: N, 1.7 mg g−1; Ptotal, 150 mg kg−1; POlsen, 7 mg kg−1; K, 139 mg kg−1 (Terreau universel, Agrofino, France). This substrate was autoclaved three times at 120 °C for 1 h, with an interval of 24 h, to eliminate microorganisms.
Inoculation procedures and growth conditions
Plants were inoculated separately during their transfer to the pots. Inoculum of each AMF isolate consisted of 20 g of mixture of rhizosphere soil containing homogenized spores, mycelium, and colonized small root fragments (six treatments, one for each AMF isolate). Six different fungal mixtures were also prepared by combining equal weights of 2, 3, 4, 5, or 6 pure AMF inocula, so that each mixture inoculum consisted finally of 20 g of soil. All treatments are presented in Table 1. Fungal species mixes formulations were chosen according to the following considerations: (i) the technical difficulty that would have been involved in testing all possible mixes; (ii) a previous experiment on Sorgho in ultramafic soil that showed the best plant growth promotion using Rhizophagus neocaledonicus, thus we chose to use R. neocaledonicus in all mixes to find the best mixes for plant growth promotion; (iii) we know that Claroideoglomus etunicatum and Scutellospora ovalis are present in many ultramafic sites in New Caledonia; we chose to use them in combination with R. neocaledonicus for mixes with two species; (iv) we know that Acaulospora species are less tolerant to ultramafic soil than Glomeraceae; we chose to use them only in three mixes. Ten replicate pots for each AMF isolate and for each combination and ten non-mycorrhizal controls were set up, for a total of 130 pots.
The pots were filled using 0.85 kg of the autoclaved substrate. The mycorrhizal inocula (20 g) were spread as a layer on the surface of the soil in the pots and covered with a thin layer of the substrate. Each pot also received 2 ml of a microbial filtrate, which was obtained from a mixture of 20 g of all different AMF inocula used in the experiment, suspended in 2 l of sterile water and passed through a 20-μm mesh (Mardhiah et al. 2016). Two-month-old AMF-free plantlets of M. laurifolia were removed from the vermiculite and planted singly in every experimental pot. The cultures were kept in a greenhouse (temperature, 21–24 °C; relative humidity, 70%) for 8 months and irrigated manually with water every 2 days.
Harvesting procedures
For each plant, the height of the main stem was periodically measured, from the soil level to the tip of the stem. After 8 months, the plants were harvested. The period of 8 months was necessary to obtain sufficient mycorrhizal colonization, but waiting longer would have constrained root growth in faster-growing treatments. Roots were removed from the soil by washing, and shoots and roots separated. Approximately 300 mg per plant of fresh roots was retained for quantification of colonization and approximately 50 mg of fresh roots of each plant were pooled per treatment, frozen in liquid nitrogen, and stored at − 80 °C for molecular analyses. The rest of plant tissues were weighed, dried at 60 °C for 74 h and re-weighed.
Quantification of AMF root colonization
A subsample of fresh roots was stained using trypan blue according to the method of Phillips and Hayman (1970) modified by BŁaszkowski et al. (2006): tissue acidification was performed using 20% hydrochloric acid instead of 1%, and trypan blue concentration was 0.1% instead of 0.05%. AMF colonization of 30 root fragments (approximately 1-cm length for each fragment) per pot was quantified using the method of estimation of mycorrhizal colonization according to Trouvelot et al. (1986), under a compound microscope (Olympus BX 50, × 200 magnification). The 10 pots of each treatment were analyzed. The intensity of arbuscular mycorrhizal colonization, i.e., mycorrhizal density (M %), was determined using the formula: M (%) =  where n5 is the number of root fragments with more than 90% colonization, n4 between 90 and 50% colonization, n3 between 50 and 10% colonization, n2 between 10 and 1% colonization, n1 less than 1% colonization. We present only mycorrhizal intensity because all values of mycorrhizal frequency reached 100% or were close to 100%.
where n5 is the number of root fragments with more than 90% colonization, n4 between 90 and 50% colonization, n3 between 50 and 10% colonization, n2 between 10 and 1% colonization, n1 less than 1% colonization. We present only mycorrhizal intensity because all values of mycorrhizal frequency reached 100% or were close to 100%.
AMF spore extraction and quantification
A subsample of substrate from each pot was air-dried to quantify spore abundance of each species. Spores were extracted by wet sieving and sucrose density gradient centrifugation, using a modified method from Daniels and Skipper (1982). For each pot culture, 10 g of dried substrate was wet sieved, and then the harvested material was suspended in 20 mL of water in a 50-mL Falcon tube. A 25-mL sucrose solution (70% v/w) was injected to the bottom of the tube, forming a stepped density gradient that was centrifuged at 900g for 3 min. Spores of AMF were collected from the interface with the sucrose solution, washed with tap water on a 36-μm sieve for 2 min, and transferred to a 50-mL Falcon tube with 10 mL of water. Finally, the Falcon tube was vortexed and 10 successive aliquots of 100 μL were immediately transferred to Petri dishes for spore counting and identification under a stereomicroscope. Spore numbers were estimated per gram of soil for each pot.
Identification of AMF using molecular markers
The partial 18S-5.8S-partial 28 S region of nuclear rRNA or the partial 28 S region of each species were obtained from Genbank under accession numbers: KY362431, KY362432, KY362433; KY362428, KY362429, KY362430; KY927386, KY927387; KY934450, KY934451, KY934452; KY362436, KY362437, KY362438; KY362434, KY362435 (Crossay et al. 2017, 2018). Sequences were aligned using MAFFT 7 (http://mafft.cbrc.jp/alignement/server; Katoh and Standley 2013) to design primer pairs to specifically amplify each species of AMF. This approach was used only to evaluate the presence or absence of each fungus species in roots.
DNA extracts from spores of each species were used to cross check that the specific primers only amplified the targeted species. One to three spores of each species from the single-species cultures on S. vulgare were crushed using a pipette tip in a 1.5 Eppendorf tube containing 10 μl of ultrapure water, and 2 μl was used for polymerase chain reaction (PCR). A DNA fragment of around 1545 bp, covering partial SSU, the whole ITS and the variable D1 and D2 regions of the LSU, was amplified using the AMF-specific primers developed by Krüger et al. (2009). In the first round of PCR, the primers SSUmAf and LSUmAr were used. In the second, nested round of PCR, the specific primers (Table S1) were used with 1 μl of the first PCR round product as a template. The PCR mix included 0.4 U of AmpliTaq® 360 DNA polymerase (Applied Biosystems), 1X AmpliTaq® 360 PCR buffer (Applied Biosystems), 0.2 mM of each dNTP, 0.4 μM of each primer, and 1 μl of the template in a final volume of 25 μl. The cycling parameters for the first PCR were 3 min at 98 °C followed by 35 cycles of 10 s at 98 °C, 30 s at 60 °C, and 1 min at 72 °C. The program was concluded by a final extension phase of 10 min at 72 °C. The cycling parameters for the second PCR were 3 min at 98 °C followed by 35 cycles of 30 s at 95 °C, 20 s at 60 °C, and 40 s at 72 °C. The program was concluded by a final extension phase of 7 min at 72 °C. The amplified DNA fragments were visualized on a 1% (w/v) agarose gel in TBE buffer to ensure that the specific primers amplify only the target species.
DNA extraction of root samples (100 mg) was performed using the FastDNA® Spin Kit for plant (MP Biomedicals, Solon, OH) according to the manufacturer’s instructions. The primers were used in a nested polymerase chain reaction (PCR). The first PCR was performed as explained above using primers SSUmAf and LSUmAr and the second PCR was performed as explained above with species specific primers (Table S1). The amplified DNA fragments were visualized on a 1% (w/v) agarose gel in TBE buffer.
When a weak amplification or no amplification was obtained, we performed a third nested PCR (Jansa et al. 2003). In this case, the primers SSUmAf and LSUmAr and SSUmCf and LSUmBr were used for the first and the second PCR respectively. For the second, nested round of PCR, the primers SSUmCf and LSUmBr were used with 1 μl of the first PCR round product as a template. The PCR mix included 0.4 U of AmpliTaq® 360 DNA polymerase (Applied Biosystems), 1X AmpliTaq® 360 PCR buffer (Applied Biosystems), 0.2 mM of each dNTP, 0.4 μM of each primer, and 1 μl of the template in a final volume of 25 μl. The cycling parameters for the second PCR were the same as in the first PCR (see above) except for annealing temperature (63 °C). The second PCR product (1 μL) was used as template for the third reaction using specific primers (Table S1). PCR conditions are similar to those described above.
Mineral analyses of plants
Aliquots of dried plant tissues (shoot or root) were collected for mineral analyses, which were performed by ICP-OES as reported in Perrier et al. (2006) and by CHN, mass spectrometry using an Integra2 spectrometer (Sercon Instruments, www.sercongroup.com) linked to a Sercon elemental analyzer. All elements analyses (Ca, Mg, K, P, Na, Co, Cr, Fe, Mn, Ni, N, C) were performed by the Laboratoire des Moyens Analytiques (LAMA), Centre IRD de Nouméa, New Caledonia. Phosphorus, potassium, and nitrogen content were calculated by the following formulae: P, K, or N content = P, K, or N concentration × dry matter weight. The concentrations of each metal element in the shoots and the roots were used to calculate the translocation factor (TF), defined as the ratio of metal concentration in shoots to that in roots. The value of TF is < 1 when the metal transfer to aerial parts is reduced.
Statistical analysis
All statistical analyses were performed using R software version 3.3.1 (R Core Team 2018) (version 1.2-4, available at http://CRAN.R-project.org/package=agricolae ). Plant height data (Table S2) were analyzed using the parametric tests: one-way ANOVA with 13 levels and a repeated measures ANOVA (Fig. S1), followed by Tukey’s HSD post hoc test. For all other data, assumptions were not fulfilled using ad hoc tests even after Box-Cox data transformation (Shapiro and Kolmogorov-Smirnov goodness-of-fit tests for normality and Levene’s homogeneity of variances test); therefore, we used nonparametric statistical methods (Kruskal-Wallis test). If significant effects were detected, Mann-Whitney post hoc tests were used.
Hierarchical clustering on principal components (HCPC) from principal component analysis (PCA) using Ward’s method were carried out to describe the relationships between the different inoculation treatments and the measured variables (biomass, mycorrhizal colonization, sporulation, root and shoot element concentrations, etc.). The convex hull of the set of points defined in each cluster by the HCPC method was displayed on the first two principal components (Gordon 1999; Jolliffe 2002; Husson et al. 2017). The objective was to estimate the values of the biomass shoot variable on the basis of selected principal components of the explanatory variables: mycorrhizal variables (root colonization and sporulation), plant nutrition (main elements), and heavy metal translocation. The position of the tested AMF treatments on the abscissa was then determined by the result of these explanatory variables.
The combination of principal component analysis and quantile regression is a robust alternative method of extracting important variables (in the form of components) from a large set of variables available in a data set. As multicollinearity has an effect on the least squares estimates in a multiple linear regression model, principal component regression was used. Principal component regression is a regression method based on principal component analysis leading to dimensionality reduction, mitigating overfitting and avoiding multicollinearity among predictors. As least squared regression is also sensitive to outliers, we propose a robust principal component regression method based on the quantile regression method (Durrieu and Briollais 2009; Briollais and Durrieu 2014; Durrieu and Briollais 2017; Koenker et al. 2017). Here, we consider the regression median which is known to be much more efficient than the least squares estimator in a linear regression model with a non-Gaussian error term. The computations were performed using the “FactoMineR,” “factoextra,” and “quantreg” R packages.
Results
Plant growth and mycorrhizal colonization
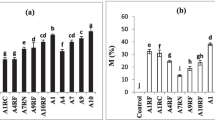
Summarized plant growth data are presented in Fig. 1. Full data are accessible in Electronic Supplementary Material (ESM) Tables S2 and S3 and Figs. S1 and S2. When comparing all treatments together to the control, the biomass of M. laurifolia was significantly enhanced at the end of the experiment (p < 0.05). Shoot dry weight was increased 4.9-fold (A. saccata) to 21.6-fold (Mix 5). Treatments using A. fragilissima and S. ovalis were not significantly different from the control. Root dry weight was significantly increased 3 times (S. ovalis) to 11.9 times (Mix 5), except for plants inoculated with A. fragilissima. The highest biomasses were obtained using AMF Mix 5 and Mix 6, containing the largest diversity. However, Mix 6 performed significantly less than Mix 5 on root biomass. The general AMF effect on plant height (Table S2) was significant 120 days after inoculation (p < 0.05). All AMF-inoculated plants were significantly higher than controls from 186 days to the end of experiment.
Metrosideros laurifolia (a) control versus treatment Mix 5 (representing the extremes) after 8 months of growth on ultramafic substrate. b Dry weight of shoot and root, respectively. Bars represent means, and error bars represent standard error of means (n = 10 except for control treatment n = 9). Means followed by the same letter above columns do not differ significantly by the Mann-Whitney post hoc test (p ≤ 0.05)
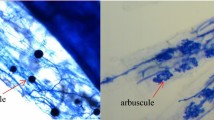
Mycorrhizal frequency, intensity, and percentage of arbuscules were analyzed. Results of mycorrhizal intensity are provided in Fig. 2. Full data are accessible in Table S4. All plants except controls were colonized by AMF; weak colonization was found for the two Acaulospora species and S. ovalis. Mycorrhizal colonization was superior to 80% for all AMF mixes and for mono-inoculation using R. neocaledonicus. Arbuscules were clearly visible and well-developed in all AMF treatments with arbuscular colonization values higher than 70% within infected areas for A. saccata, R. neocaledonicus, and all AMF mixes.
Mycorrhizal colonization (intensity of mycorrhization) for each fungal treatment after 8 months of growth in ultramafic substrate. Bars represent means, and error bars represent standard error of means (n = 10 except for control n = 9). Means followed by the same letter above columns do not differ significantly by Mann-Whitney post hoc test (p ≤ 0.05)
Spores of all species inoculated alone or combined were recovered and counted after plant growth (Table 2). Rhizophagus neocaledonicus produced more spores than other species with an average of 23.5 ± 4 spores g−1 of dry soil. The maximum number of spores was obtained for the AMF treatment Mix 2 with 79.2 ± 19 spores g−1 of dry soil (74.5 ± 18.1 spores of R. neocaledonicus and 4.7 ± 0.7 spores of S. ovalis).
Primers developed for the molecular species detection were validated on pure spore DNA extracts of all species, except for S. ovalis which failed to be amplified with its specific primers. In mono-inoculation, all species were detected by PCR in roots of M. laurifolia except for plants inoculated with S. ovalis (Table 2). Curiously, in two fungal treatments (mixes 3 and 6), S. ovalis was detected in roots with primers which failed to amplify DNA from spores. In plants inoculated with Mix 1, the two species C. etunicatum and R. neocaledonicus were detected. For Mix 2, only R. neocaledonicus was detected in roots. For Mix 3 and Mix 6, all species were detected. Only C. etunicatum was detected in roots of plants inoculated with Mix 4 and only A. saccata and C. etunicatum and P. simplex were detected for Mix 5.
Plant mineral nutrition analyses
The major elements needed for plant nutrition (i.e., P, K, N) were analyzed in shoots and roots of inoculated and control plants of M. laurifolia. AMF treatments differently affected major element concentrations in shoots and roots tissues (Fig. 3; Table S5). Interestingly, phosphorus concentration (Fig. 3a) was enhanced in shoot tissues exclusively by AMF mixes 1, 2, 3, 5, 6 (by 23% to 37%) and not by inoculation using any single species. A significant reduction of P concentration in root tissues (49 to 58%) was observed for A. fragilissima, A. saccata, C. etunicatum, and Mix 4. For the remaining treatments, P concentration was not significantly different when compared with controls. Potassium concentration in shoots (Fig. 3b) was significantly enhanced (20 to 27%) by all AMF mixes and by mono-inoculation using A. saccata and R. neocaledonicus. In root tissues, K concentration was increased (18 to 32%) for all AMF mixes except Mix 4. For the other treatments, K concentration in root tissues was not significantly different when compared with controls. Nitrogen concentration in shoot tissues (Fig. 3c) was generally reduced by AMF inoculation (16 to 40% less). This reduction was significant for R. neocaledonicus and mixes 2, 3, 4, 5, and 6. N concentration was also lower in root tissues (19 to 34% less) for A. saccata and all mixes.
Major element (P, K, N) concentration in plant shoots and roots of M. laurifolia expressed as mg kg−1 or g kg−1 of dry weight tissue; and major element (P, K, N) content in the whole plant dry weight tissue expressed as mg plant−1. a Phosphorus, b potassium, c nitrogen, d N, P, K content. Bars represent means and error bars represent standard error of means (n = 5). Means followed by the same letter above columns do not differ significantly by the Mann-Whitney post hoc test (p ≤ 0.05)
Phosphorus, potassium, and nitrogen total contents per plant were significantly higher than control in all inoculated treatments, except for plants inoculated with A. fragilissima, A. saccata, and S. ovalis, with greatest improvements for AMF mixes (Fig. 3d).
A significant increase of the Ca/Mg ratio (29 to 35%) was induced by all AMF mixes and mono-inoculation with P. simplex in shoot tissues of M. laurifolia (Fig. 4). In root tissues Ca/Mg ratio was enhanced by 35 to 43% in the presence of P. simplex, C. etunicatum, and mixes 2, 3, and 4. For the other treatments, the Ca/Mg ratio was not significantly different when compared with controls.
Heavy metal translocation
Five metals, present at high concentrations in ultramafic soils (Fe, Mn, Ni, Cr, and Co), were analyzed in shoots and roots of plants. The translocation factors (TF) are presented in Table 3, while full data are reported in Table S6. Metal accumulation was higher in roots than in shoots, but a significant decrease of most heavy metal concentrations was observed in shoots for inoculated plants (p < 0.05).
Iron concentration in shoots ranges from 545 to 2232 mg kg−1; root concentration of this metal was a lot higher than in shoots (11,708 to 33,571 mg kg−1). The translocation factor is in the range of 0.02 to 0.1. Seven treatments showed a significantly reduced TF value: A. fragilissima, R. neocaledonicus, and all AMF mixes except Mix 4. Manganese concentration in plant organs was different depending on AMF treatment (from 611 to 2224 mg kg−1 in shoots and from 1027 to 2642 mg kg−1 in roots). Five AMF treatments did not have any significant effect on the Mn TF value: A. fragilissima, R. neocaledonicus, Mix 1, Mix 5, and Mix 6. The other treatments significantly increased the TF value of this metal (from 0.69 for the control to a maximum of 1.53 for A. saccata). Nickel concentration in shoots (19.8 to 79.4 mg kg−1) is lower than in roots (333 to 790 mg kg−1). Nickel TF values were reduced in plants inoculated with R. neocaledonicus, Mix 1 and Mix 5. The other treatments did not significantly affect Ni translocation. Chromium was accumulated in roots (284 to 1124 mg kg−1); generally, less than 20% was translocated to shoots. Two AMF treatments reduced its TF value three to four times: R. neocaledonicus and Mix 1. Mix 3 and Mix 4 significantly increased this value. Cobalt concentration in plant organs was lower than the other metals (2.6 to 13.0 mg kg−1 in shoots and 71.5 to 171.7 mg kg−1 in roots). AMF inoculation generally decreased the TF value of this metal: for example, Mix 5 reduced it more than six times. Only A. saccata, S. ovalis, and Mix 4 did not significantly reduce Co translocation.
Global statistical analyses
Significant total correlations between analyzed variables were obtained (Table 4). Mycorrhizal variables (intensity of mycorrhization, percentage of arbuscules, and spore production) showed a significantly positive correlation with dry root and shoot biomass, P concentration in shoot, K concentration in shoot, and Ca/Mg ratio in shoot. These mycorrhizal parameters had a significantly negative correlation with Mg concentration in shoot. Intensity of mycorrhization was negatively correlated with the tested metal concentrations in shoots except for Cr. P values were generally very low, particularly for mycorrhizal intensity.
Two clusters were clearly differentiated by hierarchical classification on principal component projections (Fig. 5). Cluster 1 was composed by control, C. etunicatum, S. ovalis, P. simplex, A. saccata, A. fragilissima, and Mix 4 treatments. This cluster was characterized by a lower biomass, higher N, Mg, and Mn concentrations in roots and shoots, higher Co, Fe, and Ni concentrations in shoots, when compared with the second cluster. Cluster 2 was represented by Mix 1, Mix 2, Mix 3, Mix 5, and Mix 6 treatments. This cluster was characterized by a higher biomass, a higher mycorrhizal intensity, a greater arbuscule abundance in the root system, a higher Ca/Mg ratio in roots and shoots, and higher P, K, and Ca concentrations in root and shoot tissues. The R. neocaledonicus treatment was recovered between clusters 1 and 2. The results also indicated that the different AMF species are scattered in the first cluster and showed different abilities. For example, P. simplex and C. etunicatum were more efficient in increasing the plant Ca/Mg ratio (Fig. 4); A. saccata was more efficient in K absorption (Fig. 3); R. neocaledonicus was efficient in the reduction of heavy metal translocation (Table 3) and in K absorption (Fig. 3); A. fragilissima, situated high in the axis 2 of the PCA induced high Mg levels in roots and shoots and reduction of iron and cobalt translocation (see also Table 3).
Hierarchical clustering on principal components (HCPC) from principal components analysis (PCA): the convex hull of the set of points defined in each cluster is shown in panel “a.” In panel “b,” the PCA projections of the variables with the correlation circle are displayed. The filled circles and triangles correspond to the PCA individual value projections in cluster 1 and in cluster 2, respectively. The symbols with treatment names represent the mean values for each treatment. The barycentric coordinates of the two clusters are represented by the largest filled triangle and circle. PCA and HCPC were performed on 26 variables: dry weight tissue of shoot (biomass S) and roots (biomass R), intensity of mycorrhization (I Myco), arbuscule abundance in roots system (Arb), total sporulation (Nb Spores), major element concentration in shoot (e.g., N_S) and roots (e.g., N_R) (N, P, K, Ca, Mg, Ca/Mg), and metal concentration in shoot (e.g., Co_F) and roots (e.g., Co_R) (Co, Cr, Fe, Mn, Ni)
We propose a global model using robust principal component regression to predict plant biomass as a function of mycorrhizal and nutritional variables considering the two first principal components of the explanatory variables. The scatterplot representation of plant biomass observation against model prediction (Fig. 6) revealed that the proposed model is satisfactory and significant. The prediction is highly significant (R2 = 0.90, p < 0.00001) since all the points are closely dispersed around the first bisector. This means that the performance of the symbiosis (in terms of biomass increase) was correctly modeled as a function of the different AMF properties, in relation to the absorption of major elements, Ca/Mg ratio and heavy metal absorption. The abscissa values which represent the results of these explanatory variables were significantly higher for Mix 5 and 6 and lowest for A. fragilissima and the non-inoculated treatment which had the value 0. The model confirmed clearly that AMF co-inoculations induce better plant performance than single-species inocula, especially when the number of AMF species is high (Mix 5 and 6). Differences appeared also between AMF isolates.
Representation of observed total plant biomass (g) values vs. model-predicted values: the circles and blue squares are the individual and mean values for each treatment respectively, and the first bisector line is shown in the graph. The model predicts the values of plant biomass as the additive effect of all the tested variables (mycorrhizal parameters, absorption of main elements, Ca/Mg ratio and heavy metal absorption), for each treatment (AMF inoculations)
Discussion
This study is one of the first to analyze in detail synergistic effects of AMF species on plant growth and mineral nutrition, suggesting fungal functional complementarity. That we used a highly mycorrhiza-dependent host plant with native fungal isolates in an ultramafic soil with multiple stress factors may have contributed to the strong synergistic effects we observed and sets our study apart from others. As reported by Gosling et al. (2016), the benefits of AMF diversity may be non-apparent in single stress factor environments. We found that combined inoculation of AMF species belonging to different families: (i) improved plant growth more than mono-inoculation, (ii) enhanced mineral nutrition of the plant, (iii) improved the Ca/Mg ratio, and (iv) limited metal translocation to shoots.
AMF mono-inoculation versus co-inoculation
The highest increase of biomass obtained here using the endemic plant species M. laurifolia inoculated with five AMF isolates is substantial (total biomass 18 times higher than control). In comparison, Amir et al. (2019) tested a mix of three AMF isolates with the same plant species, in a field experiment in ultramafic soil and reported biomass four times higher than control plants. Orłowska et al. (2011), using an ultramafic soil in South Africa, showed that the Ni-hyperaccumulating plant Berkheya coddii produced six to 12 times more biomass than control plants when inoculated with AMF. AMF inoculation of Knautia arvensis in an ultramafic soil in the Czech Republic resulted in a doubled plant dry weight (Doubková et al. 2013).
Our results are in accordance with those reported by Maherali and Klironomos (2007): these authors tested AMF co-inoculations of four to eight species belonging to one, two or three different families and showed that mixes containing three families were most efficient. Yang et al. (2016) reported a meta-analysis review of this topic, pointing out the same conclusion concerning the better performance of AMF mixes with high family richness. In our experiment, when two species of the same genus (Acaulospora) were used (Mix 6), the biomass value was reduced when compared with the same inoculation treatment with only one species of Acaulospora (Mix 5). A functional redundancy between close species, inducing competition, could explain this lower efficiency, as already reported by other authors (Thonar et al. 2014; Gosling et al. 2016; Yang et al. 2016).
A significant difference between the tested AMF species and co-inoculations for the ability to colonize root systems was obtained, confirming the conclusions of other studies (Hart and Reader 2002; Maherali and Klironomos 2007; Bennett and Bever 2009). The highest values of root colonization were recovered with R. neocaledonicus and mixed AMF species. The two species of Acaulospora spp. and S. ovalis were characterized by weak root colonization and this seemed to be related to their low efficiency in plant growth promotion. This conclusion is reinforced by the highly significant positive correlation obtained between plant biomass and mycorrhizal colonization.
In each fungal treatment, spores of the inoculated species were found at the end of the experiment, showing that all inoculated AMF species had grown and achieved their life cycle. Our molecular approach allowed the detection of each species of AMF in mono-inoculation except for S. ovalis. In pots with AMF mixes, not all inoculated species were always recovered in roots, suggesting interactions such as competition among the different species. This competition could have resulted in sparse colonization by the less-competitive species rendering them undetectable by PCR (Sutlovic et al. 2008; Opel et al. 2010).
Mineral nutrition and synergistic effects of AMF isolates
Shoot and root phosphorus concentrations were not improved by any of the six AMF isolates in mono-inoculation, but shoot P concentration was significantly increased by five of the six tested mixes indicating synergistic or additive effects on plant P absorption. Jansa et al. (2007) and Thonar et al. (2014) equally found that combined inocula exclusively improved P concentration in tissues of Allium porrum and Medicago truncatula, respectively. All AMF mixes and two mono-inoculations increased shoot potassium concentration, whereas only five mixes enhanced root concentration of this element. Amir et al. (2019) reported an increase of K concentration in M. laurifolia plants inoculated using a mix of three AMF isolates in a field experiment in an ultramafic area. Porras-Soriano et al. (2009) also showed an increase of K concentration in olive trees inoculated using C. claroideum, F. mosseae, and R. intraradices, under high salt conditions.
Treatments with the highest biomass (Mix 5 and 6) showed the most significant reduction of N tissue concentration when compared with controls. Wu et al. (2017) observed the same effect and concluded that inoculation of plants with AMF improved photosynthesis and that AMF effect was mostly determined by the relative allocation of nitrogen to photosynthesis and not by the leaf nitrogen concentration. However, we can also explain these results by the greater dilution of N in a larger volume of tissues due to the high increase of biomass. Indeed, total N content of the plant was clearly improved by AMF inoculation for nine of the 12 tested treatments. This increase of mineral content by AMF-inoculated plants was generally clear for the three tested major elements.
The low Ca/Mg ratio is an important limiting factor in ultramafic soils because of the competition between Mg and Ca in plant nutrition (Jaffré and L’Huillier 2010b). As Mg is always in excess in these soils and tends to reduce Ca absorption, the high values of this ratio particularly in aerial parts are better for plant health and growth. Generally, Ca/Mg values range between 1 and 7 in New Caledonian woody plants (Jaffré and L’Huillier 2010b). Our results showed that this ratio was improved principally by AMF mixes, particularly in relation to a reduction of Mg assimilation. The increase of Ca/Mg ratio by AMF inoculation in ultramafic soils was reported recently (Amir et al. 2019). A significant positive correlation between mycorrhizal colonization and Ca/Mg ratio of Knautia arvensis plants in different ultramafic and non-ultramafic areas was previously reported by Doubková et al. (2011).
All these results clearly demonstrate that a mix of different AMF isolates, belonging to different families, stimulated the mineral nutrition and improved the Ca/Mg ratio of M. laurifolia more than each isolate taken separately.
Heavy metal translocation
In this study, we found that ultramafic AMF isolates reduced plant metal translocation in ultramafic soil conditions which may reduce metal toxicity as shown by Amir et al. (2013). The mechanisms involved in metal homeostasis and detoxification of heavy metals by AMF are described in detail by Ferrol et al. (2016). Heavy metal tolerance can be due to several processes: (i) binding mechanisms on external mycelium and spores, (ii) chelating heavy metal in cytosol, (iii) sequestration in the fungal vacuole, and (iv) reduction of metal accumulation by activation of specific transport. Our results argue in favor of the hypothesis that AMF regulates metals in the rhizosphere and diminishes metal translocation to the shoot: metals were generally drastically reduced in plant shoots and increased in roots. Manganese was found to be accumulated in shoot tissues of M. laurifolia approximately at the same level as in roots and AMF inoculation either did not have any reducing effect on its translocation or increased it. This probably means that Mn is not toxic for this plant at the levels observed in our experiment. Indeed, these levels are in accordance with those reported by Wulff et al. (2010) for this species under natural conditions. In summary, AMF mixes and R. neocaledonicus treatments were generally the most efficient to reduce metal concentrations in shoot tissues.
Multivariate analysis and synergistic effects of AMF isolates
The multivariate analyses clearly confirmed that AMF mixes, particularly those with the highest family diversity, were the most efficient for plant growth and adaptation. It was also relatively clear that the AMF species tested showed different abilities (mycorrhizal colonization, increase of plant mineral element content, improvement of plant Ca/Mg ratio, and/or reduction of heavy metal translocation) as already reported in a literature meta-analysis (Yang et al. 2016). These authors highlighted that Glomeraceae were more efficient for improved nutrient content and fungal pathogen inhibition, Gigasporaceae for growth under heavy metal stress and Claroideoglomeraceae for nematode impact reduction. Our results are not in accordance with some of these assertions, as R. neocaledonicus (Glomeraceae) was not more efficient than the other taxa for P and N content and S. ovalis (Gigasporaceae) did not show a better performance than the other AMF species for the reduction of heavy metal translocation.
The principal component regression model (Fig. 6) showed that the combination of the contributions of these different abilities (explanatory variables), in relation to mycorrhizal colonization, mineral nutrition, and heavy metal translocation, allowed us to predict accurately and with a very high correlation the biomass production of the plant host under multistress conditions (ultramafic soil). This model clearly shows that AMF mixes are more efficient than AMF single isolates although there is no simple correlation between species number and efficiency. This model showed that these explanatory variables are important as a whole and act with complementarity to produce the biomass increase and that the AMF treatments which were efficient for different variables composed the best treatments.
Very few studies have reported synergistic effects of different AMF taxa (Maherali and Klironomos 2007; Powell et al. 2009; Yang et al. 2016). The latter have linked these effects to the functional complementarity among phylogenetically distant taxa. This functional complementary was also clear in our results and might have the same significance even if we did not perform the corresponding control comprising several AMF isolates belonging to one family. As stressed by some authors (Powell et al. 2009; Gosling et al. 2016; Yang et al. 2016), the use of closely related AMF taxa can induce functional redundancy which can limit the synergistic effects of AMF.
All these results suggest that when a plant faces multiple stress factors, the use of an AMF mix containing different taxa is more efficient than mono-inoculation to improve biomass, mineral nutrition, Ca/Mg ratio, and tolerance to heavy metals of plants in ultramafic soil. Our results support the hypothesis that there is a positive correlation between AMF family diversity and enhancement of plants growth (Hart and Reader 2002; Maherali and Klironomos 2007; Yang et al. 2016). These results also clearly underline the importance of mycorrhizal symbiosis diversity in multistress environments. AMF symbiosis can have a substantial effect on plant growth and adaptation when combined native AMF taxa were inoculated to a native plant species, and this opens a new perspective for the use of AMF for ecological restoration of ultramafic soils.
References
Amir H, Pineau R, Violette Z (1997) Premiers résultats sur les endomycorhizes des plantes de maquis miniers de Nouvelle-Calédonie. In: Jaffré T, Reeves RD, Becquer T (eds) The ecology of ultramafic and metalliferous areas. Edition ORSTOM. Documents Scientifiques et Techniques III2, Noumea, pp 79–85
Amir H, Jasper DA, Abbott LK (2008) Tolerance and induction of tolerance to Ni of arbuscular mycorrhizal fungi from New Caledonian ultramafic soils. Mycorrhiza 19:1–6. https://doi.org/10.1007/s00572-008-0197-y
Amir H, Ducousso M (2010) Les bactéries et les champignons du sol sur roches ultramafiques. In : L’Huillier L, Jaffré T, Wulff A (eds) Mines et environnement en Nouvelle-Calédonie : les milieux sur substrats ultramafiques et leur restauration, IAC Ed Nouméa, New Caledonia, pp 129–145
Amir H, Lagrange A, Hassaïne N, Cavaloc Y (2013) Arbuscular mycorrhizal fungi from New Caledonian ultramafic soils improve tolerance to nickel of endemic plant species. Mycorrhiza 23:585–595. https://doi.org/10.1007/s00572-013-0499-6
Amir H, Cavaloc Y, Laurent A, Pagand P, Gunkel P, Lemestre M, Médevielle V, Pain A, McCoy S (2019) Arbuscular mycorrhizal fungi and sewage sludge enhance growth and adaptation of Metrosideros laurifolia on ultramafic soil in New Caledonia: a field experiment. Sci Total Environ 651:334–343. https://doi.org/10.1016/j.scitotenv.2018.09.153
Augé RM, Toler HD, Saxton AM (2015) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza 25:13–24. https://doi.org/10.1007/s00572-014-0585-
Baker AJM (1987) Metal tolerance. New Phytol 106:93–111
Bárzana G, Aroca R, Paz JA, Chaumont F, Martinez-Ballesta MC, Carvajal M, Ruiz-Lozano JM (2012) Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann Bot 109:1009–1017. https://doi.org/10.1093/aob/mcs007
Bennett AE, Bever JD (2009) Trade-offs between arbuscular mycorrhizal fungal competitive ability and host growth promotion in Plantago lanceolata. Oecologia 160:807–816. https://doi.org/10.1007/s00442-009-1345-6
Berruti A, Lumini E, Balestrini R, Bianciotto V (2016) Arbuscular mycorrhizal fungi as natural biofertilizers: let’s benefit from past successes. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.01559
Błaszkowski J, Kozłowska A, Crossay T, Symanczik S, al-Yahya'ei MN (2017) A new family, Pervetustaceae with a new genus, Pervetustus, and P. simplex sp. nov. (Paraglomerales), and a new genus, Innospora with I. majewskii comb. nov. (Paraglomeraceae) in the Glomeromycotina. Nova Hedwigia 105:397–410. https://doi.org/10.1127/nova_hedwigia/2017/0419
BŁaszkowski J, Renker C, Buscot F (2006) Glomus drummondii and G. walkeri, two new species of arbuscular mycorrhizal fungi (Glomeromycota). Mycol Res 110:555–566. https://doi.org/10.1016/j.mycres.2006.02.006
Briollais L, Durrieu G (2014) Application of quantile regression to recent genetic and -omic studies. Hum Genet 133:951–966. https://doi.org/10.1007/s00439-014-1440-6
Brooks RR (1987) The serpentine factor. In: Brooks RR (ed) Serpentine and its vegetation. A multidisciplinary approach. Dioscorides, Portland
Crossay T, Antheaume C, Redecker D, Bon L, Chedri N, Richert C, Guentas L, Cavaloc Y, Amir H (2017) New method for the identification of arbuscular mycorrhizal fungi by proteomic-based biotyping of spores using MALDI-TOF-MS. Sci Rep 7:14306. https://doi.org/10.1038/s41598-017-14487-6
Crossay T, Cilia A, Cavaloc Y, Amir H, Redecker D (2018) Four new species of arbuscular mycorrhizal fungi (Glomeromycota) associated with endemic plants from ultramafic soils of New Caledonia. Mycol Progress 17:1–16. https://doi.org/10.1007/s11557-018-1386-5
Crossay T (2018) Caractérisation taxonomique des champignons mycorhiziens à arbuscules natifs des sols ultramafiques de Nouvelle-Calédonie; analyse de leur synergie permettant l’adaptation des plantes à ces milieux extrêmes. PhD thesis. New Caledonia University, Nouméa
Daniels BA, Skipper HD (1982) Methods for the recovery and quantitative estimation of propagules from soil. In: Schenck NC (ed) Methods and principles of mycorrhizal research. The Amercian Phytopath Society, St Paul, Minnesota, U.S.A, pp 29–35
Durrieu G, Briollais L (2009) Sequential design for microarray experiments. J Am Stat Assoc 104:650–660. https://doi.org/10.1198/jasa.2009.0135
Durrieu G, Briollais L (2017) Some recent statistical methods applied in genetics/genomics. In: Koenker R, Chernozhukov V, Xumin H, Limin P (eds) Handbooks of modern statistical methods. Chapman and Hall/CRC, New York, pp 409–427
Doubková P, Suda J, Sudová R (2011) Arbuscular mycorrhizal symbiosis on serpentine soils: the effect of native fungal communities on different Knautia arvensis ecotypes. Plant Soil 345:325–338. https://doi.org/10.1007/s11104-011-0785-z
Doubková P, Vlasáková E, Sudová R (2013) Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant Soil 370:149–161. https://doi.org/10.1007/s11104-013-1610-7
Feddermann N, Finlay R, Boller T, Elfstrand M (2010) Functional diversity in arbuscular mycorrhiza – the role of gene expression, phosphorous nutrition and symbiotic efficiency. Fungal Ecol 3:1–8. https://doi.org/10.1016/j.funeco.2009.07.003
Ferrol N, Tamayo E, Vargas P (2016) The heavy metal paradox in arbuscular mycorrhizas: from mechanisms to biotechnological applications. J Exp Bot 67:6253–6265. https://doi.org/10.1093/jxb/erw403
Gordon AD (1999) Classification, 2nd edn. Chapman and Hall, Boca Raton
Gosling P, Jones J, Bending GD (2016) Evidence for functional redundancy in arbuscular mycorrhizal fungi and implications for agroecosystem management. Mycorrhiza 26:77–83. https://doi.org/10.1007/s00572-015-0651-6
Graham RC (2009) Serpentine geoecology of Western North America: geology, soils, and vegetation. Soil Sci 174:193. https://doi.org/10.1097/SS.0b013e318199f342
Hart M, Reader RJ (2002) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153:335–344. https://doi.org/10.1046/j.0028-646X.2001.00312.x
Hawkins H-J, Johansen A, George E (2000) Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil 226:275–285. https://doi.org/10.1023/A:1026500810385
Heijden MGA, Martin F, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423. https://doi.org/10.1111/nph.13288
Husson F, Lê S, Pagès J (2017) Exploratory multivariate analysis by example using R. 2nd edition. Chapman & Hall/CRC, Boca Raton
Jaffré T, L’Huillier L (2010aa) La végétation des roches ultramafiques ou terrains miniers. In : L’Huillier L, Jaffré T, Wulff A (eds) Mines et Environnement en Nouvelle-Calédonie : les milieux sur substrats ultramafiques et leur restauration. IAC Ed, Noumea, New Caledonia, pp 45–103
Jaffré T, L’Huillier L (2010bb) Conditions de milieu des terrains miniers. In : L’Huillier L, Jaffré T, Wulff A (eds) Mines et Environnement en Nouvelle-Calédonie : les milieux sur substrats ultramafiques et leur restauration. IAC Ed, Noumea, New Caledonia, pp 33–44
Jansa J, Mozafar A, Kuhn G, Anken T, Ruh R, Sanders IR, Frossard E (2003) Soil tillage affects the community structure of mycorrhizal fungi in maize roots. Ecol Appl 13:1164–1176. https://doi.org/10.1890/1051-0761(2003)13[1164:STATCS]2.0.CO;2
Jansa J, Smith FA, Smith SE (2007) Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi. New Phytol 177:779–789. https://doi.org/10.1111/j.1469-8137.2007.02294.x
Jolliffe I (2002) Principal component analysis. Springer 2nd edn
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Kazakou E, Dimitrakopoulos PG, Baker AJM, Reeves RD, Troumbis AY (2008) Hypotheses, mechanisms and trade-offs of tolerance and adaptation to serpentine soils: from species to ecosystem level. Biol Rev 83:495–508. https://doi.org/10.1111/j.1469-185X.2008.00051.x
Kiers ET, Duhamel M, Beesetty Y, et al (2011) Reciprocal Rewards Stabilize Cooperation in the Mycorrhizal Symbiosis. Science 333:880–882. https://doi.org/10.1126/science.1208473
Koch AM, Antunes PM, Maherali H, Hart MM, Klironomos JN (2017) Evolutionary asymmetry in the arbuscular mycorrhizal symbiosis: conservatism in fungal morphology does not predict host plant growth. New Phytol 214:1330–1337. https://doi.org/10.1111/nph.14465
Koenker R (2017) Quantile Regression: 40 Years On. Annual Review of Economics 9:155–176. https://doi.org/10.1146/annurev-economics-063016-103651
Koske RE, Gemma JN (1997) Mycorrhizae and succession in plantings of Beachgrass in sand dunes. Am J Bot 84:118–130. https://doi.org/10.2307/2445889
Krüger M, Stockinger H, Krüger C, Schüßler A (2009) DNA-based species level detection of Glomeromycota: one PCR primer set for all arbuscular mycorrhizal fungi. New Phytol 183:212–223. https://doi.org/10.1111/j.1469-8137.2009.02835.x
Lagrange A, Ducousso M, Jourand P, Majorel C, Amir H (2011) New insights into the mycorrhizal status of Cyperaceae from ultramafic soils in New Caledonia. Can J Microbiol 57:21–28. https://doi.org/10.1139/W10-096
Lagrange A, Amir H, L’Huillier L (2013) Mycorrhizal status of Cyperaceae from New Caledonian ultramafic soils: effects of phosphorus availability on arbuscular mycorrhizal colonisation of Costularia comosa in field conditions. Mycorrhiza 23:655–661
Luçon S, Marion F, Niel JF, Pelletier B (1997) Rehabilitation des sites miniers sur roches ultramafiques en Nouvelle-Calédonie. In: Jaffré T, Reeves RD, Becquer T (eds) Écologie des milieux sur roches ultramafiques et sur sols métallifères. ORSTOM Ed. New Caledonia, Noumea, pp 297–303
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748. https://doi.org/10.1126/science.1143082
Mardhiah U, Caruso T, Gurnell A, Rillig MC (2016) Arbuscular mycorrhizal fungal hyphae reduce soil erosion by surface water flow in a greenhouse experiment. Appl Soil Ecol 99:137–140. https://doi.org/10.1016/j.apsoil.2015.11.027
Opel KL, Chung D, McCord BR (2010) A study of PCR inhibition mechanisms using real time PCR. J Forensic Sci 55:25–33. https://doi.org/10.1111/j.1556-4029.2009.01245.x
Orłowska E, Ryszka P, Jurkiewicz A, Turnau K (2005) Effectiveness of arbuscular mycorrhizal fungal (AMF) strains in colonisation of plants involved in phytostabilisation of zinc wastes. Geoderma 129:92–98. https://doi.org/10.1016/j.geoderma.2004.12.036
Orłowska E, Przybyłowicz W, Orlowski D, Turnau K, Mesjasz-Przybyłowicz J (2011) The effect of mycorrhiza on the growth and elemental composition of Ni-hyperaccumulating plant Berkheya coddii Roessler. Environ Pollut 159:3730–3738. https://doi.org/10.1016/j.envpol.2011.07.008
Perrier N, Amir H, Colin F (2006) Occurrence of mycorrhizal symbioses in the metal-rich lateritic soils of the Koniambo Massif, New Caledonia. Mycorrhiza 16:449–458. https://doi.org/10.1007/s00572-006-0057-6
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–IN18. https://doi.org/10.1016/S0007-1536(70)80110-3
Porras-Soriano A, Soriano-Martín ML, Porras-Piedra A, Azcón R (2009) Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J Plant Physiol 166:1350–1359. https://doi.org/10.1016/j.jplph.2009.02.010
Powell JR, Parrent JL, Hart MM, et al (2009) Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. Proc R Soc Lond B Biol Sci 276:4237–4245. https://doi.org/10.1098/rspb.2009.1015
Pozo MJ, Azcón-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:393–398. https://doi.org/10.1016/j.pbi.2007.05.004
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna https://www.R-project.org/
Ruiz-Lozano JM, Aroca R (2010) Modulation of aquaporin genes by the arbuscular mycorrhizal symbiosis in relation to osmotic stress tolerance. In: Symbioses and stress. Springer, Dordrecht, pp 357–374
Shrivastava G, Ownley BH, Augé RM, et al (2015) Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 65:65–74. doi: https://doi.org/10.1007/s13199-015-0319-1
Singh DP, Kumar N, Bhargava SK, Barman SC (2010) Accumulation and translocation of heavy metals in soil and plants from fly ash contaminated area. J Environ Biol 31:421–430
Smith SE, Read DJ (2008) Mineral nutrition, toxic element accumulation and water relations of arbuscular mycorrhizal plants. In: Mycorrhizal symbiosis, 3rd edn. Academic Press, London, pp 145–187
St-Arnaud M, Vujanovic V (2007) Effect of the arbuscular mycorrhizal symbiosis on plant diseases and pests. In: Hamel C, Plenchette C (eds) Mycorrhizae in crop production. Haworth Food and Agricultural Products Press, New York, pp 67–122
Sutlovic D, Gamulin S, Definis-Gojanovic M, Gugic D, Andjelinovic S (2008) Interaction of humic acids with human DNA: proposed mechanisms and kinetics. ELECTROPHORESIS 29:1467–1472
Thonar C, Frossard E, Šmilauer P, Jansa J (2014) Competition and facilitation in synthetic communities of arbuscular mycorrhizal fungi. Mol Ecol 23:733–746. https://doi.org/10.1111/mec.12625
Trouvelot A, Kough J, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhisation VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson GS (ed) Mycorrhizae: physiology and genetics. INRA, Paris, pp 217–221
Vandenkoornhuyse P, Mahé S, Ineson P, et al (2007) Active root-inhabiting microbes identified by rapid incorporation of plant-derived carbon into RNA. PNAS 104:16970–16975. https://doi.org/10.1073/pnas.0705902104
Wang S, Shi X, Sun H, Chen Y, Pan H, Yang W, Rafiq T (2014) Variations in metal tolerance and accumulation in three hydroponically cultivated varieties of Salix integra treated with lead. PLoS One 9(9):e108568. https://doi.org/10.1371/journal.pone.0108568
Wu F, Zhang H, Fang F, Liu H, Tang M (2017) Arbuscular mycorrhizal fungi alter nitrogen allocation in the leaves of Populus × canadensis ‘Neva’. Plant Soil 421:477–491. https://doi.org/10.1007/s11104-017-3461-0
Wulff A, L’Huillier L, Véa C, Jaffré T (2010) Espèces indigènes utilisables en revégétalisation. In: L’Huillier L, Jaffré T, Wulff A (eds) Mines et Environnement en Nouvelle-Calédonie : les milieux sur substrats ultramafiques et leur restauration. IAC Ed. New Caledonia, Noumea, pp 231–344
Yang H, Zhang Q, Koide RT, Hoeksema JD, Tang J, Bian X, Hu S, Chen X, Cahill J (2016) Taxonomic resolution is a determinant of biodiversity effects in arbuscular mycorrhizal fungal communities. J Ecol 105:219–228. https://doi.org/10.1111/1365-2745.12655
Funding
This study received financial support from CNRT “Nickel et son Environnement.”
Author information
Authors and Affiliations
Contributions
T.C. designed the research, provided AMF cultures, conducted the molecular and greenhouse experiment, analyzed data and wrote the manuscript; C.M. conducted the molecular analysis; D.R. contributed to the research supervision and wrote the manuscript; S.G. contributed to the statistical analysis; V.M. contributed to the greenhouse experiment; G.D. conducted statistical analysis; Y.C. contributed to the research supervision; H.A. supervised and designed the research, provided the funding, and wrote the manuscript.
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1141 kb)
Rights and permissions
About this article
Cite this article
Crossay, T., Majorel, C., Redecker, D. et al. Is a mixture of arbuscular mycorrhizal fungi better for plant growth than single-species inoculants?. Mycorrhiza 29, 325–339 (2019). https://doi.org/10.1007/s00572-019-00898-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-019-00898-y