Abstract
Many clonal plants live in symbiosis with ubiquitous arbuscular mycorrhizal (AM) fungi, however, little is known about their interaction with respect to clonal reproduction and resource acquisition. The effects of arbuscular mycorrhiza on the growth and intraclonal integration between ramets of two stoloniferous species were studied experimentally in a nutritionally homogenous soil environment. Two species coexisting at the same field site, Potentilla reptans and Fragaria moschata, were selected as model plants for the study. Pairs of their ramets were grown in neighbouring pots with each ramet rooted separately. Four inoculation treatments were established: (1) both mother and daughter ramets remained non-inoculated, (2) both ramets were inoculated with a mixture of three native AM fungi from the site of plant origin, (3) only mother or (4) daughter ramet was inoculated. The stolons connecting the ramets were either left intact or were disrupted. Despite the consistent increase in phosphorus concentrations in inoculated plants, a negative growth response of both plant species to inoculation with AM fungi was observed and inoculated ramets produced fewer stolons and fewer offspring ramets and had lower total shoot dry weights as compared to non-inoculated ones. A difference in the extent of the negative mycorrhizal growth response was recorded between mother and daughter ramets of P. reptans, with daughter ramets being more susceptible. Due to AM effect on ramet performance, and thereby on the source-sink relationship, inoculation also significantly influenced biomass allocation within clonal fragments. Physiological integration between mother and daughter ramets was observed when their root systems were heterogeneous in terms of AM colonization. These results hence indicate the potential of mycorrhizal fungi to impact clonal growth traits of stoloniferous plant species, with possible consequences for their population dynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Clonal plant species account for a considerable part of the flora in temperate communities such as grasslands (Klimeš et al. 1997). Their distinctive feature is a formation of a network of genetically identical offsprings termed ramets. Physical connections between separately rooted ramets enable intraclonal physiological integration, which means the translocation of water, photosynthates, mineral nutrients or signalling molecules between different units of the clonal network (Pitelka and Ashmun 1985; Marshall 1990; Stuefer et al. 2004). The phenomenon of resource sharing is widely agreed to increase the survival and growth of clonal plants in non-homogeneous environment. This has been repeatedly demonstrated for both stoloniferous or rhizomatous plants subjected to non-uniform conditions, either in terms of light, nutrient or water availability, salinity, sand burial, herbivore attack or pathogen distribution (e.g. Salzman and Parker 1985; Stuefer et al. 1994; de Kroon et al. 1996; D’Hertefeldt and van der Putten 1998; Alpert et al. 2003; Yu et al. 2004; Gómez and Stuefer 2006). In general, ramets experiencing advantageous conditions support the growth of ramets facing a more adverse environment, however, the extent of resource sharing is species- and genotype-dependent (Stuefer and Huber 1998; Alpert 1999; Alpert et al. 2003).
Soil is a highly variable environment, the heterogeneity of which may concern not only abiotic factors such as nutrient or water availability, but also biotic agents, including soil microorganisms. Among these, arbuscular mycorrhizal (AM) fungi play a special role, inhabiting roots of more than 80% of plant species and forming the most common type of mycorrhiza (and symbiosis in general) (Trappe 1987; Smith and Read 1997). AM fungi are fully dependent on plants for carbohydrates while their wide-spreading extraradical mycelium, in turn, provides the host nutrients from the soil, particularly relatively immobile ones such as phosphorus or zinc (Marschner 1995). Colonization of plant roots with AM fungi may then promote plant growth by enhancing nutrient uptake, particularly when the supply of phosphorus is a limiting factor. Under certain conditions (e.g. low light or high nutrient supply), the cost for maintaining AM symbiosis can, however, outweigh the benefits, resulting in a detrimental effect on host plant growth. In general, plant growth response to mycorrhizal colonization thus ranges widely from positive to negative and largely depends on environmental factors and a combination of particular plant and fungus (Johnson et al. 1997).
Although Onipchenko and Zobel (2000) suggested a trade-off between plant vegetative mobility and mycorrhizal symbiosis based on their results from alpine grasslands, many stoloniferous and rhizomatous plant species are highly colonized by AM fungi in natural populations. However, little is known about the role of AM fungi in clonal plant establishment, vegetative reproduction and resource acquisition. A potential of AM fungi to considerably affect clonal growth traits was demonstrated in a pioneering study by Streitwolf-Engel et al. (1997). They assessed the response of two stoloniferous species with stolons extending a few centimetres from the parent ramet, Prunella vulgaris and P. grandiflora (Lamiaceae), to AM inoculation and observed that stolon length and branching were significantly influenced by mycorrhiza in both plant species, with considerable variability among AM isolates. In their next study, Streitwolf-Engel et al. (2001) showed that effects of AM fungi on plant growth and clonal reproduction were comparable with those caused by genotypic variation of the host plant. These findings point to the potential of AM fungi to modify the extent and pattern of clonal reproduction, which might subsequently influence spatial distribution and competitive fitness of the species.
The distribution of AM propagules in the soil is not fully uniform, neither on temporal nor spatial scales (Brundrett and Abbott 1994, 1995; Carvalho et al. 2003; Wolfe et al. 2007). Thus, different parts of the clone, especially in plant species with long stolons and widely spaced ramets, may be located in microhabitats differing greatly in their potential to initiate mycorrhizal colonization of the roots. As a result, differences in AM-mediated growth effects can be expected for individual ramets and, consequently, the performance of the whole interconnected system may be modified due to physiological integration. Moreover, the development of mycorrhizal symbiosis in the roots of clonal plants may depend on the ramet age as suggested from results by Watson et al. (2001). They found that offspring ramets of a rhizomatous species Podophyllum peltatum (Berberidaceae) were not colonized in the first growth stage. As long as offspring ramets do not develop their own mycorrhiza at early developmental stages, it can be speculated that they exhibit dependence on the parent ramet, especially in species with a high mycorrhizal dependency.
This study aimed to provide answers to the following questions: (1) Do AM fungi affect clonal growth traits of stoloniferous plant species with far-reaching stolons? (2) Is there physiological integration between mother and daughter ramets when their root systems are heterogeneous in terms of colonization with AM fungi? To address these questions, a cultivation experiment involving a natural soil-plant-fungi combination was conducted where ramet pairs of two selected species were subjected to AM inoculation and stolon disruption in a nutritionally homogeneous soil environment.
Material and methods
Plants
The experiment was conducted with two stoloniferous perennial herbs, Potentilla reptans L. and Fragaria moschata (Duchesne) Weston (Rosaceae). Both species are naturally highly colonized by AM fungi and are similar with respect to their developmental pattern: a parent rosette produces long, horizontally growing stolons consisting of ramets separated by stolon internodes. In contact with the soil, ramets may root and form further offspring rosettes. In the absence of physical disturbance, ramets remain interconnected throughout one growing season (Stuefer et al. 2002; Klimešová and Klimeš 2006).
Plants of both species were originally collected from their natural populations inhabiting a mown mesotrophic meadow in the vicinity of a broad-leaved forest [Litožnice, Central Bohemia, Czech Republic (50°04′10″ N, 14°36′30″ E; altitude 230 m)]. Ten rosettes per species were taken from an approximately 5 × 5 m area and analysed for genetic variation using isozyme analysis based on eight enzymatic systems (EST, LAP, PGM, 6-PGDH, ADH, NADH-DH, G-6-PDH, SHDH; according to Soltis and Soltis 1989). Genotype uniformity of the sampled plant material was confirmed (Plačková and Sudová, unpublished). To obtain stock plants with roots free of AM fungi, the original plants from the field were planted into pots and new ramets that did not come into contact with the substrate were separated as soon as stolons started to grow out of the pots. Subsequently, they were rooted in new pots filled with a γ-sterilized (25 kGy) mixture of river sand and soil from the field site (1:1, v/v) with no living AM propagules and clonally multiplied in the greenhouse to produce sufficient numbers of replicates. For the experiment, similarly sized ramet pairs were taken from the stock plants, always from the same position on primary stolons. At the establishment of the experiment, mother and daughter ramets bore two leaves and one leaf, respectively.
AM fungi
High rates of mycorrhizal colonization were commonly observed in the roots of P. reptans and F. moschata from the field site, i.e., 70 ± 3% and 73 ± 1%, respectively (means ± SE, n = 30). From the rhizosphere and roots of both plant species, three native AM isolates were obtained using a series of trap and multispore cultures with P. reptans and F. moschata as host plants. The isolates were identified based on spore morphology as Glomus intraradices Schenck and Smith, G. mosseae (Nicolson and Gerdemann) Gerd. and Trappe and G. microaggregatum Koske, Gemma and Olexia. A mixture of these isolates was then used to inoculate plants in the experiment (see below).
Experimental design
Ramet pairs of both species were grown in neighbouring plastic pots (12 × 12 × 10 cm), each rooted separately. The older ramet is referred to as a ‘mother ramet’, and the younger one as a ‘daughter ramet’ throughout the text. Eight different treatments resulting from a combination of three experimental factors (AM inoculation of mother ramet, inoculation of daughter ramet, and stolon disruption) were established, each consisting of six replicates (Fig. 1). Four inoculation treatments were established: (1) both ramets remained non-inoculated (treatment referred to as –AM/–AM), (2) both ramets were inoculated with AM fungi (+AM/ +AM), (3) mother ramet was inoculated while the daughter one was non-inoculated (+AM/–AM), (4) mother ramet was non-inoculated while the daughter one was inoculated (–AM/ +AM). Interconnecting stolons either remained intact or were disrupted by a pair of scissors and so mother and daughter ramets were either connected or separated. Stolon disruption was done at the time when both ramets were rooted, i.e., 2 weeks after experiment establishment.
Experimental set-up. Ramet pairs of Potentilla reptans or Fragaria moschata were grown in separated pots, with interconnecting stolons intact (−) or disrupted (+). Four inoculation treatments were established: a both mother and daughter ramets remained non-inoculated (−AM/−AM), b only daughter ramets were inoculated with AM fungi (−AM/+AM), c only mother ramets were inoculated with AM fungi (+AM/−AM) or d both ramets were inoculated with AM fungi (+AM/+AM). Plastic diaphragm with an aperture for interconnecting stolons served as a protection against cross-contamination between neighbouring pots
The experiment was conducted from April to July 2005 in a greenhouse under natural light conditions with supplementary 12-h illumination provided by metal halide lamps (400W). The plants were grown in a γ-sterilized soil from the field site, which was 1:1 (v/v) diluted with sterile river sand to reduce soil compaction in the pots. The resulting substrate had the following characteristics: pH (KCl) 5.6, organic carbon C 2.5%, N 0.2%, P (Olsen) 15.6 mg kg−1.
In inoculated treatments, each plant was inoculated with 10 ml of a suspension of a mixture of three AM isolates obtained from the site of plant origin (each at one third the volume). The inoculum was prepared by wet sieving (Gerdemann and Nicolson 1963) from mature pure cultures with abundant sporulation and contained colonized root segments, extraradical mycelium (ERM) and spores. The non-inoculated treatments received the same amount of the autoclaved inoculum (121°C, 2×25 min). Non-inoculated plants were also treated with a filtrate from the non-sterile mycorrhizal inoculum in an attempt to balance composition of the microbial community between AM inoculated and non-inoculated treatments. The filtrate was obtained by passing a 1:10 (w/v) suspension of the soil inocula through a filter paper (Whatman No. 1, UK) to remove AM propagules. Moreover, all pots were treated with a filtrate from a non-sterile soil from the field site to mimic natural composition of microbial community (preparation of the filtrate as above). From the third week, the plants were irrigated (50 ml per pot weekly) with a nutrient solution P2N3 (Gryndler at al. 1992) in an attempt to avoid nutrient limitation due to soil mixing with sand. To prevent cross-contamination in asymmetrically inoculated ramet pairs (e.g. during irrigation), a plastic diaphragm with an aperture for interconnecting stolons was used to separate the ramets (this precaution was done in all inoculation treatments, incl. non-inoculated ones, to avoid differences in plant shading). In the course of the experiment, both mother and daughter ramets produced new (secondary) stolons with secondary ramets, which are referred to as ‘offspring ramets’ throughout the text. These ramets were not allowed to root because the secondary stolons were left trailing from the pots. Numbers of secondary stolons and offspring ramets were recorded in a time span of one month from the beginning of the experiment.
Harvest
After 16 weeks of growth, plant shoots were cut off and the roots were thoroughly washed to remove substrate particles. Sub-samples of the roots were taken for evaluation of mycorrhizal colonization. For the aboveground biomass, numbers of stolon apices and offspring ramets, and stolon length were recorded for each mother and daughter ramet. Leaf area was assessed using an area meter (LI-3100, LI-COR, USA) and dry weights of the shoots and roots were recorded after drying at 75°C. Then, shoot biomass was ground and digested in HNO3 and H2O2 to analyse for phosphorus concentrations. Phosphorus concentrations were assessed spectrophotometrically (UV4-100; Unicam, UK) at wavelength of 630 nm (Olsen et al. 1954). Mycorrhizal colonization was evaluated microscopically following staining with 0.05% trypan blue in lactoglycerol (Koske and Gemma 1989) using a grid-line intersect method at ×100 magnification. ERM length was estimated using a modified membrane filtration technique (Jakobsen et al. 1992). Only the hyphae bearing the characteristics of AM mycelium were taken into account (i.e., with dichotomical branching and irregular walls, mostly non-septate). The total ERM length was assessed under a compound microscope, using an ocular grid at ×100 magnification, and expressed in meters of hyphae in 1g of air-dried substrate. The background length of hyphae found in non-inoculated treatments was subtracted from the values in inoculated treatments.
Statistical analysis
All statistical analyses were performed using S-Plus 2000 (MathSoft Inc., USA). Data on mycorrhizal colonization and ERM length of inoculated plants were evaluated using two-way analysis of variance (ANOVA), with stolon disruption and ramet type (mother vs. daughter) as independent variables (percentage of root colonization was arcsine transformed prior to the analysis). Plant growth parameters of the whole ramet pairs (total stolon length, total number of offspring ramets, total leaf area, total shoot and root dry weight) were analysed by a three-way ANOVA, using inoculation of mother ramet, inoculation of daughter ramet and stolon disruption as independent variables. For statistical analysis of the growth parameters of either mother or daughter ramet, a three-way analysis of covariance (ANCOVA) was used to take into account that mother and daughter ramets were not independent of each other. Inoculation of mother ramet, inoculation of daughter ramet, stolon disruption and their interactions then served as independent variables and biomass of neighbouring (mother or daughter) ramet as a covariate. Data on the numbers of stolon apices were analysed using a generalized linear model (GLM) assuming Poisson distribution of the dependent variable. Prior to ANCOVA/ANOVA, data were checked for normality and homogeneity of variance and logarithmically transformed when necessary. Relative biomass allocation within ramet pairs was evaluated by ANCOVA, using inoculation of mother ramet, inoculation of daughter ramet and stolon disruption as independent variables, total biomass of ramet pairs as a covariate and proportional contribution of daughter ramets to total biomass yield as the dependent variable (after arcsine transformation). Comparisons between means were carried out using the Least Significant Difference test at a significance level of P < 0.05.
Results
Mycorrhiza development
No mycorrhizal structures were observed in the roots of non-inoculated plants. In both plant species, AM inoculation resulted in a consistently very high percentage of root colonization with abundant arbuscules and vesicles. On average, 95 ± 1% (mean ± SE) and 91 ± 1% of root length of P. reptans and F. moschata, respectively, were inhabited by AM fungi. In the substrate, high densities of extraradical mycelium were recorded: on average 7.3 ± 0.3 and 7.5 ± 0.6 m g−1 dry soil for P. reptans and F. moschata, respectively. Mother and daughter ramets did not significantly differ either in the level of root colonization (P. reptans: P = 0.589, F. moschata: P = 0.144) or length of the associated ERM (P. reptans: P = 0.440, F. moschata P = 0.840). Similarly, stolon disruption had no significant effect on mycorrhizal colonization (P. reptans: P = 0.183; F. moschata: P = 0.860) and ERM length (P. reptans: P = 0.906; F. moschata: P = 0.824). Interaction of stolon disruption and ramet type did not have significant effect on mycorrhizal parameters of either species.
Effect of AM inoculation on plant growth and P uptake
AM inoculation had a significant adverse effect on the growth of both plant species, which was evident from the beginning of the experiment. Non-inoculated plants produced secondary stolons markedly earlier than their inoculated counterparts. After two months of cultivation, on average by 37% and 50% fewer offspring ramets were recorded for the inoculated ramet pairs of P. reptans and F. moschata, respectively (data not shown). At harvest, the extent of the negative response differed between both species and also between mother and daughter ramets (Tables 1 and 2). In P. reptans, AM inoculation of mother ramets had only little impact on their growth parameters (significant only for root dry weight, P < 0.001). A significant effect of mother ramet inoculation was however observed for total shoot and root dry weight of ramet pairs (P < 0.05 and P < 0.001, respectively). In contrast to mother ramets, daughter ramets of P. reptans responded to AM inoculation by a pronounced growth depression and produced significantly fewer stolon apices (P < 0.001) and offspring ramets (P < 0.001), had shorter stolon length (P < 0.001), lower leaf area (P < 0.05) and both shoot (P < 0.001) and root dry weights (P < 0.01) when inoculated (Table 1, Fig. 2). For comparison, dry weight of inoculated daughter ramets was on average 30% lower than in the non-inoculated treatment. Inoculation of daughter ramets also resulted in a significantly shorter stolon length of mother ramets (P < 0.05) and influenced the ratio by which daughter ramets contributed to the total biomass (P < 0.001). The proportion of inoculated daughter ramets on the total yield of ramet pairs was almost the same compared with mother ramets. On the contrary, higher biomass of daughter ramets as compared to the mother ones (at the ratio of about 3:2) was observed in the treatment where daughter ramets remained non-inoculated. The effect of AM inoculation of daughter ramets was manifested not only on their growth parameters but also at the level of the whole ramet pairs. With inoculated daughter ramets, the ramet pairs showed significantly fewer stolon apices (P < 0.001) and offspring ramets (P < 0.001), shorter total stolon length (P < 0.001), lower leaf area (P < 0.05) and shoot dry weight (P < 0.001).
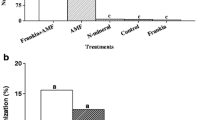
Shoot dry weights of ramet pairs of Potentilla reptans as affected by inoculation with arbuscular mycorrhizal (AM) fungi [both ramets inoculated (+AM) or non-inoculated (−AM) or only mother or daughter ramet inoculated] and stolon disruption (mother and daughter ramets connected or separated). Data for mother and daughter ramets are presented in the upper and lower parts of the graph, respectively. Data are means (±SE) of six replicates. Columns marked with the same letters are not significantly different according to the Least Significant Difference test at P<0.05
In F. moschata, mother and daughter ramets did not differ with respect to their growth response to AM inoculation. Both mother and daughter ramets had significantly lower number of offspring ramets (P < 0.05), lower leaf area (P < 0.001) and shoot (P < 0.001) and root dry weights (P < 0.001) when inoculated (Table 2, Fig. 3). In daughter ramets, inoculation also significantly reduced the number of stolon apices and the mean stolon length (both at P < 0.05). On average, inoculated mother and daughter ramets showed 35% and 32%, respectively, lower total dry weight than their counterparts in the non-inoculated treatment. Inoculation of either mother or daughter ramet of F. moschata significantly reduced their contribution to the total biomass (P < 0.001). At the level of the ramet pairs, inoculation of mother or daughter ramet significantly decreased total shoot (P < 0.01) and root dry weight (P < 0.001).
Shoot dry weights of ramet pairs of Fragaria moschata as affected by inoculation with arbuscular mycorrhizal (AM) fungi [both ramets inoculated (+AM) or non-inoculated (−AM) or only mother or daughter ramet inoculated] and stolon disruption (mother and daughter ramets connected or separated). Data for mother and daughter ramets are presented in the upper and lower parts of the graph, respectively. Data are means (±SE) of six replicates. Columns marked with the same letters are not significantly different according to the Least Significant Difference test at P<0.05
In both plant species, both mother and daughter ramets showed significantly higher shoot P concentrations when inoculated (P < 0.001; Tables 1 and 2), regardless of the negative effect of AM fungi on plant growth. Inoculation of mother ramets of F. moschata also led to a significant increase in shoot P concentrations of daughter ramets (P < 0.05; Table 2). In P. reptans, AM inoculation also positively influenced total shoot P content, both in mother and daughter ramets (P < 0.001 and P < 0.05, respectively).
Effect of stolon disruption on plant growth and P uptake
The effect of stolon disruption on plant growth was generally much weaker than that of AM inoculation (Tables 1 and 2). In P. reptans, only marginally significant negative effects of stolon disruption on total stolon length (P = 0.070), total shoot (P = 0.064) and root dry weight (P = 0.083) were recorded. Mother ramets of F. moschata profited from disconnecting from daughter ramets and produced significantly more secondary stolons than when connected (P < 0.05; Table 2). Similar trend, i.e. better performance of mother ramets after stolon disruption, was obvious also for the number of offspring ramets (P = 0.051) and stolon length (P = 0.078). Stolon disruption did not influence phosphorus concentrations in shoot biomass of either species.
Effect of AM inoculation × stolon disruption interaction on plant growth and P uptake
In P. reptans, the interaction between AM inoculation of mother ramet and stolon disruption significantly influenced the length of secondary stolons (P < 0.01) and number of offspring ramets (P < 0.01) produced by daughter ramets, shoot dry weight of daughter ramets (P < 0.01) as well as total stolon length and total number of offspring ramets (both at P < 0.05). When mother ramets remained non-inoculated, stolon disruption negatively influenced the performance of the whole ramet pair as well as of daughter ramets (Table 1, Fig. 2). With inoculated mother ramets, daughter ramets significantly profited from stolon disruption, as indicated by their significantly higher proportion on total biomass (P < 0.01). Similarly, the effect of the interaction of daughter ramet inoculation with stolon disruption was significant for stolon length (P < 0.01), shoot (P < 0.05) and root dry weight (P < 0.05) and shoot P concentration (P < 0.05) of mother ramets as well as for the performance of the whole ramet pairs (total stolon length at P < 0.01, total number of stolon apices, total leaf area and total shoot dry weight at P < 0.05). Mother ramets significantly benefited from integration with non-inoculated daughter ramets. In contrast, no significant effect of stolon disruption on the growth of mother ramets was observed when daughter ramets were inoculated.
In F. moschata, none of plant growth parameter was significantly affected by the interaction of stolon disruption with AM inoculation of mother ramet. Concerning the interaction between stolon disruption and daughter ramet inoculation, it significantly influenced root dry weight of daughter ramets (P < 0.05). When daughter ramets of F. moschata remained non-inoculated, stolon disruption resulted in an increase in their root dry weight, while no significant effect of stolon disruption was observed in the inoculated treatment. With inoculated daughter ramets, there was a trend to higher shoot dry weight of mother ramets in the treatment with stolon disruption (P = 0.097).
Discussion
The results of our study clearly demonstrate the potential of mycorrhizal fungi to influence clonal growth traits of stoloniferous species. The effect of AM inoculation on the performance of ramet pairs of both plant species was considerably stronger compared to that of stolon disruption. However, contrary to our expectation, inoculation of P. reptans and F. moschata with native AM fungi resulted in a remarkable negative growth response. Although AM fungi were effective in supplying phosphorus to plants, elevated shoot P concentrations were not associated with growth stimulation, indicating that nitrogen rather than phosphorus was likely a limiting factor of plant growth. In literature, no information on the effects of mycorrhiza on the growth of either species tested is, to our knowledge, yet available. There are only reports on AM interaction with another wild Fragaria species, showing either positive effects of AM fungi on F. vesca growth (Mark and Cassells 1996) or absence of any growth response to AM inoculation (Zobel and Moora 1995).
Costs of forming and maintaining mycorrhiza (usually accounting for 4% to 20% of a plant’s total carbon; Johnson et al. 1997) apparently exceeded plant benefits from being mycorrhizal in the present experiment. A negative effect of AM inoculation on plant performance is not a scarce phenomenon and has been reported repeatedly (e.g. Koide 1985; Graham and Abbott 2000; Reynolds et al. 2006). It is mostly explained by the following reasons (Smith and Smith 1996; Johnson et al. 1997; Graham and Abbott 2000): (1) light deficiency resulting in an insufficient photosynthetic rate, (2) too high phosphorus availability making AM fungi superfluous in terms of its acquisition, and (3) incompatibility of fungi and their hosts resulting from a non-natural combination of plant and fungal species or isolates. In our case, the first explanation does not seem probable, taking into account that the plants, frequently occurring in partially shaded habitats in nature, were grown in the greenhouse with supplementary lighting under benign light conditions of the spring–summer season. As for the available phosphorus, the substrate used had by about a third lower P concentration than the soil at the field site of plant and fungi origin where high levels of mycorrhizal colonization were recorded for both plant species. Growth depression in response to inoculation with the same AM fungal isolates was, however, observed also in another experiment where plants were grown under the same cultivation conditions directly in the original undiluted soil from the field (Sudová, unpublished). All AM isolates used in the present study were obtained from the roots and rhizosphere of P. reptans and F. moschata, however, it must be admitted that these isolates can represent only part of the whole native fungal community as the isolation procedure might discriminate in favour of r-strategists as suggested by Sýkorová et al. (2007). Our observation of the negative growth effect of native AM fungi corresponds with other studies showing that AM-induced growth depressions are probably quite frequent also in natural combinations of plants and fungi (e.g. Wilson and Hartnett 1998; Klironomos 2003). The latter author even observed that the range of plant responses to AM inoculation (on the scale from parasitism to mutualism) was broader for associations between local plants and fungi than between foreign plant and fungal genotypes. Using Klironomos’s data, Landis and Fraser (2008) recently proposed an alternative hypothesis to explain the variation in plant response to AM inoculation. According to their model of phosphorus and carbon transfers, P and C are not exchanged in a constant ratio between the symbionts, but independently of each other based solely on symbionts’ internal needs, thus resulting in a range of mutualistic or parasitic interactions.
Mycorrhizal inoculation not only influenced the growth of individual ramets in the present study but also modified their relative proportion on the total biomass produced by ramet pairs. Whereas AM inoculation of either mother or daughter ramet of F. moschata reduced their proportional contribution to the total yield, a different pattern was observed for P. reptans. Due to different mycorrhizal growth response of mother and daughter ramets, AM inoculation functioned as a buffer balancing the growth of mother and daughter ramets of P. reptans, proportionally increasing the profit of mother ramets compared to the non-inoculated treatment. The reason for the difference in mycorrhizal growth response between differently aged ramets remains unknown and it can only be hypothesised that the younger ramets were more susceptible to losses of carbohydrates required for symbiosis establishment.
Physiological integration between mother and daughter ramets was obvious when their root systems were heterogeneous in terms of arbuscular mycorrhizal inoculation. In the treatment with intact stolons and asymmetric inoculation of mother and daughter ramets, the negative effect of mycorrhiza on plant growth was manifested not only in the ramets directly subjected to AM inoculation but also in their non-inoculated neighbours. Resource-rich (non-inoculated) ramets subsidised the growth of resource-poor (inoculated) ramets, similarly to other studies where clonal fragments were subjected to differently advantageous habitats (e.g. Hartnett and Bazzaz 1983; Salzman and Parker 1985; Peterson and Chesson 2002).
To conclude, though the effect of mycorrhiza on clonal growth was negative in the present experiment, the potential of mycorrhizal fungi to change the fitness of ramet units is evident. Therefore, modifications in source-sink relationships and subsequently also in resource investment within a clonal network are to be expected due to the AM growth effect, either negative or positive. Several facts should, however, be mentioned with respect to the results of the present study. First, it should be emphasised that only two stoloniferous plant species and a mixture of three AM isolates were tested. It is likely that AM effects on clonal growth traits differ for various combinations of plant and fungi genotypes, as inferred from the study by Streitwolf-Engel et al. (1997). Secondly, it must also be taken into account that benefits from being mycorrhizal might be long-term and connected with changes other than biomass production, e.g., plant protection against pathogens (Newsham et al. 1994). Finally, studies on AM effect on clonal growth traits should be extended to a nutritionally heterogeneous soil environment that is typically inhabited by clonal plants.
Abbreviations
- AM:
-
arbuscular mycorrhiza
- ERM:
-
extraradical mycelium
References
Alpert P (1999) Clonal integration in Fragaria chiloensis differs between populations: ramets from grassland are selfish. Oecologia 120:69–76
Alpert P, Holzapfel C, Slominski C (2003) Differences in performance between genotypes of Fragaria chiloensis with different degrees of resource sharing. J Ecol 91:27–35
Brundrett MC, Abbott LK (1994) Mycorrhizal fungus propagules in the jarrah forest. 1. Seasonal study of inoculum levels. New Phytol 127:539–546
Brundrett MC, Abbott LK (1995) Mycorrhizal fungus propagules in the jarrah forest.2. Spatial variability in inoculum levels. New Phytol 131:461–469
Carvalho LM, Correia PM, Ryel RJ, Martins-Loução MA (2003) Spatial variability of arbuscular mycorrhizal fungal spores in two natural plant communities. Plant Soil 251:227–236
de Kroon H, Fransen B, van Rheenen JWA, van Dijk A, Kreulen R (1996) High levels of inter-ramet water translocation in two rhizomatous Carex species, as quantified by deuterium labelling. Oecologia 106:73–84
D’Hertefeldt T, van der Putten WH (1998) Physiological integration of the clonal plant Carex arenaria and its response to soil-borne pathogens. Oikos 81:229–237
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet-sieving and decanting. Trans Br Myc Soc 46:235–244
Gómez S, Stuefer JF (2006) Members only: induced systemic resistance to herbivory in a clonal plant network. Oecologia 147:461–468
Graham JH, Abbott LK (2000) Wheat responses to aggressive and non-aggressive arbuscular mycorrhizal fungi. Plant Soil 220:207–218
Gryndler M, Vejsadová H, Vančura V (1992) The effect of magnesium ions on the vesicular-arbuscular mycorrhizal infection of maize roots. New Phytol 122:455–460
Hartnett DC, Bazzaz FA (1983) Physiological integration among intraclonal ramets in Solidago canadensis. Ecology 64:779–788
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol 120:371–380
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–585
Klimeš L, Klimešová J, Hendriks R, van Groenendael J (1997) Clonal plant architecture: a comparative analysis of form and function. In: de Kroon H, van Groenendael J (eds) The ecology and evolution of clonal plants. Backhuys Publishers, Leiden, the Netherlands, pp 1–29
Klimešová J, Klimeš L (2006) CLO-PLA3. http://clopla.butbn.cas.cz/
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Koide R (1985) The nature of growth depressions in sunflower caused by vesicular–arbuscular mycorrhizal infection. New Phytol 99:449–462
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res 92:486–505
Landis FC, Fraser LH (2008) A new model of carbon and phosphorus transfers in arbuscular mycorrhizas. New Phytol 177:466–479
Mark GL, Cassells AC (1996) Genotype-dependence in the interaction between Glomus fistulosum, Phytophthora fragariae and the wild strawberry (Fragaria vesca). Plant Soil 185:233–239
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London, Great Britain
Marshall C (1990) Source-sink relations of interconnected ramets. In: van Groenendael J, de Kroon H (eds) Clonal growth in plants: regulation and function. SPB Academic Publ., The Hague, pp 23–42
Newsham KK, Fitter AH, Watkinson AR (1994) Root pathogenic and arbuscular mycorrhizal fungi determine fecundity of asymptomatic plants in the field. J Ecol 82:805–814
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Dept Agric Circ 939:1–19
Onipchenko VG, Zobel M (2000) Mycorrhiza, vegetative mobility and responses to disturbance of alpine plants in the Northwestern Caucasus. Folia Geobot 35:1–11
Peterson AG, Chesson P (2002) Short-term fitness benefits of physiological integration in the clonal herb Hydrocotyle peduncularis. Austral Ecology 27:647–657
Pitelka LF, Ashmun JW (1985) Physiology and integration of ramets in clonal plants. In: Jackson JBC, Buss LW, Cook RE (eds) Population biology and evolution of clonal organisms. Yale University Press, New Haven, Connecticut, USA, pp 399–435
Reynolds HL, Vogelsang KM, Hartley AE, Bever JD, Schultz PA (2006) Variable responses of old-field perennials to arbuscular mycorrhizal fungi and phosphorus source. Oecologia 147:348–358
Salzman AG, Parker MA (1985) Neighbors ameliorate local salinity stress for a rhizomatous plant in a heterogeneous environment. Oecologia 65:273–277
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic Press, London, UK
Smith FA, Smith SE (1996) Mutualism and parasitism: Diversity in function and structure in arbuscular“(VA) mycorrhizal symbiosis. Adv Bot Res 22:1–43
Soltis DE, Soltis PS (1989) Isozymes in plant biology. Dioscorides Press, Portland, Oregon, USA
Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1997) Clonal growth traits of two Prunella species are determined by co-occurring arbuscular mycorrhizal fungi from a calcareous grassland. J Ecol 85:181–191
Streitwolf-Engel R, van der Heijden MGA, Wiemken A, Sanders IR (2001) The ecological significance of arbuscular mycorrhizal fungal effects on clonal reproduction in plants. Ecology 82:2846–2859
Stuefer JF, Huber H (1998) Differential effects of light quantity and spectral light quality on growth, morphology and development of two stoloniferous Potentilla species. Oecologia 117:1–8
Stuefer JF, During HJ, de Kroon H (1994) High benefits of clonal integration in two stoloniferous species, in response to heterogeneous light environment. J Ecol 82:511–518
Stuefer JF, van Hulzen JB, During HJ (2002) A genotypic trade-off between the number and size of clonal offspring in the stoloniferous herb Potentilla reptans. J Evol Biol 15:880–884
Stuefer JF, Gómez S, van Molken T (2004) Clonal integration beyond resource sharing: implications for defence signalling and disease transmission in clonal plant networks. Evol Ecol 18:647–667
Sýkorová Z, Ineichen K, Wiemken A, Redecker D (2007) The cultivation bias: different communities of arbuscular mycorrhizal fungi detected in roots from the field, from bait plants transplanted to the field, and from a greenhouse trap experiment. Mycorrhiza 18:1–14
Trappe JM (1987) Phylogenetic and ecological aspects of mycotrophy in the angiosperms from an evolutionary stand point. In: Safir GR (ed) Ecophysiology of VA mycorrhizal plants. CRC Press, Boca Raton, USA, pp 5–25
Watson MA, Scott K, Griffith J, Dieter S, Jones CS, Nanda S (2001) The developmental ecology of mycorrhizal associations in mayapple, Podophyllum peltatum, Berberidaceae. Evol Ecol 15:425–442
Wilson GWT, Hartnett DC (1998) Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am J Bot 85:1732–1738
Wolfe BE, Mummey DL, Rillig MC, Klironomos JN (2007) Small-scale spatial heterogeneity of arbuscular mycorrhizal fungal abundance and community composition in a wetland plant community. Mycorrhiza 17:175–183
Yu F, Dong M, Krüsi B (2004) Clonal integration helps Psammochloa villosa survive sand burial in an inland dune. New Phytol 162:697–704
Zobel M, Moora M (1995) Interspecific competition and arbuscular mycorrhiza—importance for the coexistence of 2 calcareous grassland species. Folia Geobot Phytotax 30:223–230
Acknowledgement
We thank Ivana Plačková and Marie Albrechtová for their help with isozyme and chemical analyses, respectively. We are also grateful to Zuzana Münzbergová for statistical advice, and to Jan Suda, Jana Rydlová, David Püschel, and two anonymous reviewers for their valuable comments on an earlier draft of the manuscript. Financial support by the Czech Science Foundation (project No. 526/05/P063) and the Grant Agency of the Academy of Sciences of the Czech Republic (AV0Z60050516) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: F. Andrew Smith.
Rights and permissions
About this article
Cite this article
Sudová, R., Vosátka, M. Effects of inoculation with native arbuscular mycorrhizal fungi on clonal growth of Potentilla reptans and Fragaria moschata (Rosaceae). Plant Soil 308, 55–67 (2008). https://doi.org/10.1007/s11104-008-9605-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9605-5