Abstract
Background
Chronic kidney disease (CKD) is characterized as a progressive dysfunction of the kidney, and it might have a close relationship with insulin resistance. We utilized the triglyceride–glucose (TyG) index, a reliable marker of insulin resistance, to evaluate the association between the TyG index and CKD in adults from the general population.
Methods
This was a cross-sectional study obtaining data from the 2015–2018 National Health and Nutrition Examination Survey. The estimated glomerular filtration rate (eGFR) and urinary albumin-to-creatinine ratio (UACR) served as kidney function indicators. We defined CKD as the existence of either low eGFR (eGFR < 60 mL/min/1.73 m2 BSA) or albuminuria (UACR > 30 mg/g). Multivariate regressions, correlated subgroup analyses, and interaction terms were performed in this study.
Results
For 4361 recruited participants, the mean TyG index was 8.60 ± 0.68, and the prevalence of CKD was 13.35%. Participants with a higher TyG index showed a higher UACR level (β = 25.10, 95% CI: 6.76, 43.44, P = 0.0074) and higher levels of CKD (OR = 1.34, 95% CI: 1.13, 1.59, P = 0.0006). The positive relationship between the TyG index and CKD became stronger and remained significant in the overweight (OR = 1.61, 95% CI: 1.18, 2.20, P = 0.0027) and obese (OR = 2.48, 95% CI: 1.95, 3.15, P < 0.0001) groups and in people with diabetes (OR = 1.94, 95% CI: 1.46, 2.56, P < 0.0001).
Conclusions
Higher TyG index was strongly associated with a higher UACR level and higher values of albuminuria and CKD, which might be useful in kidney function screening especially among people in disadvantageous socioeconomic conditions with no availability for direct measurement of kidney function. However, more well-designed studies are still needed to validate this relationship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) has become a significant public health and economic burden, extensively affecting approximately 8% to 16% of the global population. The Global Burden of Disease Study 2017 have reported that there were approximately 697.5 million patients with CKD worldwide, which had caused 1.2 million deaths directly [1]. Additionally, the global mortality from CKD also shows a 41.5% increasing tendency [2]. People with CKD are characterized by chronic and progressive kidney dysfunction and were susceptible to progressing to end-stage renal disease (ESRD) [3]. In fact, the most important adverse outcomes of CKD include not only the complications of a decreased glomerular filtration rate (GFR) and progression to kidney failure but also an increased risk of cardiovascular disease (CVD) [4]. Although the exact pathological process of CKD remains unclear, but many studies have confirmed that several autoimmune diseases, genetic disorders, environmental pollution and increasing prevalence of diabetes and hypertension are critical risk factors for CKD [5,6,7,8].
Accumulating studies have indicated that insulin resistance (IR) might have a close relationship with CKD occurrence and progression. IR represents insensitivity to insulin in the human body and is very common in people with type 2 diabetes mellitus (T2DM) [9]. A prospective cohort study reported that there was a positive association between IR condition and CKD severity [10]. R A DeFronzo et al. pointed out that IR also existed in nondiabetic patients with ESRD [11]. Several studies demonstrated that severe IR partially contributed to the increase in cardiovascular mortality in patients with CKD [12, 13]. Kayo Shinohara et al. further concluded that IR could be used as an independent predictor of CVD mortality in patients with ESRD [14].
The triglyceride–glucose (TyG) index is a novel, plausible, and reliable indicator of IR based on fasting blood glucose and fasting triglycerides in clinical practice [15, 16]. The TyG index even performed better in assessing IR conditions than the traditional homeostasis model assessment of insulin resistance (HOMA-IR) and the triglyceride/HDL-cholesterol ratio [17, 18], partially because the distribution of body fat was heterogeneous in the whole human body [19]. Previous studies showed that a higher TyG index was associated with more severe metabolic syndrome, T2DM, liver fibrosis, and an increased risk of CVD [20,21,22,23]. However, the relationship between the TyG index and CKD remains unclear. Therefore, we utilized the publicly available database National Health and Nutrition Examination Survey (NHANES) cycle 2015–2016 and 2017–2018 to evaluate the TyG index levels in U.S. adults and explore its potential associations with kidney function indicators from a large-scale population perspective.
Materials and methods
Study population
We obtained data from NHANES 2015–2016 and 2017–2018 and enrolled a total of 4361 participants. NHANES is an ongoing cross-sectional and national survey managed by researchers in the National Center for Health Statistics (NCHS) at the U.S. Centers for Disease Control and Prevention (CDC). Samples in NHANES represented the health and nutritional status of the general U.S. population well and employed a carefully conducted multistage and stratified probability design. NHANES is publicly available at www.cdc.gov/nchs/nhanes/.
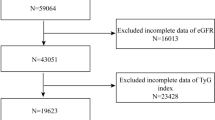
All participants ≥ 20 years old with full information on fasting glucose, serum triglyceride and creatinine, and urinary albumin creatinine ratio (UACR) from NHANES 2015–2018 were included in this study. NHANES 2015–2018 primarily included 19,225 participants, and we excluded participants below 20 years old (N = 7937), participants without serum triglyceride and fasting glucose levels to calculate the TyG index (N = 6473), participants without serum creatinine levels to calculate eGFR and participants who lacked UACR data (N = 454). The flow diagram of sample collection is shown in Fig. 1.
Exposure and outcome definitions
We treated the TyG index as an exposure variable, and it was calculated by Ln (triglycerides (mg/dl) * fasting glucose (mg/dl)/2) [23]. According to the NHANES protocol, both triglycerides and fasting glucose were measured through enzymatic assays in an automatic biochemistry analyser. Serum triglyceride and fasting glucose were measured utilizing the Roche Modular P and Roche Cobas 6000 chemistry analysers and the hexokinase-mediated reaction on Roche/Hitachi Cobas C 501 chemistry analysers, respectively [24].
The outcome variables included eGFR, UACR, low eGFR, and albuminuria to serve as kidney function indicators. The CKD-EPI formula was used to calculate the eGFR (mL/min/1.73 m2 BSA), and "low eGFR" was defined as eGFR < 60 mL/min/1.73 m2 BSA [25, 26]. The measurement of UACR (mg/g) was clearly reported elsewhere, and albuminuria was defined as UACR > 30 mg/g [27, 28]. According to Kidney Disease: Improving Global Outcomes 2012 recommendations, CKD was defined as the existence of either eGFR < 60 mL/min/1.73 m2 BSA, or markers of kidney damage (e.g. albuminuria), or both, of at least 3 months duration, regardless of the underlying cause.[29]. CKD was categorized into 5 stages: CKD stage 1: eGFR ≥ 90 ml/min/1.73 m2 BSA; stage 2: 60 ml/min/1.73 m2 ≤ eGFR < 90 ml/min/1.73 m2 BSA; stage 3: 30 ml/min/1.73 m2 ≤ eGFR < 60 ml/min/1.73 m2 BSA; stage 4: 15 ml/min/1.73 m2 ≤ eGFR < 30 ml/min/1.73 m2 BSA; and stage 5: eGFR < 15 ml/min/1.73 m2 BSA [29].
Covariates
Continuous variables included age, energy and protein intake, systolic and diastolic pressure, glycohemoglobin, and serum levels of uric acid, phosphorus, total calcium and cholesterol. Categorical variables included sex, race, education level, marital status, ratio of family income to poverty (RIP), alcohol intake, body mass index (BMI), serum cotinine (smoking status), diabetes, and hypertension. RIP was classified into two groups (RIP > 1 or RIP ≤ 1) [30]. Alcohol intake was defined by 24 h dietary recall; if a participant reported any alcohol consumption, he or she would be grouped into "Yes", and vice versa. Participants were divided into three groups based on their BMI, including normal (BMI < 25 kg/m2), overweight (25 ≤ BMI ≤ 30 kg/m2) and obese (BMI > 30 kg/m2) [31]. We defined smoking exposure through serum cotinine levels: Smoker, serum cotinine > 10 ng/mg; Second-hand smoker, 0.011 ≤ serum cotinine ≤ 10 ng/mg; Nonsmoker, serum cotinine < 0.011 ng/mg [32]. Participants with a self-reported diabetes diagnosis, HbAlc ≥ 6.5%, a plasma glucose level ≥ 200 mg/dL at 2 h after oral glucose tolerance test (OGTT), a fasting glucose level ≥ 126 mg/dL, or use of oral hypoglycemic agents and/or insulin were all categorized into "diabetes" [30]. Hypertension was defined as a self-reported hypertension diagnosis, diastolic blood pressure ≥ 90 mmHg, and/or systolic blood pressure ≥ 140 mmHg [33].
Statistical analysis
We strictly followed CDC guidelines when conducting all statistical analyses, and a suitable sample weight for each participant was employed for the NHANES complex multistage cluster survey design [34]. Categorical variables are displayed as percentages, and continuous variables are presented as the mean ± standard deviation (S.D.). We assessed the difference among TyG tertiles through ANOVA for continuous variables and weighted chi-square tests for categorical variables. As for UACR (a non-parametric variable), we presented statistics in Median (P25, P75) and used Kruskal–Wallis H test for comparison. Multivariate linear regressions were employed to assess the association between the TyG index and UACR and eGFR, whereas multivariate logistic regressions were performed to evaluate the relationship between the TyG index and albuminuria and low eGFR. Three different models were assigned to explore the potential covariate impact on this association (Model 1: unadjusted; Model 2: adjusted for age, gender, and race; Model 3: adjusted for age, race, sex, energy intake, protein intake, systolic pressure, diastolic pressure, glycohemoglobin, serum uric acid, total calcium, serum phosphorus, total cholesterol, education level, RIP, marital status, alcohol intake, BMI, hypertension, diabetes, and serum cotinine). Interaction terms were also conducted to test the heterogeneity among different subgroups. All our analyses were performed by EmpowerStats software (www.empowerstats.com; X & Y solutions, Inc., Boston MA) and R version 4.0.5 (http://www.R-project.org, The R Foundation).
Results
Baseline characteristics of participants
Weighted baseline characteristics of participants grouped by TyG index tertiles are displayed in Table 1. A total of 4361 participants had an average age of 51 ± 18 years, of whom 53.34% were females and 46.66% were males. The mean TyG index was 8.60 ± 0.68, and the ranges of TyG index tertiles were 5.85 ~ 8.27, 8.27 ~ 8.85, and 8.85 ~ 12.39, respectively. Although the difference in energy intake among TyG index tertiles was not significant, people in tertile 3 tended to consume more protein (80.08 ± 46.64 g VS 75.82 ± 39.30 g, P = 0.0322) than those in TyG index tertile 1. Patients in TyG index tertile 3 had higher systolic and diastolic blood pressure, and the prevalence of hypertension was more serious in higher tertiles (Tertile 1: 10.60%; Tertile 2: 21.65%; Tertile 3: 27.06%, P < 0.0001). A significant increase in mean glycohemoglobin and a higher prevalence rate of diabetes were also observed in TyG tertile 3 (P < 0.0001). The mean ± SD eGFR was 92.80 ± 14.12 ml/min/1.73 m2 BSA and its difference among tertiles was not significant, but a dramatic increase in UACR (Tertile 1, 6.57(4.46, 29.49) mg/g; Tertile 2, 7.23(4.60, 12.98) mg/g; Tertile 3, 8.79(5.38, 19.80) mg/g, P < 0.0001) and a higher likelihood of albuminuria (Tertile 1, 8.33%; Tertile 2, 12.00%; Tertile 3, 17.85%, P < 0.0001) were identified among different TyG index tertiles. The prevalence rate of CKD in the general population was 13.35%, and it increased in line with a higher TyG index. Furthermore, people in the highest TyG index tertile were more likely to have a higher serum uric acid and to be males, overweight and obese, and smokers.
Association between the TyG index and CKD
Associations between the TyG index and UACR and eGFR with a hierarchal covariate-adjusting model are shown in Table 2. Our results revealed a positive association between the TyG index and UACR (Model 1, β = 43.84, 95% CI: 29.84, 57.84, P < 0.0001; Model 2, β = 43.12, 95% CI: 29.10, 57.15, P < 0.0001; Model 3, β = 25.10, 95% CI: 6.76, 43.44, P = 0.0074), while that of eGFR was not significant. Then the TyG index was grouped into tertiles (sensitivity analysis), and we only found significant results in TyG index tertile 3 compared with the lowest TyG index tertile in model 1 (β = 55.06, 95% CI: 31.73, 78.39, P < 0.0001) and model 2 (β = 53.80, 95% CI: 30.44, 77.16, P < 0.0001). However, this association did not remain significant in the fully adjusted model (model 3). This result might indicate that the positive relationship between the TyG index and UACR could, at least, partially explained by extra covariates in model 3 in relative to model 2. No significant difference in eGFR among TyG index tertiles was observed.
We then investigated the association between the TyG index and the likelihood of albuminuria, low eGFR, and CKD in Table 3. The positive association between the TyG index and CKD was stable in three different models (Model 1, OR = 1.79, 95% CI: 1.59, 2.03, P < 0.0001; Model 2, OR = 1.79, 95% CI: 1.59, 2.02, P < 0.0001; Model 3, OR = 1.34, 95% CI: 1.13, 1.59, P = 0.0006). For model 3, the fully adjusted model, the OR reached 1.06 in tertile 2 (95% CI: 0.80, 1.42, P = 0.6750) and 1.41 in tertile 3 (95% CI: 1.05, 1.91, P = 0.0244) relative to TyG index tertile 1. This positive association was similar to the albuminuria prevalence. However, no marked correlation between TyG index and the prevalence rate of low eGFR was found.
Subgroup analysis
BMI-stratified subgroup analysis was then conducted to investigate the relationship between the TyG index and the likelihood of CKD, which is presented in Table 4. No statistically significant difference in the prevalence of albuminuria was found among the normal-weight group. However, the positive association between the TyG index and CKD became stronger in the overweight (OR = 1.61, 95% CI: 1.18, 2.20, P = 0.0027) and obese groups (OR = 2.48, 95% CI: 1.95, 3.15, P < 0.0001). In the obese group, the OR was 3.08 for participants in tertile 3, which was larger than the other groups (95% CI: 1.74, 5.43, P < 0.0001). However, there seemed to be no obvious significance between tertile 1 and tertile 2 in the obese group (P = 0.5432). Notably, there was no dependence on BMI categories for this association.
Table 5 displays the diabetes condition stratified association between the TyG index and the prevalence rate of CKD. Similarly, interaction terms (P for interaction = 0.4128) showed that diabetes condition did not affect this association. Interestingly, the TyG index was not correlated with the likelihood of CKD in people without diabetes in either the primary or sensitivity analysis. However, for people with diabetes, a higher TyG index was associated with a higher level of CKD (OR = 1.94, 95% CI: 1.46, 2.56, P < 0.0001). People in TyG index tertile 3 showed a 402% higher level of albuminuria (OR = 5.02, 95% CI: 1.58, 16.02, P = 0. 0.0063).
Discussion
This cross-sectional study enrolled a total of 4361 participants from NHANES 2015–2016 and 2017–2018 and investigated the association between the TyG index and CKD. We identified that a higher TyG index was strongly associated with a higher UACR level and a higher level of CKD, especially in overweight and obese participants and/or individuals with diabetes. However, TyG index failed to predict the incidence of renal insufficiency, implying that the TyG index was more suitable for early prediction of CKD.
The TyG index has become an accessible and attractive option to assess IR condition due to the highly available and inexpensive biochemical markers needed for its calculation since it did not need quantification of insulin and might apply to all participants with different health condition [15, 16, 19, 35]. Previous studies showed that the TyG index even performed better in assessing IR conditions than the traditional IR indicators, such as homeostasis model assessment of insulin resistance (HOMA-IR) and the triglyceride/HDL-cholesterol ratio [17, 18]. Moreover, accumulating studies have identified that TyG index was not only a good indicator of IR condition but also had some specific superiorities in assessing the occurrence and progression of several diseases. Kun Zhang et al. independently used HOMA-IR and TyG index to assess the relationship between IR and male hypogonadism in China and concluded that the TyG index had a better predictability than HOMA-IR [36]. A systematic review pointed that the TyG index correlated with other IR prediction methods well and appears to be more superior in predicting IR risk and other cardiometabolic risk factors in children and adolescents [37]. Researches from Mexico conducted another systematic review to evaluate the diagnostic accuracy of the TyG index in assessing IR condition, where a moderate-to-low quality evidence about the usefulness of the TyG index as a marker of IR was identified [38]. In total, the TyG index performed well in assessing IR condition with different population settings, but its predictability of the occurrence of CKD have not been studied.
To our knowledge, this is the first cross-sectional study exploring the association between the TyG index and CKD. A higher TyG index indicated a more serious IR condition, which might lead to multiple unfavorable health outcomes. Two similar cross-sectional studies using the NHANES database reported that a higher TyG index was associated with more severe abdominal aortic calcification and an increased risk of subclinical myocardial injury in U.S. adults [24, 39]. For kidney health management, a study from the Austrian Vorarlberg Health Monitoring and Promotion Program (VHM&PP) identified that the TyG index appeared to be related to the occurrence of end-stage kidney disease (ESKD) and mediated almost 50% of the total association between BMI and ESKD in the general population [40]. Liangjing Lv et al. reported that the TyG index was a potential predictor for diabetic kidney disease in T2DM patients through a combined cross-sectional and longitudinal analysis [41].
Our results indicated that the TyG index was positively associated with UACR level and albuminuria (a kidney damage marker), while it did not correlate with eGFR level, indicating a possible relationship among insulin resistance (IR)-diabetes-albuminuria. And several studies have also confirmed that IR followed by hyperinsulinemia did exist in an early phase of CKD and was prior to clinically significant GFR decline [42,43,44]. IR was also found to be associated with early glomerular hyperfiltration and then led to late glomerular damage in the early stage of diabetic nephropathy [45]. Therefore, we posit that TyG index could be a very good early-stage kidney damage indicator, especially diabetic kidney damage. However, the exact mechanism behind the association between the TyG index and CKD was still not clear. Many previous studies have investigated and explored a close interaction between CKD and IR. On the one hand, the progression of CKD characterized by metabolic acidosis, intestinal dysbiosis, activation of chronic inflammatory pathways or production of uremia toxin substances might lead to IR. Thomas SS et al. revealed that the common inflammatory condition of CKD patients could facilitate and aggravate IR conditions [46]. Elevated muscle cell inflammatory cytokines and mass transmembrane glycoproteins (signal regulatory protein-α, SIRP-α) via the NF-\(\kappa\) B-dependent pathway in CKD patients were identified. SIRP-α reacted with insulin receptors and insulin receptor substrate-1 (IRS-1), whose overexpression could reduce insulin signal transduction, promote protein hydrolysis, and then lead to a more serious IR condition [46]. SIRP-α knockdown with siRNAs in skeletal muscle cells improved IR conditions with increased insulin sensitivity [46]. CKD also caused defects in signaling through the IRS/phosphatidylinositol 3-kinase (PI3-K)/Akt pathway and then led to insulin insensitivity and IR [47]. White adipose tissue (WAT) is recognized as an essential source of oxidative stress and inflammatory cytokines. Immune cell labeling experiments showed ascending expression of CD68, IL-6, SOCS-3 and oxidative stress genes in subcutaneous WAT in patients with CKD, indicating that CKD was also associated with the inflammation of adipose tissue, thereby promoting IR [48,49,50]. Furthermore, subgroup analysis showed that higher TyG index was associated with higher levels of CKD only in overweight/obese or diabetic participant instead of people with normal BMI or without diabetes, indicating that TyG index might be more utilizable in detecting early-stage kidney damage (albuminuria) among people who have already had abnormal metabolic condition (e.g., diabetes or obesity). Additionally, our results were consistent with previous studies that people with higher TyG index tended to have higher risks of obesity and diabetes, which were also closely linked with kidney damage, so there might be multidirectional relationship among TyG index (IR condition), obesity, diabetes, and kidney damage [51, 52].
Our studies possess multiple strengths. This is the first large-scale cross-sectional study based on NHANES data with full consideration of NHANES sample design and CDC analytical guidelines. We combined two continuous NHANES cycles to improve the sampled cohort and lend greater stability to the data estimates. Our results showed that a higher TyG index could be a good predictor of CKD occurrence, which might be highly useful among people in disadvantageous socioeconomic conditions with no availability for direct measurement of kidney function. Moreover, higher TyG index (more serious IR) was believed to be not only associated with poorer renal outcome, like ESKD, but also higher risks of other common complications of CKD, including metabolic syndrome (MetS) and CVD [53]. TyG index served as an accessible and practical IR indicators for early-stage kidney damage (albuminuria), and the early detection and treatment for CKD (asymptomatic until its later stages) has been an important way to prevent kidney dysfunction progression, associated complications and reduce the impact of CKD on public health resources, indicating its possible utility in large-scale kidney function screening. Furthermore, we reduced the use of self-report questionnaire data to avoid recall bias as much as possible. For instance, we defined smoking exposure through serum cotinine instead of smoking questionnaire. However, there were also some limitations in this study. First and foremost, we could not obtain the causal relationship between the TyG index and the risk of CKD due to the cross-sectional design, and the relationship among IR, metabolic disorders and kidney function indeed seemed to be multi-directional based on previous studies [42]. To verify their relationships, more-well designed longitudinal studies are needed. And as for TyG index itself, though it has been considered as a feasible IR assessment tool in many studies, TyG index is still novel and its comparison to hyperglycemic clamp (gold standard for IR; expensive and laborious) or other well established IR measurement like HOMA-IR (based on non-routinely collected fasting insulin) in predicting kidney function might need further investigation. Additionally, we could not clarify type I or type II diabetes and then manage correlated subgroup analysis due to a lack of related data. To consider the impact of protein intake on kidney dysfunction (albuminuria), we used 24 h dietary recall data, which means that recall bias is still inevitable. We could not consider all residual confounders due to unmeasured or unknown variables. Last, we only incorporated participants aged more than 20 years old, so more studies based on a wider population setting should be performed.
Conclusions
Higher TyG index was strongly associated with a higher UACR level and higher values of albuminuria and CKD, which might be useful in kidney function screening especially among people in disadvantageous socioeconomic conditions with no availability for direct measurement of kidney function. However, more well-designed studies are still needed to validate this relationship.
Availability of data and materials
Data described in the manuscript, codebook, and analytic code will be made publicly and freely available without restriction at www.cdc.gov/nchs/nhanes/.
References
Dikaiakou E, Vlachopapadopoulou EA, Paschou SA, Athanasouli F, Panagiotopoulos Ι, Kafetzi M, Fotinou A, Michalacos S (2020) Τriglycerides-glucose (TyG) index is a sensitive marker of insulin resistance in Greek children and adolescents. Endocrine 70:58–64. https://doi.org/10.1007/s12020-020-02374-6
Bikbov B, Purcell CA, Levey AS, Smith M et al (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395:709–733. https://doi.org/10.1016/s0140-6736(20)30045-3
Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ (2007) Chronic kidney disease: common, harmful and treatable–World Kidney Day 2007. Am J Nephrol 27:108–112. https://doi.org/10.1159/000099801
Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ (2007) Chronic kidney disease: common, harmful, and treatable–World Kidney Day 2007. J Am Soc Nephrol 18:374–378. https://doi.org/10.1681/asn.2006121305
Smyth LJ, Duffy S, Maxwell AP, McKnight AJ (2014) Genetic and epigenetic factors influencing chronic kidney disease. Am J Physiol Renal Physiol 307:F757-776. https://doi.org/10.1152/ajprenal.00306.2014
Neugut YD, Mohan S, Gharavi AG, Kiryluk K (2019) Cases in precision medicine: APOL1 and genetic testing in the evaluation of chronic kidney disease and potential transplant. Ann Intern Med 171:659–664. https://doi.org/10.7326/m19-1389
Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL et al (2010) Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329:841–845. https://doi.org/10.1126/science.1193032
Orr SE, Bridges CC (2017) Chronic kidney disease and exposure to nephrotoxic metals. Int J Mol Sci. https://doi.org/10.3390/ijms18051039
Lebovitz HE (2001) Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes 109(Suppl 2):S135-148. https://doi.org/10.1055/s-2001-18576
Navaneethan SD, Kirwan JP, Remer EM, Schneider E, Addeman B, Arrigain S, Horwitz E, Fink JC, Lash JP, McKenzie CA et al (2021) Adiposity, physical function, and their associations with insulin resistance, inflammation, and adipokines in CKD. Am J Kidney Dis 77:44–55. https://doi.org/10.1053/j.ajkd.2020.05.028
DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J (1981) Insulin resistance in uremia. J Clin Invest 67:563–568. https://doi.org/10.1172/jci110067
Pham H, Utzschneider KM, de Boer IH (2011) Measurement of insulin resistance in chronic kidney disease. Curr Opin Nephrol Hypertens 20:640–646. https://doi.org/10.1097/MNH.0b013e32834b23c1
Siew ED, Ikizler TA (2008) Determinants of insulin resistance and its effects on protein metabolism in patients with advanced chronic kidney disease. Contrib Nephrol 161:138–144. https://doi.org/10.1159/000130659
Shinohara K, Shoji T, Emoto M, Tahara H, Koyama H, Ishimura E, Miki T, Tabata T, Nishizawa Y (2002) Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol 13:1894–1900. https://doi.org/10.1097/01.asn.0000019900.87535.43
Singh B, Saxena A (2010) Surrogate markers of insulin resistance: A review. World J Diabetes 1:36–47. https://doi.org/10.4239/wjd.v1.i2.36
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F (2008) The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 6:299–304. https://doi.org/10.1089/met.2008.0034
Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B (2011) TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract 93:e98–e100. https://doi.org/10.1016/j.diabres.2011.05.030
Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, Lee JH, Yim HW, Kang MI, Lee WC et al (2014) Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS ONE 9:e90430. https://doi.org/10.1371/journal.pone.0090430
Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X (2014) Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol 13:146. https://doi.org/10.1186/s12933-014-0146-3
Mirr M, Skrypnik D, Bogdański P, Owecki M (2021) Newly proposed insulin resistance indexes called TyG-NC and TyG-NHtR show efficacy in diagnosing the metabolic syndrome. J Endocrinol Invest 44:2831–2843. https://doi.org/10.1007/s40618-021-01608-2
da Silva A, Caldas APS, Rocha D, Bressan J (2020) Triglyceride–glucose index predicts independently type 2 diabetes mellitus risk: A systematic review and meta-analysis of cohort studies. Prim Care Diabetes 14:584–593. https://doi.org/10.1016/j.pcd.2020.09.001
Tutunchi H, Naeini F, Mobasseri M, Ostadrahimi A (2021) Triglyceride glucose (TyG) index and the progression of liver fibrosis: A cross-sectional study. Clin Nutr ESPEN 44:483–487. https://doi.org/10.1016/j.clnesp.2021.04.025
Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, Kim JH, Park JS (2019) Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care 42:1569–1573. https://doi.org/10.2337/dc18-1920
Chen Y, Chang Z, Zhao Y, Liu Y, Fu J, Zhang Y, Liu Y, Fan Z (2021) Association between the triglyceride–glucose index and abdominal aortic calcification in adults: A cross-sectional study. Nutr Metab Cardiovasc Dis 31:2068–2076. https://doi.org/10.1016/j.numecd.2021.04.010
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Sanders AP, Mazzella MJ, Malin AJ, Hair GM, Busgang SA, Saland JM, Curtin P (2019) Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in NHANES 2009–2014. Environ Int 131:104993. https://doi.org/10.1016/j.envint.2019.104993
Guo J, Wu C, Zhang J, Chang X, Zhang Y, Cao Y, Zhou Z (2020) Associations of melamine and cyanuric acid exposure with markers of kidney function in adults: Results from NHANES 2003–2004. Environ Int 141:105815. https://doi.org/10.1016/j.envint.2020.105815
Webster AC, Nagler EV, Morton RL, Masson P (2017) Chronic kidney disease. Lancet 389:1238–1252. https://doi.org/10.1016/s0140-6736(16)32064-5
Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013 3:19–62. doi:https://doi.org/10.1038/kisup.2012.64.
Qin Z, Zhao J, Li J, Yang Q, Geng J, Liao R, Su B (2021) Low lean mass is associated with lower urinary tract symptoms in US men from the 2005–2006 national health and nutrition examination survey dataset. Aging (Albany NY) 13:21421–21434. https://doi.org/10.18632/aging.203480
Liu N, Ma F, Feng Y, Ma X (2021) The Association between the Dietary Inflammatory Index and Thyroid Function in U.S. Adult Males. Nutrients. https://doi.org/10.3390/nu13103330
Wiener RC (2015) Serum cotinine and chronic pain NHANES 2003–2004. J Drug Abuse. https://doi.org/10.21767/2471-853X.10003
Egan BM, Li J, Shatat IF, Fuller JM, Sinopoli A (2014) Closing the gap in hypertension control between younger and older adults: National Health and Nutrition Examination Survey (NHANES) 1988 to 2010. Circulation 129:2052–2061. https://doi.org/10.1161/circulationaha.113.007699
Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR (2013) National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2:1–24
Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, Yang H, Ren LB, Qi W, Li WY et al (2020) Triglyceride–glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol 19:80. https://doi.org/10.1186/s12933-020-01054-z
Zhang K, Chen Y, Liu L, Lu M, Cheng J, Gao F, Wang N, Shen Z, Lu Y (2017) The Triglycerides and glucose index rather than HOMA-IR is more associated with Hypogonadism in Chinese men. Sci Rep 7:15874. https://doi.org/10.1038/s41598-017-16108-8
Brito ADM, Hermsdorff HHM, Filgueiras MS, Suhett LG, Vieira-Ribeiro SA, Franceschini S, Novaes JF (2021) Predictive capacity of triglyceride–glucose (TyG) index for insulin resistance and cardiometabolic risk in children and adolescents: a systematic review. Crit Rev Food Sci Nutr 61:2783–2792. https://doi.org/10.1080/10408398.2020.1788501
Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, Álvarez-Villalobos NA, González-González JG (2020) Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review. Int J Endocrinol 2020:4678526. https://doi.org/10.1155/2020/4678526
Liu Y, Wu M, Xu J, Sha D, Xu B, Kang L (2020) Association between Triglyceride and glycose (TyG) index and subclinical myocardial injury. Nutr Metab Cardiovasc Dis 30:2072–2076. https://doi.org/10.1016/j.numecd.2020.06.019
Fritz J, Brozek W, Concin H, Nagel G, Kerschbaum J, Lhotta K, Ulmer H, Zitt E (2021) The triglyceride–glucose index and obesity-related risk of end-stage kidney disease in Austrian Adults. JAMA Netw Open 4:e212612. https://doi.org/10.1001/jamanetworkopen.2021.2612
Lv L, Zhou Y, Chen X, Gong L, Wu J, Luo W, Shen Y, Han S, Hu J, Wang Y et al (2021) Relationship between the TyG index and diabetic kidney disease in patients with Type-2 diabetes mellitus. Diabetes Metab Syndr Obes 14:3299–3306. https://doi.org/10.2147/dmso.s318255
Spoto B, Pisano A, Zoccali C (2016) Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol 311:F1087-f1108. https://doi.org/10.1152/ajprenal.00340.2016
Bergman RN, Ider YZ, Bowden CR, Cobelli C (1979) Quantitative estimation of insulin sensitivity. Am J Physiol 236:E667-677. https://doi.org/10.1152/ajpendo.1979.236.6.E667
Kawagishi T, Nishizawa Y, Konishi T, Kawasaki K, Emoto M, Shoji T, Tabata T, Inoue T, Morii H (1995) High-resolution B-mode ultrasonography in evaluation of atherosclerosis in uremia. Kidney Int 48:820–826. https://doi.org/10.1038/ki.1995.356
Qin Y, Tang H, Yan G, Wang D, Qiao Y, Luo E, Hou J, Tang C (2020) A High Triglyceride–glucose index is associated with contrast-induced acute kidney injury in Chinese patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne) 11:522883. https://doi.org/10.3389/fendo.2020.522883
Thomas SS, Dong Y, Zhang L, Mitch WE (2013) Signal regulatory protein-α interacts with the insulin receptor contributing to muscle wasting in chronic kidney disease. Kidney Int 84:308–316. https://doi.org/10.1038/ki.2013.97
Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE (2006) Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol 17:1388–1394. https://doi.org/10.1681/asn.2004100842
Kawazoe Y, Naka T, Fujimoto M, Kohzaki H, Morita Y, Narazaki M, Okumura K, Saitoh H, Nakagawa R, Uchiyama Y et al (2001) Signal transducer and activator of transcription (STAT)-induced STAT inhibitor 1 (SSI-1)/suppressor of cytokine signaling 1 (SOCS1) inhibits insulin signal transduction pathway through modulating insulin receptor substrate 1 (IRS-1) phosphorylation. J Exp Med 193:263–269. https://doi.org/10.1084/jem.193.2.263
Okumura F, Matsuzaki M, Nakatsukasa K, Kamura T (2012) The role of elongin BC-containing ubiquitin ligases. Front Oncol 2:10. https://doi.org/10.3389/fonc.2012.00010
Pereira BJ, Sundaram S, Snodgrass B, Hogan P, King AJ (1996) Plasma lipopolysaccharide binding protein and bactericidal/permeability increasing factor in CRF and HD patients. J Am Soc Nephrol 7:479–487. https://doi.org/10.1681/asn.v73479
Ndisang JF, Vannacci A, Rastogi S (2017) Insulin resistance, Type 1 and Type 2 diabetes, and related complications 2017. J Diabetes Res 2017:1478294. https://doi.org/10.1155/2017/1478294
Gu S, Wang A, Ning G, Zhang L, Mu Y (2020) Insulin resistance is associated with urinary albumin-creatinine ratio in normal weight individuals with hypertension and diabetes: The REACTION study. J Diabetes 12:406–416. https://doi.org/10.1111/1753-0407.13010
Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, Sowers JR (2021) Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 119:154766. https://doi.org/10.1016/j.metabol.2021.154766
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: NL and JT. Methodology: NL and CL. Formal analysis and investigation: NL, CL, JT and ZQ. Writing—original draft preparation: NL, CL and ZQ. Writing—review and editing: JT. Funding acquisition: none. Resources: NL. Supervision: JT.
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declared no competing interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (National Center for Health Statistics (NCHS) at the U.S. Centers for Disease Control and Prevention (CDC)) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, N., Liu, C., Qu, Z. et al. Association between the triglyceride–glucose index and chronic kidney disease in adults. Int Urol Nephrol 55, 1279–1289 (2023). https://doi.org/10.1007/s11255-022-03433-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03433-9