Abstract
Aims
Examining the connection between the triglyceride-glucose (TyG) index and chronic kidney disease (CKD) was the aim of this investigation.
Methods
Data from the National Health and Nutrition Examination Survey (NHANES) covering the years 1999–2020 were analyzed in this study. The TyG index was calculated as Ln (triglycerides (mg/dl) * fasting glucose (mg/dl)/2). The two criteria used to diagnose CKD were low estimated glomerular filtration rate (eGFR) (eGFR < 60 mL/min/1.73m2) or albuminuria (urine albumin-to-creatinine ratio (ACR) ≥ 30 mg/g). To look into the independent associations between TyG index levels with CKD, albuminuria, and low-eGFR, weighted multivariable logistic regression and generalized additive models were employed. To assess and contrast the diagnostic ability, receiver operating characteristic (ROC) curves were employed.
Results
Out of 18,078 total participants recruited, 48.54% were male. 8.48 + 0.68 was the mean value of the TyG index. CKD, albuminuria, and low-eGFR were common, with respective prevalences of 17.06%, 11.26%, and 8.03%, respectively. The TyG index and CKD were observed to positively correlate (OR = 4.03; 95% CI 1.81, 8.96). In US adults between the ages of 41 and 60, a J-shaped connection was found between the two. Furthermore, a higher TyG index is associated with a higher prevalence of albuminuria (OR = 6.11; 95% CI 2.64, 14.14). Subgroup analyses and interaction tests revealed that different stratifications did not significantly affect the relationship between TyG index and CKD, albuminuria, and low-eGFR. Comparing the TyG index to other indicators [lipid accumulation product (LAP), Visceral adiposity index (VAI), and the triglyceride glucose–body mass index (TyG-BMI)], it may be more accurate and discriminative in predicting CKD and albuminuria.
Conclusion
When predicting CKD and albuminuria, the TyG index may be a more useful marker when compared to other markers (LAP, VAI, and TyG-BMI index). In addition, in American adults aged 41–60, the TyG index shows a J-shaped relationship with CKD. As a result, when assessing the kidney health of US adults, we must pay close attention to the significance of the TyG index.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is becoming more common, with the majority of cases being caused by diabetes and hypertension. It affects 15–20% of adults globally, raising the possibility of unfavorable outcomes [1,2,3]. Globally, CKD is the main source of catastrophic health expenditures, or medical costs that are more than 40% of household income [4]. By 2040, it is expected to rise to the fifth rank among the world’s major causes of death [5]. Thus, renal health is something we should take very seriously. According to studies, CKD is significantly influenced by insulin resistance (IR) [6, 7]. For clinical purposes, the hyperinsulinemic-normoglycemic clamp test, which is the gold standard for evaluating IR, is too time-consuming and expensive [8]. Therefore, alternative markers for IR have emerged.
Using fasting triglycerides and glucose, one may quickly and simply determine IR by calculating the triglyceride-glucose (TyG) index [9]. The TyG index can predict the occurrence of CKD, according to earlier studies. A cohort research involving 11,712 Japanese participants discovered a link between a higher TyG index and a greater prevalence of CKD [10]. TyG index and CKD prevalence were reported to be positively correlated in Chinese hypertension patients by Shi et al. [11]. TyG index and end-stage kidney disease (ESKD) were found to be positively correlated in an Austrian study [12]. Previous studies also explored the significant association between high levels of the TyG index and high levels of CKD and albuminuria in the US population [13, 14]. However, there are no studies that delve into the potential association between the TyG index and CKD in US adults while also assessing its predictive value for kidney disease.
As such, this study aims to investigate the relationship between the TyG index and CKD using data from the National Health and Nutrition Examination Survey (NHANES).
Materials and methods
Study design and population
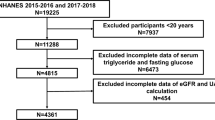
NHANES is a research study that gathers health and nutrition information from US households through a population-based inquiry [15]. The NHANES 1999–2020 was the source of participants for our research. There were 18,078 eligible individuals who remained in the trial after patients who were < 20 years of age (n = 48,975), had cancer (n = 1316), were pregnant (n = 229), and lacked information regarding ACR (n = 8506), eGFR (n = 16,013), and TyG index (n = 23,428) were eliminated (Fig. 1). Both the NHANES survey protocols and the informed consent forms signed by each study participant were approved by the National Center for Health Statistics (NCHS) research ethics review committee.
Definition of the TyG index and CKD
The TyG index, which was derived using Ln [triglycerides (mg/dl) * fasting glucose (mg/dl)/2], was considered as an exposure variable [16]. According to the NHANES protocol, enzymatic assays were used in an automatic biochemistry analyzer to measure triglycerides and fasting glucose. We also compare the predictive power of TyG index with other markers such as Visceral adiposity index (VAI)(male [WC/39.68 + (1.88 × BMI)] × (TG/1.03) × (1.31/HDL-C); female [WC/36.58 + 1.89 × (BMI)] × (TG/0.81) × (1.52/HDL-C)), lipid accumulation product (LAP)(male [WC − 65] × TG; female [WC − 58] × TG), and triglyceride glucose–body mass index (TyG-BMI) (TyG index × BMI) [17, 18].
To diagnose CKD, an albuminuric or eGFR of less than 60 mL/min/1.73 m2 is necessary [19]. In 2009, eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) algorithm for standardized creatinine [20]. ACR ≥ 30 mg/g was used to characterize albuminuria. In our study, low-eGFR, albuminuria, and CKD were the outcome variables. During an individual interview, a standardized medical condition questionnaire was used to gather self-reported physician diagnoses, which led to the diagnosis of cardiovascular disease (CVD). The inquiry posed to the participants was, “Have you ever received a diagnosis of congestive heart failure, coronary heart disease, angina pectoris, myocardial infarction, or stroke from a doctor or other health expert?” Answering “yes” to any of the aforementioned questions was considered an indication of CVD.
Selection of covariates
We adjusted for a number of demographic factors in our analysis, such as education level, race, age, and sex. We also included a number of laboratory and anthropometric covariates, including BMI, waist circumference (WC), smoking status, alcohol drinking (number of days of alcohol consumption in the past year), systolic blood pressure (SBP), diastolic blood pressure (DBP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum uric acid, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and serum total calcium.
Variations in health status, such as diabetes and hypertension, were also included as factors in our analysis. This study defines hypertension using three different criteria. “Ever told you you had hypertension” is a questionnaire item that serves as the basis for an assessment. In the second section, the mean diastolic or systolic blood pressure exceeding 130 or 80 mmHg is measured [21]. Using the item “taking hypertension prescription” program, participants with hypertension are identified in the third section. Three components were also included in the definition that was applied to diabetes. The first portion concerned self-reported diabetes, whereas the second concerned the use of insulin or other diabetes treatments. The last step was determining which patients had diabetes based on hemoglobin A1c (HbA1c) (%) > 6.5 and fasting glucose (mmol/l) ≥ 7.0.
Statistical analysis
In compliance with the recommendations given by the US Centers for Disease Control and Prevention (CDC), every statistical analysis takes into account the intricate sample design of a multi-stage cluster survey [22]. The standard deviation and mean were displayed for continuous values, and percentages were used for categorical variables. For either continuous or categorical data, differences between groups of the TyG index (tertiles) were analyzed using weighted chi-square tests or t tests. A weighted multivariable regression model was used to investigate three different models that looked at the link between the TyG index and low-eGFR, albuminuria, and CKD. There was no covariate adjustment made in Model 1. Age, sex, and race adjustments were made to Model 2. Sex, age, race, BMI, WC, education level, smoking status, alcohol consumption, SBP, DBP, AST, ALT, serum uric acid, TC, LDL-C, HDL-C, serum total calcium, hypertension, and diabetes status were among the variables that were taken into consideration while adjusting Model 3. The non-linear problems were solved by smooth curve fitting and generalized additive models (GAM). Using subgroup analysis and a stratified multivariable logistic regression model stratified by sex, age, BMI, hypertension, and diabetes, the relationship between TyG index and CKD, albuminuria, and low-eGFR was also investigated. The predictive efficacy of the TyG index and other markers (LAP, VAI, and TyG-BMI index) was further evaluated using receiver operating characteristic (ROC) curves and area under the curve (AUC) values. Based on the data available, mode imputation was applied to missing values in categorical variables and median imputation to missing values in continuous variables. R 4.1.3 was used for all statistical analyses, along with the Empower software. One utilized a two-tailed p value of less than 0.05 to determine statistical significance.
Results
Participants characteristics at baseline
18,078 participants in total, 51.46 percent female and 48.54% male, with an average age of 48.93 ± 18.24 years for the analysis. 8.48 ± 0.68 was the average TyG index value. For prevalence of albuminuria it was 11.26%, for CKD it was 17.06%, and for low-eGFR it was 8.03%. Individuals in the upper tertile of the TyG index had greater rates of CKD, albuminuria, and low-eGFR prevalence (all p < 0.05). Age, BMI, WC, smoking status, hypertension, diabetes, SBP, DBP, TC, HDL-C, LDL-C, ACR, eGFR, albuminuria, low-eGFR, and CKD stages were all significantly different between tertiles (all p < 0.05)(Table 1). We also looked at the characteristics of the population which are the unavailability of data related to the TyG index (n = 23,428) (Supplementary Table S1).
The association between the TyG index and CKD
Table 2 shows the correlation between CKD and the TyG index. In both crude and minimally adjusted models, our findings show a positive connection between the two. The positive correlation remains stable (OR = 4.03; 95% CI 1.81, 8.96) following full adjustment, suggesting that there is a 3.03-fold increase in the prevalence of CKD among the subjects for every unit rise in the TyG index. For a sensitivity analysis, the continuous variable was additionally converted to a categorical variable (tertiles) using the TyG index. The prevalence of CKD was more common in the higher tertiles than in the lower tertiles of the TyG index (p for trend < 0.05).
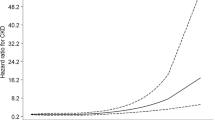
The TyG index and CKD did not indicate a non-linear connection, according to the smooth curve fitting (Fig. 2). Among those in the 41–60 age range, we discovered a non-linear correlation between the two. There was a definite breakpoint of 8.21. The TyG index and CKD did not seen to have a significant relationship with the breakpoint’s left side (OR = 0.68, 95% CI 0.37, 1.24). A positive correlation was seen between the two to the right of the breakpoint (OR = 6.04, 95% CI 1.60, 22.80) (Table 3).
The association between the TyG index and albuminuria
Additionally, a higher prevalence of albuminuria was observed to be correlated with a higher TyG index (OR = 6.11; 95% CI 2.64, 14.14). There was still a statistically significant link even after switching the TyG index to tertiles. The higher tertiles had a higher prevalence of albuminuria than the lower tertiles of the TyG index, respectively (p for trend < 0.05) (Table 2).
The TyG index and albuminuria were revealed to have a non-linear association based on GAM and smooth curve fitting (Fig. 3). Our calculations resulted in a breakpoint of 8.72. The TyG index and albuminuria were positively correlated on either side of the breakpoint (left side: OR = 4.19, 95% CI 1.68, 10.47; right side: OR = 17.16, 95% CI 4.24, 69.46) (Table 4).
The association between the TyG index and low-eGFR
The TyG index and low-eGFR were found to be significantly positively correlated in both the crude model (Model 1: OR = 1.65, 95% CI 1.53, 1.78) and the minimally adjusted model (Model 2: OR = 1.82, 95% CI 1.69, 1.97). The positive connection did not, however, achieve statistical significance after full adjustment (OR = 0.97, 95% CI 0.31, 3.00) (Table 2).
The TyG index and low-eGFR did not show any non-linear connection, according to smooth curve fitting (Fig. 3).
Subgroup analysis
CKD, albuminuria, and low-eGFR are not consistently correlated with the TyG index, according to our findings. TyG index and CKD were found to have significant relationships in each subgroup broken down by sex, diabetes, and hypertension (all p < 0.05). In the overweight and 41–60 age group, the TyG index did, however, demonstrate a positive but non-significant relationship with CKD. The interaction tests demonstrated that there was no significant influence of age, sex, BMI, hypertension, or diabetes on the connection between the TyG index with CKD, albuminuria, and low-eGFR (all p for interaction > 0.05) (Fig. 4).
ROC analysis
For CKD, albuminuria, and low-eGFR, we calculated the AUC values to assess the prediction accuracy of the TyG index with other markers (LAP, VAI, and TyG-BMI index) (Fig. 5). Comparing the TyG index to the other indicators, our results show that it had higher AUC values. Furthermore, Table 4 demonstrates that the TyG index and other indicators had statistically significant differences in AUC values in the prediction of CKD and albuminuria (all p < 0.05) (Table 5). These results demonstrate that when compared to other indicators (LAP, VAI, and TyG-BMI index), the TyG index may have a greater discriminative capacity and accuracy in predicting CKD and albuminuria.
ROC curves and the AUC values of the four markers (TyG index, LAP, VAI, and TyG-BMI index) in diagnosing CKD, albuminuria and low-eGFR. A Four inflammatory markers were assessed to identify CKD. B Four inflammatory markers were assessed to identify albuminuria. C Four inflammatory markers were assessed to identify low-eGFR
The association between the TyG index and CVD
We looked more closely at the connection between CVD and the TyG index. The two were found to positively correlate (Supplementary Table S2). We used smooth curve fitting and GAM to find the nonlinear relationship between the two (Supplementary Fig. S1). Supplementary Table S3 shows that 9.52 was its breakpoint. The interaction test results showed that age, sex, BMI, hypertension, or diabetes did not significantly affect the link between the two (Supplementary Fig. S2). ROC analysis revealed that for CVD prediction, the TyG index performed better in terms of AUC values than the LAP, VAI, and TyG-BMI index (Supplementary Fig. S3, Table S4).
Discussion
The prevalences of CKD and albuminuria was positively correlated with the TyG index level in this cross-sectional investigation of 18,078 adults. Additionally, we discovered a J-shaped connection between the TyG index and CKD in the 41–60 age range (K = 8.21). If, that is, the TyG index was greater than 8.21, the prevalence of CKD in the 41–60 age group of American participants increased significantly. There was no discernible impact of population differences on the relationship between the TyG index and low-eGFR, albuminuria, and CKD, according to subgroup analysis and interaction testing. Additionally, in comparison to other markers (LAP, VAI, and TyG-BMI index), the TyG index may have greater discriminative power and accuracy in predicting CKD and albuminuria.
In several populations and regions, the link between the TyG index and CKD has been researched [23,24,25]. One study found a connection between a rise in the TyG index and deteriorating kidney function in senior Chinese persons [26]. The TyG index and ESKD (end-stage renal disease) were found to be significantly correlated in cohort research from Austria [12]. In a cohort analysis of 11,712 patients in Japan, the TyG index was found to have a positive relationship with CKD [10]. In China, the TyG index and CKD of hypertension patients were positively associated [11]. A higher TyG index was linked to a higher prevalence of CKD and albuminuria, according to US studies [13, 14]. Our study has a number of advantages over earlier studies as well. Firstly, in the US population of people aged 41–60, which has received less attention in other studies on kidney health in middle-aged adults, our study discovered for the first time a J-shaped link between the TyG index and CKD. Secondly, whereas earlier research on the connection between low-eGFR and the TyG index has produced contentious findings, our analysis of data on US adults revealed no significant connection between the two [10, 13, 26, 27]. Thirdly, we explored territory by conducting ROC analyses to assess the predictive abilities of the TyG index, LAP, VAI, and TyG-BMI index for CKD, albuminuria, and low-eGFR in US adults. This is a fundamental difference from previous studies. Additionally, our study delved into the TyG index’s predictive significance for cardiovascular disease (CVD) prevalence in US adults. This aspect, previously explored primarily for its prognostic implications, adds another layer to our understanding [28, 29].
Our study, in addition to showing a positive correlation between TyG index levels with CKD, demonstrated a J-shaped link between the two in the group of people aged 41–60 (K = 8.21). The twos had a negative correlation, although it was not statistically significant, on the left side of the breakpoint. Nonetheless, every unit increase in the TyG index was linked to a 5.04-fold rise in the prevalence of CKD on the right side of the breakpoint. Therefore, Americans aged 41–60 with a TyG index > 8.21 should pay close attention to their kidney health. TyG index and CKD have been established in earlier research to have a nonlinear association in patients with impaired glucose metabolism and hypertension [30]. A non-linear association between the TyG index and the prevalence of diabetic nephropathy was also discovered by Shang et al. [31]. However, we need more prospective studies to confirm our findings.
According to our research, the TyG index and albuminuria are positively and nonlinearly correlated in US people. The link was seen on both sides of a breakpoint (K = 8.72), with a high positive correlation on the right side and a much weaker positive correlation on the left. That is, the prevalence of albuminuria considerably rises when the TyG index is higher than 8.72. Nonlinear relationships between the TyG index and other diseases have been discovered in prior research. Jiang et al. found a nonlinear correlation between the TyG index and the prevalence of kidney stones [32]. Adult US deaths from cardiovascular and all-cause were shown to have a nonlinear relationship with the TyG index by Liu et al. [33]. The relationship between low-eGFR and the TyG index in previous studies remains controversial [10, 13, 26, 27]. We could not find any relationship between low-eGFR and the TyG index. We believe that different results can be obtained from different demographic characteristics, including population, race, area, sample size, and eGFR calculation method.
IR has been demonstrated to have a significant correlation with CKD [6, 7]. However, the gold standard test for diagnosing IR can be difficult to perform in clinical settings [8]. TyG index, as a simple and feasible IR indicator, has been shown to be significantly better than other traditional IR indicators. Research indicates that the TyG index outperforms the VAI and lipid accumulation product index (LAPI) in predicting CKD occurrence [34]. In our study, we also examined the TyG index’s superiority. ROC analysis showed that the TyG index was a more reliable indicator of CKD and albuminuria than other indicators (LAP, VAI, and TyG-BMI index). To sum up, the TyG index has a lot of potential for clinical use in CKD prediction and might be a more straightforward and accurate IR indicator.
CKD was more likely to occur in female participants, according to our subgroup analysis (Males: OR = 3.62, 95% CI 1.37, 9.53; Females: OR = 4.36, 95% CI 1.88, 10.12). This result has been supported by earlier research [10, 34, 35]. However, the fundamental cause of the sex gap is yet unknown, necessitating additional research. Importantly, the independent associations between the TyG index and CKD, albuminuria, or low eGFR persisted across age, sex, BMI, hypertension, and diabetes. These associations may be applicable to diverse populations, reinforcing the adverse impact of the TyG index on renal function.
Inflammation and oxidative stress brought on by IR may be the basis for the relationship between the TyG index and CKD. IR inhibits the insulin signaling pathway, causes a rise in monocyte chemoattractant protein-1 (MCP-1) synthesis, and encourages inflammation in adipose tissue. Tumor necrosis factor (TNF) -alpha and interleukin-6 (IL-6) are two pro-inflammatory cytokines that are produced when macrophages are activated by inflammatory stimuli in adipose tissue [36, 37]. Endothelial dysfunction is related to CKD and is facilitated by TNF-alpha and IL-6 [10, 38]. Additionally, oxidative stress and IR are related [38]. The activation of nuclear factor erythroid-2-related factor-2 (Nrf-2), which defends renal tissue, might be compromised by oxidative stress and inflammation [39]. The precise mechanisms underlying this relationship, however, need more research.
CKD increases the risk of various adverse outcomes, especially CVD [40]. As a result, we looked into the possibility of a connection between CVD and the TyG index. According to our findings, for every unit rise in the TyG index, the prevalence of CVD increased by 61%. Similar findings were noted in earlier research [28, 41, 42]. Prior research also revealed that the TyG index outperformed VAI as a predictor of CVD risk [43]. In addition, our research revealed that, when compared to the LAP, VAI, and TyG-BMI index, the TyG index had the greatest AUC value for predicting CVD. Therefore, we need to pay equal attention to the importance of the TyG index for renal and cardiovascular health in US adults.
The advantages of our research are numerous. Initially, the NHANES data is a nationwide population-based survey. The second reason is that our study is more reliable and representative because of its large sample size and adjustment for confounding factors. However, there are several shortcomings in our investigation. The cross-sectional design, for example, made it unable to show a causal relationship between the TyG index and CKD. Second, we are unable to completely exclude the influence of additional potential confounding variables, even after adjusting for a number of significant confounders. Third, because the US population survey NHANES is cross-sectional in nature, extrapolating our findings to other ethnic groups or the larger population may prove difficult.
Conclusion
When predicting CKD and albuminuria, the TyG index may be a more useful marker when compared to other markers (LAP, VAI, and TyG-BMI index). In addition, in American adults aged 41–60, the TyG index shows a J-shaped relationship with CKD. As a result, when assessing the kidney health of US adults, we must pay close attention to the significance of the TyG index.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
References
Andreucci M, Provenzano M, Faga T, Michael A, Patella G, Mastroroberto P et al (2021) Aortic aneurysms, chronic kidney disease and metalloproteinases. Biomolecules. https://doi.org/10.3390/biom11020194
Ramspek CL, Evans M, Wanner C, Drechsler C, Chesnaye NC, Szymczak M et al (2021) Kidney failure prediction models: a comprehensive external validation study in patients with advanced CKD. J Am Soc Nephrol 32:1174–1186. https://doi.org/10.1681/ASN.2020071077
Li J, Liu Z, Pu Y, Dai H, Peng F (2023) Association between dietary vitamin E intake and chronic kidney disease events in US adults: a cross-sectional study from NHANES 2009–2016. Clin Kidney J 16:2559–2566. https://doi.org/10.1093/ckj/sfad162
Jamison DT (2018) Disease Control Priorities, 3rd edition: improving health and reducing poverty. Lancet 391:e11–e14. https://doi.org/10.1016/S0140-6736(15)60097-6
Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M et al (2018) Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 392:2052–2090. https://doi.org/10.1016/S0140-6736(18)31694-5
de Boer IH, Mehrotra R (2014) Insulin resistance in chronic kidney disease: a step closer to effective evaluation and treatment. Kidney Int 86:243–245. https://doi.org/10.1038/ki.2014.123
Schrauben SJ, Jepson C, Hsu JY, Wilson FP, Zhang X, Lash JP et al (2019) Insulin resistance and chronic kidney disease progression, cardiovascular events, and death: Findings from the chronic renal insufficiency cohort study. BMC Nephrol 20:60. https://doi.org/10.1186/s12882-019-1220-6
Muniyappa R, Lee S, Chen H, Quon MJ (2008) Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294:E15-26. https://doi.org/10.1152/ajpendo.00645.2007
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F (2008) The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 6:299–304. https://doi.org/10.1089/met.2008.0034
Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M (2019) Triglyceride-glucose index is a predictor of incident chronic kidney disease: a population-based longitudinal study. Clin Exp Nephrol 23:948–955. https://doi.org/10.1007/s10157-019-01729-2
Shi Y, Hu L, Li M, Zhou W, Wang T, Zhu L et al (2022) Association between the surrogate markers of insulin resistance and chronic kidney disease in Chinese hypertensive patients. Front Med (Lausanne) 9:831648. https://doi.org/10.3389/fmed.2022.831648
Fritz J, Brozek W, Concin H, Nagel G, Kerschbaum J, Lhotta K et al (2021) The Triglyceride-Glucose Index and Obesity-Related risk of End-Stage kidney disease in Austrian adults. JAMA Netw Open 4:e212612. https://doi.org/10.1001/jamanetworkopen.2021.2612
Liu N, Liu C, Qu Z, Tan J (2023) Association between the triglyceride-glucose index and chronic kidney disease in adults. Int Urol Nephrol 55:1279–1289. https://doi.org/10.1007/s11255-022-03433-9
Wang Z, Qian H, Zhong S, Gu T, Xu M, Yang Q (2023) The relationship between triglyceride-glucose index and albuminuria in United States adults. Front Endocrinol (Lausanne) 14:1215055. https://doi.org/10.3389/fendo.2023.1215055
Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J (2013) National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1:1–37
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO et al (2010) The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 95:3347–3351. https://doi.org/10.1210/jc.2010-0288
Xue Y, Xu J, Li M, Gao Y (2022) Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: triglyceride glucose index-related parameters. Front Endocrinol (Lausanne) 13:951689. https://doi.org/10.3389/fendo.2022.951689
Zheng L, Sun A, Han S, Qi R, Wang R, Gong X et al (2023) Association between visceral obesity and 10-year risk of first atherosclerotic cardiovascular diseases events among American adults: National Health and Nutrition Examination Survey. Front Cardiovasc Med 10:1249401. https://doi.org/10.3389/fcvm.2023.1249401
Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). (2013) 3:19–62. https://doi.org/10.1038/kisup.2012.64
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AR, Feldman HI et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Whelton PK, Carey RM, Aronow WS, Casey DJ, Collins KJ, Dennison HC et al (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension 71:e13-115. https://doi.org/10.1161/HYP.0000000000000065
Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM et al (2013) National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2:1–24
Ren X, Jiang M, Han L, Zheng X (2023) Association between triglyceride-glucose index and chronic kidney disease: a cohort study and meta-analysis. Nutr Metab Cardiovasc Dis 33:1121–1128. https://doi.org/10.1016/j.numecd.2023.03.026
Kim B, Kim GM, Han K, Maki N, Taniguchi K, Oh S (2023) The triglyceride-glucose index is independently associated with chronic kidney disease in the geriatric population, regardless of obesity and sex. Ann Geriatr Med Res 27:258–265. https://doi.org/10.4235/agmr.23.0096
Xu X, Tang X, Che H, Guan C, Zhao N, Fu S et al (2021) Triglyceride-glucose product is an independent risk factor for predicting chronic kidney disease in middle-aged and elderly population: a prospective cohort study. Nan Fang Yi Ke Da Xue Xue Bao 41:1600–1608. https://doi.org/10.12122/j.issn.1673-4254.2021.11.02
Lei L, Liang H, Qu Y, Zhong Q, Zhang Q, Dai L et al (2022) Association between triglyceride-glucose index and worsening renal function in the elderly. Front Nutr 9:951564. https://doi.org/10.3389/fnut.2022.951564
Liu L, Xia R, Song X, Zhang B, He W, Zhou X et al (2021) Association between the triglyceride-glucose index and diabetic nephropathy in patients with type 2 diabetes: a cross-sectional study. J Diabetes Investig 12:557–565. https://doi.org/10.1111/jdi.13371
Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P et al (2022) Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol 21:124. https://doi.org/10.1186/s12933-022-01546-0
Zhang Q, Xiao S, Jiao X, Shen Y (2023) The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol 22:279. https://doi.org/10.1186/s12933-023-02030-z
Zhu Q, Chen Y, Cai X, Cai L, Hong J, Luo Q et al (2022) The non-linear relationship between triglyceride-glucose index and risk of chronic kidney disease in hypertensive patients with abnormal glucose metabolism: a cohort study. Front Med (Lausanne) 9:1018083. https://doi.org/10.3389/fmed.2022.1018083
Shang J, Yu D, Cai Y, Wang Z, Zhao B, Zhao Z et al (2019) The triglyceride glucose index can predict newly diagnosed biopsy-proven diabetic nephropathy in type 2 diabetes: a nested case control study. Medicine (Baltimore) 98:e17995. https://doi.org/10.1097/MD.0000000000017995
Jiang H, Li L, Liu J, Xu B, Chen S, Zhu W et al (2021) Triglyceride-glucose index as a novel biomarker in the occurrence of kidney stones: a cross-sectional population-based study. Int J Gen Med 14:6233–6244. https://doi.org/10.2147/IJGM.S334821
Liu XC, He GD, Lo K, Huang YQ, Feng YQ (2020) The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med 7:628109. https://doi.org/10.3389/fcvm.2020.628109
Chen T, Wang X, Wang X, Chen H, Xiao H, Tang H et al (2020) Comparison of novel metabolic indices in estimation of chronic kidney diseases in a southern Chinese population. Diabetes Metab Syndr Obes 13:4919–4927. https://doi.org/10.2147/DMSO.S286565
Khajehdehi P, Malekmakan L, Pakfetrat M, Roozbeh J, Sayadi M (2014) Prevalence of chronic kidney disease and its contributing risk factors in southern Iran: a cross-sectional adult population-based study. Iran J Kidney Dis 8:109–115
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AJ (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808. https://doi.org/10.1172/JCI19246
Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC et al (2018) Insulin resistance causes inflammation in adipose tissue. J Clin Invest 128:1538–1550. https://doi.org/10.1172/JCI96139
Bolton CH, Downs LG, Victory JG, Dwight JF, Tomson CR, Mackness MI et al (2001) Endothelial dysfunction in chronic renal failure: roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant 16:1189–1197. https://doi.org/10.1093/ndt/16.6.1189
Kim HJ, Vaziri ND (2010) Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol 298:F662–F671. https://doi.org/10.1152/ajprenal.00421.2009
Matsushita K, Ballew SH, Wang AY, Kalyesubula R, Schaeffner E, Agarwal R (2022) Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol 18:696–707. https://doi.org/10.1038/s41581-022-00616-6
Alizargar J, Bai CH, Hsieh NC, Wu SV (2020) Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol 19:8. https://doi.org/10.1186/s12933-019-0982-2
Li H, Zuo Y, Qian F, Chen S, Tian X, Wang P et al (2022) Triglyceride-glucose index variability and incident cardiovascular disease: a prospective cohort study. Cardiovasc Diabetol 21:105. https://doi.org/10.1186/s12933-022-01541-5
Jafari A, Najafipour H, Shadkam M, Aminizadeh S (2023) Evaluation of the novel three lipid indices for predicting five- and ten-year incidence of cardiovascular disease: findings from Kerman coronary artery disease risk factors study (KERCADRS). Lipids Health Dis 22:169. https://doi.org/10.1186/s12944-023-01932-x
Acknowledgements
We thank the National Health and Nutrition Examination Surveys for providing the data.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
XL: software, data analysis, and writing—original draft. LW: writing—original draft, formal analysis, and methodology. HZ: data analysis. HX: conceptualization, funding acquisition, and writing— reviewing and editing. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics statement
All research obtained approval from the NCHS Research Institutional Review Board. The user agreement data is available Online.
Informed consent
All informed consent was received from qualified subjects prior to the commencement of data collection and NHANES health screening.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11255_2024_4103_MOESM3_ESM.tif
Supplementary Fig S3 ROC curves and the AUC values of the five markers (LAP, VAI, and TyG-BMI index) in diagnosing CVD. (TIF 10040 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Wang, L., Zhou, H. et al. Association between triglyceride-glucose index and chronic kidney disease: results from NHANES 1999–2020. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-04103-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-04103-8