Abstract
Purpose
Persistent hypercalcemia after kidney transplantation (KTx) may cause nephrocalcinosis and graft dysfunction. The aim of this study was to evaluate patients with hypercalcemia and assess its effect on tubulointerstitial calcification.
Methods
A total of 247 recipients were enrolled. Transient and persistent hypercalcemia was defined as hypercalcemia (corrected serum calcium >10.2 mg/dL) persisting for 6 and 12 months after KTx, respectively. The severity of calcification in the 0-h, 6- and 12-month protocol biopsies of patients with transient (n = 8) and persistent hypercalcemia (n = 20) was compared with a matched control group (n = 28).
Results
Twenty-eight patients were hypercalcemic at 6 months posttransplantation. Serum calcium levels were normalized in eight of them at the end of the first year. Dialysis duration was a positive predictor of persistent hypercalcemia. Tubulointerstitial calcification was detected in 70.6 and 90 % of patients with persistent hypercalcemia at 6 and 12 months posttransplantation, respectively. In 20 % of patients with transient hypercalcemia, severity of calcification regressed at 12 months posttransplantation along with normalization of serum calcium levels. Graft functions and histopathological findings (ci, ct, ci + ct, cv, ah, percentage of sclerotic glomeruli) were not different at 6 and 12 months posttransplantation.
Conclusions
Hypercalcemia and persistent hyperparathyroidism are not rare after KTx. Tubulointerstitial calcification is more common and progressive among patients with persistent hypercalcemia. Normalization of calcium levels may contribute to regression of calcification in some patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nephrocalcinosis is characterized by tubular and interstitial deposits of calcium in the form of calcium oxalate or calcium phosphate. Calcium oxalate deposition can be secondary to hereditary diseases like primary hyperoxaluria or acquired causes such as enteric hyperoxaluria, toxic exposures and excessive dietary intake of oxalate [1]. The most common underlying causes of calcium phosphate deposition are conditions related to hypercalcemia and hyperphosphatemia. The histopathological properties help to distinguish the type of calcium salts. In sections stained with hematoxylin–eosin, oxalate crystals appear translucent, whereas calcium phosphate crystals often appear as blue or purple deposits. The von Kossa stain reacts with the phosphate moiety of calcium phosphate [1, 2].
In renal allografts, tubulointerstitial calcification is a common finding and occurs secondary to hyperparathyroidism, calcineurin inhibitor toxicity and delayed graft function [3–8]. Although kidney transplantation (KTx) improves most of the metabolic disturbances of chronic kidney disease, hyperparathyroidism may persist and cause hypercalcemia, which may lead to adverse effects like allograft and vascular calcification [3, 9].
The aim of this study was to determine the incidence and clinical course of persistent hypercalcemia and to determine the effect of hypercalcemia on tubulointerstitial calcification by evaluating protocol biopsies of good functioning allografts during the first year after KTx.
Materials and methods
A total of 281 consecutive patients who underwent KTx between January 2009 and December 2011 were evaluated for the study. After the exclusion of 34 patients due to graft dysfunction (serum creatinine level ≥3 mg/dL during the first year), patient death, loss to follow-up or lack of data regarding calcium level during the first year after KTx, a total of 247 patients were enrolled. Demographic, clinical and laboratory data were collected retrospectively from medical records. Pretransplantation parathyroid hormone (PTH) levels were obtained within 6 months before transplantation. Serum calcium was normalized to a serum albumin of 4.0 mg/dL, assuming that the calcium concentration changed by 0.8 mg/dL for every 1 g/dL change in serum albumin concentration. Hypercalcemia was defined as corrected serum calcium level >10.2 mg/dL. The patients whose serum calcium levels were normal at 6 and 12 months posttransplantation were included in normocalcemia group (n = 219). Patients whose serum calcium levels were high at 6 months and had normalized at 12 months of KTx were diagnosed transient hypercalcemia (n = 8). Patients whose serum calcium levels were high at 6 and 12 months posttransplantation were included in persistent hypercalcemia group (n = 20). From the normocalcemia group, a control group (n = 28) was chosen which matched the hypercalcemic patients (both transient and persistent, total n = 28) in age (40 ± 11 years vs 42 ± 9 years), gender (number of males, n = 14 vs n = 14), dialysis duration (median 78 vs 78 months), delayed graft function (n = 9 vs n = 9), deceased donor type (n = 15 vs n = 15) and immunosuppressive regimen (CNI-based regimen, n = 26 vs n = 25) in order to evaluate the severity of the allograft tubulointerstitial calcification and chronic histopathological changes.
The policy of our center is to perform a kidney biopsy (0-h biopsy) after revascularization and protocol biopsies at 6 and 12 months after transplantation unless patients do not give consent or have a contraindication for biopsy. The 0-h, 6- and 12-month protocol biopsies of patients with transient hypercalcemia, persistent hypercalcemia and the matched control group were evaluated. Analyses included 56, 50 and 56 biopsies at 0 h, 6 and 12 months, respectively. Besides routine staining with hematoxylin–eosin, periodic acid–Schiff, silver and Masson’s trichrome, von Kossa staining was performed on formalin (4 %)-fixed paraffin-embedded biopsies for visualization of tubulointerstitial calcium phosphate deposits. Tubulointerstitial calcification was evaluated according to extent (none, mild, moderate, severe, scored from 0 to 3, respectively) by one pathologist who was unaware of the serum calcium levels of the patients (Fig. 1). Histological slides were systematically analyzed, and scoring of microcalcification was evaluated in the slides stained with von Kossa. The score of slides with no calcification was defined as none. The number of calcification foci ≤3 in serial histological slides, diameter of foci smaller than diameter of tubules and ≤3 foci in a microscopic area was defined as mild microcalcification. The number of calcification foci of >3 in serial histological slides, diameter smaller than diameter of tubules and ≤3 foci in a microscopic area was defined as moderate microcalcification. The number of calcification foci of >3 in serial histological slides, diameter larger than diameter of tubules or >3 foci in a microscopic area was defined as severe microcalcification. Biopsies were evaluated according to Banff classification [10]. The number of glomeruli and percentage of sclerotic glomeruli were recorded. Histopathological grading for interstitial fibrosis (ci), tubular atrophy (ct), fibrous intimal thickening (cv) and arteriolar hyalinosis (ah) was performed, and the sum of ci and ct was used as interstitial fibrosis and tubular atrophy (IFTA) score.
Light microscopy of allograft histopathology demonstrating the scoring of tubulointerstitial calcifications. The kidney tissue was stained with von Kossa which is specific for calcium phosphate. a None (Score 0), b Mild (Score 1), c Moderate (Score 2), d Severe (Score 3); the last slide was obtained from a native kidney biopsy (von Kossa, original magnification ×10)
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) software version 15.0 for Windows. Continuous variables were presented as mean ± standard deviation or median and interquartile ranges and categorical variables as frequency and percentage. Numerical data were compared with one-way ANOVA or with Kruskal–Wallis and Mann–Whitney U test according to distribution of the data. Chi-square tests were used to compare categorical data. Correlation analyses were performed by Spearman’s analysis. Predictors of persistent hypercalcemia were evaluated by binary regression analysis based on age, PTH level before transplantation, duration of dialysis, type and age of the donor. Predictors of presence of tubulointerstitial calcification in 12-month protocol biopsies were evaluated by age, duration of dialysis, type of donor, serum creatinine and calcium levels at 6 and 12 months, calcium phosphorus product at 6 and 12 months and PTH level before and 12 months after transplantation. A p value <0.05 was considered to be statistically significant.
Results
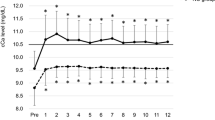
Of 247 patients, 28 (11.3 %) patients were hypercalcemic at 6 months posttransplantation. At the end of the first year, eight of those patients’ calcium levels had normalized (transient hypercalcemia group) and 20 patients remained hypercalcemic (persistent hypercalcemia group). Demographic characteristics and laboratory findings are presented in Table 1. Persistent hypercalcemia was associated with significantly longer dialysis duration and higher PTH levels before and 1 year after KTx when compared with the normocalcemia group. The binary regression analysis model included age, PTH level before transplantation, duration of dialysis, type and age of donor; duration of dialysis was found to be the only significant predictor of persistent hypercalcemia (Exp (B) = 1.018, 95.0 % CI 1.009–1.028, p < 0.001).
In transient hypercalcemia group, none of the patients received phosphorus supplementation and hypercalcemia was treated with conservative recommendations such as adequate fluid intake and low calcium diet. In persistent hypercalcemia group, two patients temporarily received phosphorus supplementation within the first month of transplantation. None of the patients received calcium-sensing receptor activators such as cinacalcet, vitamin D supplementation or vitamin D receptor activators.
Histopathological data based on tubulointerstitial calcification
The severity of calcification was evaluated in the 0-h, 6- and 12-month graft biopsies of patients with transient hypercalcemia (n = 8), persistent hypercalcemia (n = 20) and the matched control group (n = 28). Significant differences were detected in percentages of patients with different calcification scores between the groups at 6 and 12 months posttransplantation (Table 2). None of the 0-h biopsies showed microcalcification. In control group, 23 % of 6-month and 32 % of 12-month graft biopsies showed microcalcification. At 6 months posttransplantation, 57.1 % patients with transient hypercalcemia had microcalcification, but after regression of hypercalcemia, microcalcification disappeared in 20 % of the group at 12 months posttransplantation. In persistent hypercalcemia group, tubulointerstitial microcalcification was detected in 70.6 % of 6-month biopsies and 90 % of 12-month biopsies. While 35.3 and 40 % of patients with persistent hypercalcemia had moderate microcalcification at 6 and 12 months posttransplantation, respectively, the number of patients with moderate calcification was very small in control group and transient hypercalcemia group at 6 and 12 months.
Tubulointerstitial calcification at 12 months posttransplantation was positively correlated with duration of dialysis, pretransplantation PTH level and calcium levels at 6 and 12 months after KTx, PTH level 1 year posttransplantation but negatively correlated with phosphorus levels and calcium phosphorus product at 6 and 12 months of KTx (Table 3). In binary regression analysis model including age, duration of dialysis, type of donor, serum creatinine and calcium levels at 6 and 12 months, calcium phosphorus product at 6 and 12 months and PTH level before and 12 months after transplantation, serum calcium level at 12th month was the predictor of presence of microcalcification at 12-month protocol biopsies (Exp (B) = 4.972, 95.0 % CI 1.189–20.791, p = 0.028).
Means scores of allograft calcification at 0-h, 6- and 12-month protocol biopsies are presented in Table 4. Among persistent hypercalcemia group, calcification was more extensive than matched control group at 6 and 12 months posttransplantation and than transient hypercalcemia group at 12 months posttransplantation. Histopathological findings based on chronic changes (ci, ct, ci + ct, cv, ah and percentage of sclerotic glomeruli) were not significantly different between three groups at 6 and 12 months posttransplantation (Table 4). None of these changes were correlated with serum calcium levels at 6 and 12 months posttransplantation.
Discussion
In the present study including 247 kidney transplant recipients, 11.3 and 8.1 % were hypercalcemic at 6 and 12 months, respectively. Duration of dialysis was the main predictor of persistent hypercalcemia. In control group, incidences of patients with microcalcification were 23.1 and 32.1 % at 6 and 12 months posttransplantation, and majority of them were mild. In transient hypercalcemia group, mild microcalcification was detected in 57.1 % of patients at 6 months. Furthermore, in the same group, microcalcification disappeared in 20 % of patients at 12 months posttransplantation, along with normalization of serum calcium levels. The number of patients with mild and moderate microcalcification increased from 6 to 12 months posttransplantation in persistent hypercalcemia group.
The metabolic disturbances of chronic kidney disease may improve to a large extent after a successful KTx. Nonetheless, hypercalcemia and secondary hyperparathyroidism are among the most common abnormalities that persist after KTx, with a reported incidence of up to 50 % [11–16]. This variability in the incidence may be attributable to various factors such as the use of different serum calcium levels for diagnosis, evaluation of ionized or total calcium level, whether or not calcium levels were corrected based on albumin, and the timing of diagnosis. In concordance with previous studies, the rate of hypercalcemia decreased progressively during the first year in the present study.
The main cause of hypercalcemia after KTx is persistent hyperparathyroidism, which is much more likely to occur in recipients with a longer history of dialysis and therefore more severe secondary hyperparathyroidism [14, 17–19]. Our study established longer dialysis duration as a risk factor for persistent hypercalcemia. The patients with persistent hypercalcemia were transplanted mostly from deceased donors after a long period of dialysis. Two of the important consequences of longer waiting times for KTx are severe persistent hyperparathyroidism and hypercalcemia. In a previous study, patients with persistent hypercalcemia were found to have significantly elevated serum levels of PTH, calcium and phosphorus at the time of KTx and to spend a longer time on dialysis [17]. Dialysis duration and PTH level before KTx have been determined as predictors of posttransplantation hypercalcemia [19].
Calcium depositions, mostly as calcium phosphate salts, in the tubulointerstitium of the graft are frequently observed [3–6]. Microcalcification is detected in 5 % of 0-h biopsies, but the rate increases progressively after transplantation [4, 6]. In 114 patients with combined pancreas–kidney transplantation, the rate of tubular microcalcification was 40 % at 1 year and increased to 80 % by 10 years posttransplantation [4]. Delayed graft function, hyperparathyroidism, hypercalcemia and calcineurin inhibitor toxicity were associated with tubulointerstitial calcification [3–6]. In a previous study, nephrocalcinosis was observed in 17 % of patients within the first week of KTx in conjunction with delayed graft function [3]. Unlike early microcalcification, persistent hypercalcemia and hyperparathyroidism are the main causes of microcalcification developing over months or years after KTx, but the contribution of calcineurin inhibitors is controversial [4–6]. On the other hand, severe hypercalcemia may also cause calcification in the early period after KTx. In a few case reports, severe and uncontrolled hypercalcemia and hyperparathyroidism caused massive intratubular calcifications and graft dysfunction immediately after KTx [20, 21]. In one of these cases, graft dysfunction resolved after urgent parathyroidectomy [21]. Despite these catastrophic presentations, most patients with hypercalcemia have normal graft function. Nevertheless, they may have allograft calcification which can be detected only by protocol biopsies. Previous studies showed that hypercalcemia was associated with more severe calcium deposition and some demonstrated its adverse effects on graft survival [5, 7, 8]. Binary regression analysis model including age, duration of dialysis, type of donor, serum creatinine and calcium levels at 6 and 12 months, calcium phosphorus product at 6 and 12 months and PTH level before and 12 months after transplantation demonstrated serum calcium level at 12th month as a predictor of tubulointerstitial microcalcification in 12-month protocol biopsies. We could not evaluate the effect of PTH levels at 6th month posttransplantation due to lack of PTH levels in normocalcemic control group. Number of patients with microcalcification was significantly higher in hypercalcemic groups when compared with normocalcemic control group. Moreover, tubulointerstitial microcalcification was progressive in patients with persistently high calcium levels during the first year. These findings indicate that posttransplantation hypercalcemia is associated with tubulointerstitial calcification after KTx. As duration and severity of hypercalcemia increase, severity of tubulointerstitial calcification increases. However, regression of microcalcification in 12-month graft biopsies of some patients with transient hypercalcemia may indicate that tubulointerstitial microcalcification secondary to hypercalcemia may be reversible, especially in early years of KTx.
There are conflicting data in the literature regarding the association of hypercalcemia and allograft calcification with graft dysfunction. Some studies demonstrated adverse effects on graft survival [5, 7, 8, 22]. In a previous study, Gwinner et al. evaluated the effect of allograft calcification in serial protocol biopsies to analyze the relationship with graft outcome at 1 year posttransplantation. In this study, patients with calcification had significantly higher serum calcium levels and an inferior graft outcome was detected with higher PTH levels for patients with calcification [5]. Schwarz et al. [8] determined nephrocalcinosis to be a risk factor for chronic allograft nephropathy in a protocol biopsy study. Calcium oxalate deposits were detected in half of graft biopsies of recipients with early graft dysfunction. A lower 12-year graft survival rate was shown in the presence of early nephrocalcinosis related to early graft dysfunction. Although the underlying reason was different in this study, it demonstrated that calcification is an independent predictor for long-term graft functions [22]. In two different studies, persistent hypercalcemia and calcium phosphorus product were found to be risk factors for graft failure [7, 13]. In contrast, a study in which 44 % of graft biopsies showed calcification did not demonstrate a correlation between microcalcification and serum calcium levels or outcome in pediatric patients [23]. In our study, neither histopathological findings based on chronic changes and percentage of sclerotic glomeruli nor graft functions at 6 and 12 months were significantly different between patients with or without hypercalcemia. However, these time points are too early to evaluate the effect of microcalcification on graft function and chronic histopathological findings. Persistently high levels of serum calcium and subsequent tubulointerstitial microcalcification might adversely affect graft function in the long term.
This is the first study that compared the allograft tubulointerstitial calcification in 0-h, 6- and 12-month protocol biopsies of recipients with or without hypercalcemia. The main limitations include the retrospective design of the study and limited number of patients. Another limitation is that the study did not include data regarding urinary excretion of calcium and phosphorus, which may also affect calcification of the allograft. The effect of CNI levels on occurrence or progression of microcalcification was not analyzed. Lack of analysis with regard to the effects of vitamin D levels on microcalcification after KTx and lack of PTH levels at 6th month in normocalcemic matched control group are the other limitations.
In conclusion, persistent hypercalcemia is a common finding, especially among patients with long dialysis duration and severe secondary hyperparathyroidism before KTx. Tubulointerstitial calcification is more common in the presence of hypercalcemia, but may regress after normalization of serum calcium levels. In most recipients, hypercalcemia and tubulointerstitial calcification occur in the presence of good graft functions during the first years of KTx. However, they may contribute to graft dysfunction in the long term.
References
Herlitz LC, D’Agati VD, Markowitz GS (2012) Crystalline nephropathies. Arch Pathol Lab Med 136:713–720
Wiech T, Hopfer H, Gaspert A, Banyai-Falger S, Hausberg M, Schroder J, Werner M, Mihatsch MJ (2012) Histopathological patterns of nephrocalcinosis: a phosphate type can be distinguished from a calcium type. Nephrol Dial Transplant 27:1122–1131
Boom H, Mallat MJ, de Fijter JW, Paul LC, Bruijn JA, van Es LA (2004) Calcium levels as a risk factor for delayed graft function. Transplantation 77:868–873
Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR (2003) The natural history of chronic allograft nephropathy. N Engl J Med 349:2326–2333
Gwinner W, Suppa S, Mengel M, Hoy L, Kreipe HH, Haller H, Schwarz A (2005) Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications. Am J Transplant 5:1934–1941
Evenepoel P, Lerut E, Naesens M, Bammens B, Claes K, Kuypers D, Vermeersch P, Meijers B, Van Damme B, Vanrenterghem Y (2009) Localization, etiology and impact of calcium phosphate deposits in renal allografts. Am J Transplant 9:2470–2478
Ozdemir FN, Afsar B, Akgul A, Usluogullari C, Akcay A, Haberal M (2006) Persistent hypercalcemia is a significant risk factor for graft dysfunction in renal transplantation recipients. Transplant Proc 38:480–482
Schwarz A, Mengel M, Gwinner W, Radermacher J, Hiss M, Kreipe H, Haller H (2005) Risk factors for chronic allograft nephropathy after renal transplantation: a protocol biopsy study. Kidney Int 67:341–348
Messa P, Cafforio C, Alfieri C (2011) Clinical impact of hypercalcemia in kidney transplant. Int J Nephrol 2011:906832
Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y et al (1999) The banff 97 working classification of renal allograft pathology. Kidney Int 55:713–723
Torres A, Lorenzo V, Salido E (2002) Calcium metabolism and skeletal problems after transplantation. J Am Soc Nephrol 13:551–558
Heaf J, Tvedegaard E, Kanstrup IL, Fogh-Andersen N (2003) Hyperparathyroidism and long-term bone loss after renal transplantation. Clin Transplant 17:268–274
Egbuna OI, Taylor JG, Bushinsky DA, Zand MS (2007) Elevated calcium phosphate product after renal transplantation is a risk factor for graft failure. Clin Transplant 21:558–566
Evenepoel P, Van Den Bergh B, Naesens M, De Jonge H, Bammens B, Claes K, Kuypers D, Vanrenterghem Y (2009) Calcium metabolism in the early posttransplantation period. Clin J Am Soc Nephrol 4:665–672
Leca N, Laftavi M, Gundroo A, Kohli R, Min I, Karam J, Sridhar N, Blessios G, Venuto R, Pankewycz O (2006) Early and severe hyperparathyroidism associated with hypercalcemia after renal transplant treated with cinacalcet. Am J Transplant 6:2391–2395
Reinhardt W, Bartelworth H, Jockenhovel F, Schmidt-Gayk H, Witzke O, Wagner K, Heemann UW, Reinwein D, Philipp T, Mann K (1998) Sequential changes of biochemical bone parameters after kidney transplantation. Nephrol Dial Transplant 13:436–442
Evenepoel P, Claes K, Kuypers D, Maes B, Bammens B, Vanrenterghem Y (2004) Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant 19:1281–1287
Nakamura M, Tanaka K, Marui Y, Tomikawa S (2013) Clinicopathological analysis of persistent hypercalcemia and hyperparathyroidism after kidney transplantation in long-term dialysis patients. Ther Apher Dial 17:551–556
Kim YJ, Kim MG, Jeon HJ, Ro H, Park HC, Jeong JC, Oh KH, Ha J, Yang J, Ahn C (2012) Clinical manifestations of hypercalcemia and hypophosphatemia after kidney transplantation. Transplant Proc 44:651–656
Iguchi S, Nishi S, Shinbo J, Iino N, Kazama JJ, Shimada H, Ueno M, Saitou K, Tanigawa T, Takahashi K, Gejyo F (2001) Intratubular calcification in a post-renal transplanted patient with secondary hyperparathyroidism. Clin Transplant 15(Suppl 5):51–54
Sewpaul A, Sayer JA, Mohamed MA, Ahmed A, Shaw M, Prabhu VR, Wood K, Jones NA, Talbot D, Kanagasundaram NS (2007) Rapid onset intratubular calcification following renal transplantation requiring urgent parathyroidectomy. Clin Nephrol 68:47–51
Pinheiro HS, Camara NO, Osaki KS, De Moura LA, Pacheco-Silva A (2005) Early presence of calcium oxalate deposition in kidney graft biopsies is associated with poor long-term graft survival. Am J Transplant 5:323–329
Habbig S, Beck BB, Feldkotter M, Korber F, Laffeber C, Verkoelen C, Mihatsch MJ, Hoppe B (2009) Renal allograft calcification–prevalence and etiology in pediatric patients. Am J Nephrol 30:194–200
Acknowledgments
The authors also acknowledge that he or she participated sufficiently in the work to take public responsibility for its content. All authors gave final approval of the version to be published. The authors hereby confirm that neither the manuscript nor any part of it has been published or is being considered for publication elsewhere, except in abstract form.
Author contributions
Aygül Çeltik participated in study design, data collection, interpretation of the results, writing of the article and patient management. Sait Şen performed pathological analysis of the allograft biopsies. Mümtaz Yılmaz participated in data collection and patient management. Meltem Seziş Demirci participated in data collection and patient management. Gülay Aşçı participated in data collection and interpretation of the results. Abdülkerim Furkan Tamer participated in data collection and patient management. Banu Sarsık participated in data collection and interpretation of the results. Cüneyt Hoşcoşkun participated in patient management and writing of the article. Hüseyin Töz participated in study design, data collection, interpretation of the results, writing of the article and patient management. Ercan Ok participated in interpretation of the results and writing of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All the biopsies and blood samples included in this study were performed according to our clinical follow-up and biopsy protocol. Since no additional biopsy or blood samples were taken for the purpose of this retrospective study, informed consent was not obtained from any individuals included in the study and approval of the ethics committee was not required.
Rights and permissions
About this article
Cite this article
Çeltik, A., Şen, S., Yılmaz, M. et al. The effect of hypercalcemia on allograft calcification after kidney transplantation. Int Urol Nephrol 48, 1919–1925 (2016). https://doi.org/10.1007/s11255-016-1391-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1391-z