Abstract
Background
Hypercalcemia (HC) after kidney transplantation (KTx) can deteriorate both graft and patient survival. However, HC as a clinical condition and its clinical significance after KTx remain unknown. We evaluated the prevalence and risk factors of early HC after KTx.
Methods
We performed a nested case–control study using a cohort of 100 KTx patients. KTx patients were divided into the HC and normocalcemia (NC) groups based on the baseline serum-corrected calcium (cCa) levels (≥ 10.5 and < 10.5 mg/dL) within 1 year after KTx.
Results
Overall, the median value of maximum serum cCa level within 1 year after KTx was 10.1 (9.1–13.8) mg/dL. Of the 100 KTx patients within the cohort, 31 patients (31.0%) were classified as the HC group. The maximum serum cCa level was reached significantly earlier in the HC group compared with the NC group (2 vs. 4 months, p = 0.024). In univariate analysis, the risk factors of early HC after KTx were dialysis duration ≥ 10 years, serum cCa level the day before KTx, and cinacalcet administration before KTx. Among these risk factors, serum cCa level the day before KTx and cinacalcet administration before KTx were identified as significant independent risk factors of early HC after KTx in multivariate analysis.
Conclusions
One-third of the KTx patients presented early HC within 1 year after KTx. Early HC after KTx resulted from persistent hyperparathyroidism. Therapeutic strategies to manage HC after KTx must be established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In patients with end-stage kidney disease (ESKD), kidney transplantation (KTx) is expected to improve not only renal function but also chronic kidney disease–mineral and bone disorder (CKD–MBD). In fact, KTx can ameliorate most of the physiologic and metabolic derangements caused by ESKD. As a result, successful KTx improves the quality of life and increases survival of these patients, compared with the long-term dialysis treatment [1].
However, successful KTx cannot always completely normalize renal function. Indeed, even 1 year after KTx, most patients improve only to CKD stage 3T [2]. Because the abnormalities of mineral metabolism occur early after CKD onset [3], de novo CKD–MBD may arise immediately after KTx. Post-transplant CKD–MBD reflects both the effects of previous CKD–MBD persisting after KTx and de novo CKD–MBD [2].
Hypercalcemia (HC) is a common result of abnormal mineral metabolism in approximately 5–50% of KTx patients [4]. Some reports showed that HC can affect the postoperative outcomes of KTx patients. For instance, KTx patients with HC present early calcification of renal allografts [5]. KTx patients with persistent HC present progressive tubulointerstitial calcification more commonly than those without HC [6]. Further, persistent HC after KTx has been identified a significant risk factor for graft dysfunction [7]. Furthermore, HC after KTx has been identified as an independent risk for recipient death and death-censored graft loss [8]. Reportedly, HC adversely affects the graft, by leading to conditions such as nephrocalcinosis, and it may affect other organs by causing vascular calcifications, erythrocytosis, pancreatitis, among other conditions [9]. However, transplant physicians may not necessarily recognize the negative effects of HC after KTx. Further, not only the clinical significance but also the actual clinical characteristics of HC after KTx remain largely unknown. Therefore, we aimed to clarify the prevalence and risk factors of early HC after KTx by conducting a nested case–control study within a cohort.
Patients and methods
Subjects and study design
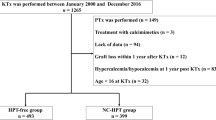
From April 2014 to March 2017, a total of 100 consecutive adult patients with ESKD underwent a KTx at Jichi Medical University Hospital. We performed a nested case–control study within a cohort of 100 KTx patients. We divided 100 KTx patients into two groups based on their baseline serum calcium (Ca) level, into the HC and normocalcemia (NC) groups. The NC group consisted of all the patients who were excluded from the HC group. We retrospectively evaluated and compared these groups, by setting the HC group as cases and the NC group as controls within the cohort.
Data collection and definition
We reviewed the medical charts of all patients to extract data on their baseline demographic and clinical characteristics, including sex, age, primary cause of ESKD, modality and duration of dialysis, donor type (living or deceased), donor age, ABO compatibility, presence of donor-specific antibodies before KTx, immunosuppressive therapy, history of parathyroidectomy, history of cinacalcet administration before KTx, and laboratory test results.
Nonfasting serum samples were collected the day before KTx and every month thereafter up to 1 year after KTx. Serum Ca, phosphorus (P), alkaline phosphatase (ALP), creatinine, and intact parathyroid hormone (iPTH) were measured using standard methods. Ca levels were adjusted to albumin levels using the Payne’s equation: corrected Ca (cCa) = Ca + [4.0 − albumin (g/dL)] if the serum albumin was < 4 g/dL. HC was defined as a baseline serum cCa level ≥ 10.5 mg/dL within 1 year after KTx. NC was defined as a serum cCa level < 10.5 mg/dL at every measurement within 1 year after KTx. The estimated glomerular filtration rate (eGFR) was measured using the Japanese GFR equation [10].
Immunosuppressive therapy and desensitization protocol
All KTx patients received immunosuppressive therapy for induction, which comprised tacrolimus (0.1 mg/kg/day), mycophenolate mofetil (30 mg/kg/day), methylprednisolone (starting dose, 250 or 500 mg/day), and basiliximab (20 mg/day) at postoperative days 0 and 4. Desensitization for ABO-incompatible and donor-specific antibody-positive patients was performed before KTx. According to the quantity of the antibody, patients underwent between 0 and 4 sessions of double-filtration plasmapheresis or plasma exchange and received 1–2 doses of rituximab 100 mg. Starting at 3 months after KTx, all patients were maintained on a triple-drug combination therapy comprising tacrolimus (trough level 5 ng/mL), mycophenolate mofetil (1000 mg/day), and methylprednisolone (4 mg/day). Patients with exacerbation of diabetes after KTx used everolimus (trough level 3–5 ng/mL) rather than methylprednisolone.
Statistical analysis
For continuous variables, we used means with standard deviations or medians with ranges as appropriate. We represented categorical variables as the number of patients and percentages. We used unpaired or paired t tests or Mann–Whitney U tests to assess continuous variables, as appropriate. We compared categorical variables using Fisher’s exact test. For correlation analysis, we used the Pearson’s product–moment correlation coefficient.
To identify the risk factors of HC, we employed a logistic regression analysis. Variables exhibiting statistically significant differences in the univariate analysis were tested in a multivariate analysis. Further, clinically relevant factors with p values of < 0.10 were included in the multivariate analysis to investigate their effects. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [11], a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, EZR is a modified version of the R commander designed to add statistical functions frequently used in biostatistics. Two-tailed p values of < 0.05 were considered statistically significant.
Results
Prevalence of early HC after KTx
In the entire study cohort, the mean and median values of maximum serum cCa within 1 year after KTx were 10.36 ± 0.79 mg/dL and 10.1 (9.1–13.8) mg/dL, respectively. Of the 100 KTx patients within the cohort, 31 patients (31.0%) were classified in the HC group because of baseline serum cCa level ≥ 10.5 mg/dL within 1 year after KTx (Fig. 1). After excluding the HC group, the NC group consisted of 69 patients (69.0%).
Patient characteristics
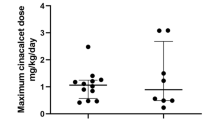
Among baseline patient characteristics the day before KTx, dialysis duration in the HC group was significantly longer than that in the NC group (Table 1). Serum cCa level in the HC group was significantly higher than that in the NC group (Table 1). Active vitamin D and cinacalcet administration in the HC group were significantly greater than those in the NC group (Table 1). Other than these, no significant differences in baseline characteristics were observed between groups the day before KTx (Table 1).
Serum cCa level (maximum, correlation before and after KTx)
The median time at which the maximum serum cCa level was reached within 1 year after KTx in the HC and NC groups was 2 (1–9) months and 4 (1–9) months, respectively (Fig. 2). The maximum serum cCa level was reached significantly earlier in the HC group than the NC group (p = 0.024).
A significant positive correlation was found between serum cCa level the day before KTx and maximum serum cCa level in the NC group (Fig. 3). Conversely, no significant correlation was found between these parameters in the HC group (Fig. 3).
Serum P, ALP, iPTH levels and graft outcomes after KTx
One year after KTx, the serum P level in the HC group was significantly lower than that in the NC group, and the serum ALP level in the HC group was significantly higher than that in the NC group (Table 2). The iPTH level was comparable between the HC and NC groups (Table 2). No patients were treated with cinacalcet after KTx in either group (Table 2). More subjects of the HC group underwent parathyroidectomy than those of the NC group (Table 2). Regarding graft outcomes, such as graft function and acute rejection, no significant differences were observed between groups (Table 2).
Risk factors of early HC after KTx
In the univariate analysis, the risk factors of early HC after KTx were dialysis duration of ≥ 10 years, serum cCa level the day before KTx, active vitamin D and cinacalcet administration before KTx (Table 3). Among these risk factors, serum cCa level the day before KTx and cinacalcet administration before KTx were identified by multivariate analysis as significant independent risk factors of early HC after KTx (Table 3). Conversely, dialysis duration and active vitamin D administration were not found to be an independent risk factor of early HC after KTx (Table 3).
Discussion
This study identified important short-term clinical aspects of CKD–MBD after KTx. To identify relevant predictors of progression of CKD–MBD that may lead to deterioration of the patient and graft survival, we examined the prevalence and risk factors of early HC after KTx. The prevalence of HC within 1 year after KTx reached 31% within this cohort. The independent risk factors of early HC after KTx were serum cCa level the day before KTx and cinacalcet administration before KTx.
Surprisingly, 31% of the KTx patients in this cohort developed HC within 1 year after KTx. Based on numbers reported previously, the prevalence of HC after KTx can range from 5 to 50% [4]. The reason for the difference in the prevalence observed in this cohort compared to that reported previously may be a difference in the definition of HC after KTx (serum Ca level > 2.55–2.95 mmol/L = 10.2–11.8 mg/dL) [9]. Within this range, we established the cut-off point of serum cCa level as 10.5 mg/dL, close to the normal upper limit, to clarify the actual prevalence of HC and predict early HC after KTx. However, it is an undeniable fact that HC is commonly observed after KTx. Moreover, in the studied cohort, HC developed as early as 2 months after KTx. Such an early HC onset after KTx is likely the result of a previous CKD–MBD that persisted after KTx. Although transplant physicians frequently encounter early-onset HC after KTx, the most important issue is that there seems to be a lack of recognition of the negative effects of HC on patient and graft outcomes. Conceivably, it might also be true that we lack effective interventions to manage HC after KTx.
Thus, we investigated the risk factors of early HC within 1 year after KTx to clarify predictors of CKD–MBD after KTx. As a result of multivariate analysis, the independent risk factors of early HC after KTx were serum cCa level the day before KTx and cinacalcet administration before KTx. Our results indicate that early HC after KTx is likely to result from persistent hyperparathyroidism. It has been reported previously that high Ca and iPTH levels before KTx were predictors of prolonged HC [12]. Moreover, iPTH was found to be more strongly correlated with HC at 1 year after KTx than any other factor [13]. Serum ALP level, a bone turnover marker, was higher in the HC group than in the NC group 1 year after KTx in the present cohort. Conversely, the iPTH level the day before KTx was not identified as a relevant risk factor by either univariate or multivariate analyses. This may be because cinacalcet was administered for hyperparathyroidism before KTx. Therefore, we should focus not on the iPTH level but on cinacalcet administration before KTx for predicting early HC after KTx. Although the serum cCa level the day before KTx was one of the independent risk factors of early HC after KTx, no significant correlation was observed between serum cCa level in the HC group the day before KTx and the time at which the maximum serum cCa level was reached. We consider that this was caused by a decline of serum Ca in the effect of cinacalcet treatment before KTx. In contrast, no patients were treated with cinacalcet after KTx in the HC and NC groups because cinacalcet has not been approved for indication in public insurance for treating tertiary hyperparathyroidism after KTx in Japan, Despite the discontinuation of cinacalcet after KTx, iPTH levels were comparable between both groups. One of the underlying reasons is that parathyroidectomy, was performed as radical therapy for patients with severe hyperparathyroidism in the HC group. Another reason may be that iPTH levels decline substantially during the first 3 months after KTx [14]. Successful KTx might improve mild or moderate hyperparathyroidism.
In addition to cinacalcet, the widespread use of new calcimimetics, such as etelcalcetide, might improve the outcomes of patients with ESKD with hyperparathyroidism. However, discontinuation of calcimimetics after KTx may lead to HC in KTx patients. Therefore, patients with ESKD with hyperparathyroidism who are candidates for KTx should undergo parathyroidectomy before KTx. Still, a therapeutic strategy for HC after KTx must be established promptly.
The current study has several limitations. First, this study was a case–control study. Therefore, our results may be subject to unintentional selection bias and observation bias. However, we designed a nested case–control study within a cohort targeting all consecutively enrolled patients within a period to eliminate selection bias and determine the prevalence of HC after KTx as precisely as possible. Second, the small number of KTx patients at a single-center limited the certainty of our results. Thus, a large-scale study is needed to confirm our findings. Nonetheless, our results accurately reflect the actual situation of early HC after KTx in a single-center.
In conclusion, a third of KTx patients presented early HC within 1 year after KTx. The independent risk factors of early HC after KTx were serum cCa level the day before KTx and cinacalcet administration before KTx. To prevent deterioration of patient and graft survival due to progression of CKD–MBD, a therapeutic strategy for HC after KTx should be established promptly.
References
Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30.
Evenepoel P. Recovery versus persistence of disordered mineral metabolism in kidney transplant recipients. Semin Nephrol. 2013;33:191–203.
Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–8.
Messa P, Cafforio C, Alfieri C. Calcium and phosphate changes after renal transplantation. J Nephrol. 2010;23:175–81.
Gwinner W, Suppa S, Mengel M, Hoy L, Kreipe HH, Haller H, Schwarz A. Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications. Am J Transpl. 2005;5:1934–41.
Çeltik A, Şen S, Yılmaz M, Demirci MS, Aşçı G, Tamer AF, Sarsık B, Hoşcoşkun C, Töz H, Ok E. The effect of hypercalcemia on allograft calcification after kidney transplantation. Int Urol Nephrol. 2016;48:1919–25.
Ozdemir FN, Afsar B, Akgul A, Usluoğullari C, Akçay A, Haberal M. Persistent hypercalcemia is a significant risk factor for graft dysfunction in renal transplantation recipients. Transpl Proc. 2006;38:480–2.
Egbuna OI, Taylor JG, Bushinsky DA, Zand MS. Elevated calcium phosphate product after renal transplantation is a risk factor for graft failure. Clin Transpl. 2007;21:558–66.
Messa P, Cafforio C, Alfieri C. Clinical impact of hypercalcemia in kidney transplant. Int J Nephrol. 2011;2011:906832.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Collaborators developing the Japanese equation for estimated GFR. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Nakamura M, Tanaka K, Marui Y, Tomikawa S. Clinicopathological analysis of persistent hypercalcemia and hyperparathyroidism after kidney transplantation in long-term dialysis patients. Ther Apher Dial. 2013;17:551–6.
Kawarazaki H, Shibagaki Y, Fukumoto S, Kido R, Ando K, Nakajima I, Fuchinoue S, Fujita T, Fukagawa M, Teraoka S. Natural history of mineral and bone disorders after living-donor kidney transplantation: a one-year prospective observational study. Ther Apher Dial. 2011;15:481–7.
Taweesedt PT, Disthabanchong S. Mineral and bone disorder after kidney transplantation. World J Transpl. 2015;5:231–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee at which the studies were conducted (IRB Approval No. A16-111) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Because this was an observational but not prospective intervention study, the Ethics Committee provided a waiver of informed consent.
About this article
Cite this article
Nanmoku, K., Shinzato, T., Kubo, T. et al. Prevalence and predictors of early hypercalcemia after kidney transplantation: a nested case–control study within a cohort of 100 patients. Clin Exp Nephrol 23, 268–274 (2019). https://doi.org/10.1007/s10157-018-1627-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-018-1627-6