Abstract
Purpose
To determine whether perirenal fat is associated with increased urinary albumin excretion and whether perirenal fat affects renal vascular endothelial function in obese rats.
Methods
Wistar rats were randomly divided into normal and obesity group, which were fed with normal and high-fat diet, respectively. Blood and urine samples were collected. Endothelial function of the aorta was determined by measuring endothelium-dependent vasodilatation. Renal tissues were collected for CD34 immunohistochemistry and free fatty acids (FFA) measurement. Levels of glomerular nitric oxide (NO) and reactive oxygen species (ROS) were measured.
Results
After 24 weeks, plasma FFA, high-sensitivity C-reactive protein, and malondialdehyde levels were elevated and were significantly higher in renal venous blood than in jugular venous blood in obese rats. Urinary albumin/creatinine ratio, glomerular CD34 expression, glomerular ROS level, and renal cortex FFA levels were higher in obese rats. Endothelial dysfunction was more severe in the infra-renal aorta than in the thoracic aorta in obese rats. Plasma adiponectin and glomerular NO levels were lower in obese rats.
Conclusion
Perirenal fat is associated with increased urinary albumin excretion in obese rats. The mechanism may be renal vascular endothelial dysfunction caused by increased oxidative stress and activation of inflammatory molecular pathways due to elevated FFA and low adiponectin levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a nutritional disorder that has emerged as a major health care challenge in the world [1, 2]. Recently, attention has been paid to the impact of excess weight and obesity on renal disease [3, 4]. The relationship between obesity and proteinuria was first reported in 1974 [5], and subsequent studies confirmed renal disease as an isolated complication of obesity [6]. Although high glucose, high blood pressure, and renal hemodynamic changes may lead to kidney damage in the obese state, the mechanisms of obesity-related renal disease have not yet been fully elucidated. Further evidence suggests that the distribution of body fat is more closely associated with kidney damage than obesity itself, with abdominal fat accumulation closely related to increased urinary albumin excretion and leading to obesity-related renal damage [7, 8]. Perirenal fat, as part of abdominal visceral fat, is thought to have a closer relationship to renal damage than other visceral fat deposits [9], because it surrounds the kidney directly, seeps into the renal sinus, and compresses the renal vessels. In our previous study, we found that perirenal fat was thicker in obese patients with microalbuminuria than in obese patients without microalbuminuria. Perirenal fat thickness may be an independent predictor of early kidney damage in obese patients [8].
Microalbuminuria was originally thought to indicate early renal damage. More recently, it has been convincingly shown that microalbuminuria is also an independent risk factor for cardiovascular morbidity and mortality. The common link between microalbuminuria and cardiovascular disease is vascular endothelial dysfunction. Thus, we speculate that in the obese state, renal vascular endothelial cell dysfunction, which in turn affects vascular hemodynamics, may lead to chronic kidney damage. Our previous study found that the interlobar arterial resistance index value could be a useful tool to assess early renal damage in obese patients [10], because the interlobar arteries are adjacent to the renal cortex, wrapped in perirenal fat, and the resistance index can detect subtle changes in renal hemodynamic status.
This conclusion and the specific mechanisms involved need further validation in animal experiments. Therefore, the aims of this study were to test whether perirenal fat is associated with increased urinary albumin excretion in obese rats and whether perirenal fat affects renal vascular endothelial function and, if so, to investigate the potential mechanisms involved.
Materials and methods
Experimental animals
Eight-week-old male Wistar rats (specific pathogen-free quality) were purchased from the Experimental Animal Center of the Weifang Medical University (Weifang, China). The study was approved by the local ethical committee for animal studies and conducted following the “Principles of laboratory animal care”. Twenty rats were randomly divided into a normal control (NC) group and an obesity (OB) group. The NC group was fed a regular diet (11.71 % fat, 65.06 % carbohydrate, 23.23 % protein, and 330 kcal/100 g), and the OB group was fed a high-fat diet (50.10 % fat, mainly saturated; 33.60 % carbohydrate; 16.30 % protein; and 493 kcal/100 g) [11]. The rats were housed under standard laboratory conditions. Body weight and food intake were monitored weekly.
Study protocol
Urine was collected from each rat in an individual metabolic cage for measurement of the urine albumin/creatinine ratio (ACR). The rats were then anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg). The intact thoracic aorta and infra-renal aorta were immediately dissected and immersed in Krebs–Henseleit bicarbonate buffer (K–H solution) for measurement of endothelial function. Jugular and renal venous blood samples were collected. Abdominal visceral fat (including perirenal fat) was harvested and weighed. Portions of the left kidney were collected for morphological observation and renal free fatty acid (FFA) measurements. The cortical tissue of the kidney was cut into small pieces, and the isolated glomeruli were collected by the sieving technique. Isolated glomeruli were collected by the mechanical graded sieving technique (a series of screens of 80 mesh, 120 mesh and 200 mesh). The suspension of glomeruli was then used for nitric oxide (NO) and reactive oxygen species (ROS) assays.
Evaluation of endothelial function
Aortic vascular reactivity was determined as described previously [12, 13]. The thoracic aorta and infra-renal aorta were immersed in ice-cold oxygenated K–H solution (95 % oxygen and 5 % carbon dioxide) at 37 °C. After equilibration for 60 min, aortic rings 3 mm in length from the two groups were challenged with norepinephrine (1 μM) to induce vasoconstriction. Once a stable contraction was achieved, acetylcholine (Ach, 10−8 to 10−4 M) or sodium nitroprusside (SNP, 10−8 to 10−4 M) was added to the organ bath to detect endothelium-dependent vasodilatation (EDV) or endothelium-independent vasodilatation (EIV) responses. A data acquisition system (PowerLab, AD Instruments, Dunedin, New Zealand) was used to record force changes.
Plasma measurements
Plasma glucose was measured by the glucose oxidase method. Plasma FFA, triglycerides (TG), and malondialdehyde (MDA) concentrations were determined by colorimetric assays. Plasma high-sensitivity C-reactive protein (hs-CRP) and adiponectin levels were measured using commercially available ELISA kits (USCN Life Sciences and Technology, Wuhan, China). Serum creatinine concentrations were measured by the Jaffe method.
Measurement of urinary albumin excretion
Urinary albumin was measured by radioimmunoassay (Beijing North Institute of Biological Technology, Beijing, China), and urinary creatinine was measured by the Jaffe method.
FFA measurements in renal cortex
The samples of renal cortex were cut into small pieces, and a solution (100 μl) of 20 mM EDTA/2 mM NaCl/50 mM sodium phosphate buffer, pH 7.4, was added to 100 mg of kidney tissue. Next, 10 μl of homogenate was mixed with 10 μl of tert-butyl alcohol and 5 μl of Triton X-100/methyl alcohol mixture (1:1 vol/vol) for lipid extraction. Renal FFA levels were measured by the enzymatic method [13].
Histology and immunohistochemistry
The renal tissues were fixed in 10 % formalin and stained with hematoxylin and eosin (HE). Formalin-fixed tissues were sectioned and used for CD34 (endothelial cell marker) immunohistochemistry. After restoring antigen retrieval, the sections were incubated with the CD34 antibody (Beijing Zhongshan Biotechnology Co., Ltd., Beijing, China) and then with the second antibody. The sections were then counterstained with hematoxylin and examined by light microscopy. A total of 12 fields per animal (three samples × four fields) were analyzed using Image-Pro Plus 6.0 software.
Measurements of NO and ROS in the isolated glomeruli
NO and ROS levels in the glomeruli were measured by the Griess and 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) methods, respectively, as described previously [14, 15]. For NO determination, the tissues were homogenized in PBS and centrifuged at 10,000g for 20 min. The resulting supernatant solutions were used to measure NO levels by colorimetric assay, according to the manufacturer’s instructions (Beyotime Biotechnology, Beijing, China). For ROS determination, isolated glomeruli were incubated in RPMI-1640 containing 0.5 mM DCFH-DA (excitation 490 nm and emission 530 nm) for 60 min. The mean fluorescence intensity of the isolated glomeruli was analyzed by a fluorescence microplate reader.
Results
Biometric and blood parameters of rats in the two groups
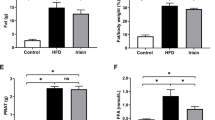
After 24 weeks, the rats in the obesity group exhibited increased body weight, visceral fat, perirenal fat, visceral fat index (visceral fat/body weight), and perirenal fat index (perirenal fat/body weight) compared with rats in the control group (Table 1). No significant difference was found in blood pressure between the two groups (NC, 119.5 ± 12.5 mmHg; OB, 123.4 ± 23.2 mmHg; P > 0.05). Plasma TG, FFA, hs-CRP, and MDA levels were elevated, and plasma adiponectin levels were reduced in the obesity group compared with the control group (P < 0.05). There were no differences in plasma glucose or total cholesterol between the two groups (P > 0.05; Table 2). To determine whether there were significant differences between peripheral blood and renal blood, we measured the FFA, hs-CRP, and MDA levels in jugular and renal venous blood. We found significantly higher serum FFA, hs-CRP, and MDA levels in renal venous blood than in jugular venous blood (Table 3).
Kidney parameters of rats in the two groups
Urine ACR and renal cortex FFA contents in the obese group were almost fourfold and threefold higher, respectively, than in the control group. The rats in the obesity group had a higher kidney weight index value (kidney weight/body weight × 1,000) than rats in the control group (Table 4).
Endothelial function of rats in the studied groups
Ach-induced EDV in both the thoracic aorta and infra-renal aorta was attenuated in the obesity group compared with the control group. There was a significant difference between the thoracic aorta and the infra-renal aorta in obese rats, with more severe endothelial dysfunction in the infra-renal aorta than in the thoracic aorta. No significant difference was found between these sections in the control group (Fig. 1). SNP-induced EIV was not impaired in the obese rats (data not shown).
Histology and immunohistochemistry
Morphological observation of kidney tissues from obese rats revealed glomerular hypertrophy, increased interstitial inflammatory cells, and Bowman’s capsular expansion. Glomerular volume increased by 56.2 % in obese rats compared with the control rats (19.27 ± 4.87 vs. 12.34 ± 3.04 μm3, P < 0.01). CD34 expression in the glomeruli was also enhanced in obese rats, indicating increased proliferation of glomerular endothelial cells [mean optical density (IOD/area): 0.136 ± 0.014 vs. 0.081 ± 0.008, P < 0.05] (Fig. 2).
Histological features and CD34 expressions of kidney in the studied groups (magnifications: × 400). HE staining in the NC group (a) and the OB group (b). Brown color indicates CD34 staining as a marker of endothelial cell in the NC group (c) and the OB group (d). Blue color indicates counterstaining for nucleus with hematoxylin
ROS and NO levels in the glomeruli
Compared with the control group, the obese rats had high ROS production and low NO levels in the glomeruli (Fig. 3).
Correlation analysis
Pearson analysis showed that ACR had a positive correlation with perirenal fat (r = 0.755, P < 0.01), plasma FFA levels (r = 0.716, P < 0.01), and renal cortex FFA levels (r = 0.729, P < 0.01). ACR had a negative correlation with adiponectin (r = −0.706, P < 0.01).
Discussion
In this study, we demonstrated that perirenal fat is related to increased urinary albumin excretion in obese rats. The mechanism by which perirenal fat affects urinary albumin may be related to renal vascular endothelial dysfunction caused by increased oxidative stress and activation of inflammatory molecular pathways due to elevated FFA and low adiponectin levels.
The relationship between obesity and proteinuria has been described in many studies [3, 5]. In our study, ACR was significantly higher in the obesity group rats than in control group rats. Morphological examination showed enlarged glomeruli and Bowman’s capsular expansion in obese rats, indicating that the most prominent morphological change was glomerulomegaly [6]. These findings further indicate that obesity is related to early renal damage. However, there is evidence that visceral fat, especially perirenal fat, is more closely related to renal damage than obesity itself [8, 16, 17]. In our study, we found that abdominal adiposity was obvious in obese rats and that the visceral fat/perirenal fat index was higher in these rats than in control rats. Correlation analysis showed that ACR had a positive correlation with perirenal fat mass. Taken together, these findings suggest that perirenal fat is strongly related to increased urinary albumin excretion and may be a good predictor of early kidney damage in obese subjects.
Microalbuminuria is associated with generalized endothelial dysfunction, which is usually seen in obese subjects. In our study, Ach-induced EDV was attenuated in both the thoracic aorta and the infra-renal aorta in obese rats. Endothelial dysfunction was more severe in the infra-renal aorta than in the thoracic aorta. EDV in the aorta could be a marker of endothelial function in the large vessels, to some extent. We also evaluated endothelial cell proliferation and glomerular NO level as markers of glomerular endothelial cells. CD34 expression in the glomeruli was enhanced and NO level was lower in obese rats, indicating greater proliferation of glomerular endothelial cells in these animals. These findings all suggest that endothelial dysfunction of both large and small vessels are associated with early kidney damage in obese subjects.
Several mechanisms may explain the associations among perirenal fat, endothelial dysfunction, and microalbuminuria in obese rats. First, perirenal fat wraps the kidney, seeps into the renal sinus, and compresses the renal vessels by developing at sites of mechanical pressure, leading to reduced renal blood flow and kidney disease [8]. In addition, excessive FFA released from perirenal fat increase renal FFA intake and lead to renal lipotoxicity by both endocrine and paracrine pathways. In our study, we found that both plasma FFA and renal FFA levels were higher in the obesity group than in the control group. Further study showed that serum FFA levels in renal venous blood were significantly higher than in jugular venous blood. Correlation analysis showed that FFA levels had a positive correlation with ACR. Third, previous studies have shown that high FFA levels and chronic inflammation can cause endothelial dysfunction by increasing oxidative stress, which can enhance oxidation of tetrahydrobiopterin, leading to l-arginine producing superoxide rather than NO by eNOS uncoupling [13]. In our study, we found high ROS production in the glomeruli of obese rats. Serum hs-CRP and MDA levels were also elevated in the obesity group compared with the control group. Serum hs-CRP and MDA levels in renal venous blood were significantly higher than in jugular venous blood.
Adiponectin is a key regulator of albuminuria, and a negative correlation has been found between adiponectin and microalbuminuria in obese subjects [18–20]. Adiponectin may play an important role in preventing the development of lipotoxicity in insulin-sensitive organs such as the kidneys [21]. Low adiponectin level seems to be linked to increase in inflammation and oxidative stress [22]. Low plasma adiponectin level was also seen in the obesity group, and correlation analysis showed that adiponectin had a negative correlation with ACR in this study.
In summary, we have shown that urinary albumin excretion was higher in obese rats than in normal rats. Perirenal fat is related to increased urinary albumin excretion in obese rats. The mechanism for this association may be renal vascular endothelial dysfunction caused by increased oxidative stress and activation of inflammatory molecular pathways due to elevated FFA and low adiponectin levels.
References
Hurt RT, Frazier TH, McClave SA, Kaplan LM (2011) Obesity epidemic: overview, pathophysiology, and the intensive care unit conundrum. JPEN J Parenter Enteral Nutr 35(5 Suppl):4S–13S
Rokholm B, Baker JL, Sorensen TI (2010) The levelling off of the obesity epidemic since the year 1999—a review of evidence and perspectives. Obes Rev 11(12):835–846
Klausen KP, Parving HH, Scharling H, Jensen JS (2009) Microalbuminuria and obesity: impact on cardiovascular disease and mortality. Clin Endocrinol (Oxf) 71(1):40–45
Kopple JD, Feroze U (2011) The effect of obesity on chronic kidney disease. J Ren Nutr 21(1):66–71
Weisinger JR, Kempson RL, Eldridge FL, Swenson RS (1974) The nephrotic syndrome: a complication of massive obesity. Ann Intern Med 81(4):440–447
Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD (2001) Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 59(4):1498–1509
Foster MC, Hwang SJ, Massaro JM, Hoffmann U, DeBoer IH, Robins SJ et al (2011) Association of subcutaneous and visceral adiposity with albuminuria: the Framingham Heart Study. Obesity (Silver Spring) 19(6):1284–1289
Sun X, Han F, Miao W, Hou N, Cao Z, Zhang G (2013) Sonographic evaluation of para- and perirenal fat thickness is an independent predictor of early kidney damage in obese patients. Int Urol Nephrol 45(6):1589–1595
Lamacchia O, Nicastro V, Camarchio D, Valente U, Grisorio R, Gesualdo L et al (2011) Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol Dial Transplant 26(3):892–898
Han F, Hou N, Miao W, Sun X (2013) Correlation of ultrasonographic measurement of intrarenal arterial resistance index with microalbuminuria in nonhypertensive, nondiabetic obese patients. Int Urol Nephrol 45(4):1039–1045
Sun X, Han F, Yi J, Hou N, Cao Z (2013) The effect of telomerase activity on vascular smooth muscle cell proliferation in type 2 diabetes in vivo and in vitro. Mol Med Rep 7(5):1636–1640
Lee RM, Lu C, Su LY, Werstuck G, Gao YJ (2009) Effects of hyperglycemia on the modulation of vascular function by perivascular adipose tissue. J Hypertens 27(1):118–131
Sun X, Yu Y, Han L (2013) High FFA levels related to microalbuminuria and uncoupling of VEGF-NO axis in obese rats. Int Urol Nephrol 45(4):1197–1207
Kono T, Saito M, Kinoshita Y, Satoh I, Shinbori C, Satoh K (2006) Real-time monitoring of nitric oxide and blood flow during ischemia-reperfusion in the rat testis. Mol Cell Biochem 286(1–2):139–145
Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T et al (2005) Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 67(5):1890–1898
Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE (2003) A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41(4):733–741
Bonnet F, Marre M, Halimi JM, Stengel B, Lange C, Laville M et al (2006) Larger waist circumference is a predictive factor for the occurrence of microalbuminuria in a non-diabetic population. Arch Mal Coeur Vaiss 99(7–8):660–662
Sharma K (2008) Adiponectin regulates albuminuria and podocyte function in mice. J Clin Investig 118(5):1645
Sharma K (2009) The link between obesity and albuminuria: adiponectin and podocyte dysfunction. Kidney Int 76(2):145–148
Li Y, Sun X, Yu Y (2013) Serum fetuin-A levels related with microalbuminuria in diet-induced obese rats. Biomed Res Int 2013:795103
Kern PA (2003) Adiponectin expression from human adipose tissue relation to obesity, insulin resistance, and tumor necrosis factor-α expression. Diabetes 52(7):1779–1785
Yamauchi T, Kadowaki T (2008) Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond) 32(Suppl 7):S13–S18
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants No. 81300688), Weifang Health Bureau Program (2013082), and the Science and Technology Innovation Fund of Affiliated Hospital of Weifang Medical University (No. K12QC1004).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, N., Han, F., Wang, M. et al. Perirenal fat associated with microalbuminuria in obese rats. Int Urol Nephrol 46, 839–845 (2014). https://doi.org/10.1007/s11255-014-0656-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-014-0656-7