Abstract
Objective
To investigate whether diabetes mellitus (DM) was associated with postoperative outcomes, including prostate-specific antigen doubling time, among men who underwent radical prostatectomy (RP) for clinically localized prostate cancer (PCa).
Methods
Data of 661 patients who underwent radical prostatectomy for node-negative prostate cancer and were followed up for ≥3 years postoperatively at our institution were analyzed. Associations between diabetes mellitus at surgery and outcomes following radical prostatectomy, such as biochemical recurrence-free survival and prostate-specific antigen doubling time, were examined. Aggressive recurrence was defined as biochemical recurrence with prostate-specific antigen doubling time <9 months.
Results
Of the 661 total subjects, DM (n = 67, 10.1 %) and non-DM group (n = 594, 89.9 %) showed no significant differences in various clinicopathologic parameters including age and PSA. DM group had lower postoperative biochemical recurrence-free survival than non-DM group, with observed difference approaching statistical significance (log-rank, p = 0.077). On multivariate analysis, DM at surgery was significantly associated with aggressive recurrence following RP (p = 0.048). Pathologic Gleason score (p = 0.008) and seminal vesicle invasion (p = 0.010) were also significantly associated with aggressive recurrence on multivariate analysis.
Conclusion
Our results show that pre-existing DM in men with PCa is associated with more aggressive recurrence, suggesting that DM may affect disease progression following RP. Further investigation would be needed to elucidate exact biologic interaction between DM and PCa and also assess causal relationships that potentially could be modified to improve long-term outcome in patients with the two diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unlike many other cancers, several published data from epidemiologic studies have shown that diabetes mellitus (DM) has an inverse association with risk of prostate cancer (PCa) [1–4]. However, it has also been suggested that association between the two disease entities may not be so simple, and controversy continues over the actual association between DM and PCa risk [5–7]. As for the potential association of pre-existing DM and outcomes of patients who underwent radical prostatectomy (RP) for PCa, reported data have shown inconsistent findings [5, 8, 9]. Debates continue regarding the influence of DM on the treatment outcomes in PCa patients as well.
Meanwhile, among the various known prognostic factor for PCa patients who underwent RP, prostate-specific antigen doubling time (PSADT), calculated following biochemical recurrence, has widely been recognized as a robust predictor of overall and PCa-specific death following RP [10–12]. Considering the apparent controversy surrounding the association of DM and prognosis after RP, it can be suggested that applying PSADT rather than biochemical recurrence or biochemical recurrence-free survival as an endpoint of relevant study may be more appropriate. As not all biochemical recurrence is considered fatal, it would be clinically important to identify patients at high risk for mortality so that they can be treated more aggressively. Looking at the literature, a paucity of data exists on the significance of DM regarding PSADT following RP. Thus, we sought to investigate whether DM was associated with various postoperative outcomes, including PSADT, among men who underwent RP for PCa.
Materials and methods
After obtaining institutional review approval, we retrieved the data of 742 patients who underwent RP for clinically localized PCa between January 2004 and June 2008 at our institution from our prospectively collected database. Patients who received neoadjuvant therapy, those who were not followed up for at least 3 years postoperatively, and those with relevant data (including DM status at surgery) missing were excluded from our study. The patients were identified as having type 1 DM and were also excluded due to the small number. Also, patients with node positive disease are likely to harbor aggressive disease; they were excluded from our analysis as previously done by others [13]. The history of DM at the time of surgery was identified from the review of medical record including prescription. In nondiabetic patients, the medication history, preoperative blood glucose levels, and/or clinical symptoms were reviewed to confirm that they were, indeed, nondiabetics at the time of surgery.
For our study, data assessed included patient age at the time of surgery, diabetic status at surgery, body mass index (BMI), preoperative prostate-specific antigen (PSA) level, biopsy and pathologic Gleason grade, clinical and pathologic tumor stage, and surgical margin status. As for BMI data, subjects were categorized according to the classification proposed for BMI of Asian population (<18.5, 18.5 to <23, 23 to <27.5, and ≥27.5 kg/m2) [14]. Biochemical recurrence was defined as a single PSA value >0.2 ng/ml, 2 measurements of 0.2 ng/ml, or secondary treatment for an elevated postoperative PSA. Men who received adjuvant treatment for an undetectable PSA were censored as not recurred at the time of treatment. PSADT was obtained as previously been reported by others [13]. PSADT was calculated assuming first-order kinetics by dividing the natural log of 2 (0.693) by the slope of the linear regression line of the natural log of PSA over time. In order to calculate PSADT, patients must have had a minimum of 2 PSA values, separated by at least 3 months, and within 2 years after biochemical recurrence. In patients who underwent salvage therapy within this time, only PSA values before salvage therapy were used to obtain PSADT. The patients who have no change of PSA and decrease of PSA had negative value of PSADT, or a long PSADT of more than 100 months was also calculated. For ease of calculation, we assigned that aforementioned PSADT <0 or PSADT >100 months were assigned a value of 100 months as Teeter et al. [13]. Associations of DM status with adverse pathologic features such as high (≥4 + 3) pathological Gleason score, extracapsular extension of tumor, seminal vesicle involvement, and biochemical outcomes were analyzed. Biochemical outcomes included the risk of postoperative biochemical recurrence, biochemical recurrence-free survival, and PSADT following biochemical recurrence.
The SPSS software package version 11.0 (Statistical Package for Social Sciences™, Chicago, IL, USA) was used for statistical analysis. For our study, patients were grouped according to DM status at RP: DM and non-DM group. The Chi-square test, independent sample t test, and Mann–Whitney test were used to compare the statistical significance of differences in proportions, as needed. One-way analysis of variance was also applied in comparing the two groups. Variables such as age and PSA after logarithmic transformation were treated as continuous variables. BMI, biopsy Gleason score, tumor stage, pathological Gleason score, seminal vesicle invasion, and surgical margin were treated as categorical variables. Univariate and multivariate logistic regression analyses were performed to assess the predictive capacities of variables shown to be significant predictor for aforementioned adverse pathological features of tumor. Adverse pathological features were defined as binary outcomes. Biochemical recurrence-free survival was compared between DM and non-DM group via Kaplan–Meier plots and the log-rank test. To estimate the relative risk of progression associated with DM, we used a Cox proportional hazards model adjusted for various known prognostic factors such as PSA, pathologic Gleason score, and pathologic stage. Examining the potential impact of DM on the aggressiveness of disease recurrence, we evaluated the association between DM and PSADT following biochemical recurrence via linear regression. PSADT was modeled as a logarithmically transformed continuous variable, and results were adjusted for the preoperative features described above. The geometric mean was back transformed for ease of interpretation. We a priori defined a PSADT <9 months as clinically aggressive as previously reported [13]. Nonaggressive disease was defined as recurrence with PSADT ≥9 months, or no evidence of biochemical recurrence. Logistic regression analysis was performed to examine the significance of DM regarding the development of disease recurrence with a PSADT <9 months adjusting for various clinicopathologic features. A 2-tailed p < 0.05 was considered significant for all analyses.
Results
Among 661 patients, a total of 67 (10.1 %) patients were identified as having DM, all type 2, at the time of surgery, and 594 (89.9 %) patients were identified as not having DM. Median age, BMI, and preoperative PSA of 661 total subjects included in our study were 66 years, 24.3 kg/m2, and 7.30 ng/ml, respectively. Sixty-seven of 661 (10.1 %) total patients showed BMI ≥27.5 kg; only 12 (1.8 %) patients showed BMI ≥30 kg/m2. As for pathologic Gleason score and stage, Gleason score of 7 (63.7 %) and pT2c (45.7 %) were the most common, respectively. Median postoperative follow-up duration of 661 total subjects was 45.0 months.
Characteristics of our subjects according to DM status at RP were listed in Table 1. No significant differences were observed in age, PSA, and other various clinicopathological parameters between DM (n = 67) and non-DM group (n = 594) in univariate analyses. Similar to the results of univariate analyses, DM group was not observed to have significantly higher rates of extracapsular extension of tumor, high pathological Gleason score, or seminal vesicle invasion than non-DM group when adjusted for multiple preoperative clinical variables, such as age, PSA, BMI, clinical stage, and biopsy Gleason score (all p > 0.05).
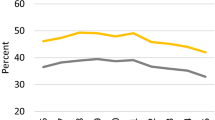
No significance difference was observed between postoperative follow-up durations of DM and non-DM group (median 43.5 vs. 45.0 months; p = 0.483). Among DM and non-DM group, biochemical recurrence occurred in 9 (13.4 %) and 51 (8.6 %) patients from each group, respectively (p = 0.190). Compared with non-DM group, DM group showed relatively lower rate of biochemical recurrence-free survival with observed difference approaching statistical significance (log-rank, p = 0.077) (Fig. 1). After adjusting for other variables, including age, BMI, PSA, pathologic Gleason score, extracapsular extension of tumor, seminal vesicle invasion, and margin positivity, DM remained not significantly associated with biochemical recurrence-free survival among the total subjects (p = 0.116). Factors shown to be significantly associated with biochemical recurrence-free survival were PSA (p = 0.014), pathologic Gleason score (p = 0.010), and seminal vesicle invasion (p = 0.022), in multivariate analysis.
Among the 60 patients with biochemical recurrence following RP, PSADT was calculable in 58 (96.7 %) patients: 9 from DM group and 49 from non-DM group. The 12 patients with a PSADT of 0 or less, that is, a decrease/no change in PSA, or a long PSADT of more than 100 months were assigned a value of 100 months. Among these 58 patients, median PSADT was 8.40 months. The median PSADT was 6.35 months in DM group and 9.24 months in non-DM group. Compared with non-DM group, DM group showed significantly shorter PSADT after RP on univariate analysis (p = 0.001). Using PSADT <9 months to define aggressive disease recurrence, DM at RP was found to be significantly associated with aggressive disease recurrence on multivariate analysis (p = 0.048) (Table 2). Pathologic Gleason score (p = 0.008) and seminal vesicle invasion (p = 0.010) were also significantly associated with aggressive recurrence on multivariate analysis.
Discussion
In our cohort of patients who underwent RP for node negative disease and were followed up for at least 3 years postoperatively, those who had DM at surgery demonstrated a trend of having lower rate of biochemical recurrence-free survival following RP than non-DM counterparts. Also, DM was observed to be significantly associated with shorter PSADT following biochemical recurrence on multivariate analysis, indicating that DM may be associated with more aggressive disease recurrence following RP. Such results suggest that DM may have a significant impact on the progression of PCa after RP.
Looking at the literature, not many have investigated the impact of pre-existing DM on the outcome following RP. Analyzing data from Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE™), a community-based prostate cancer registry study, Chan et al. [5] observed no significant association between DM and biochemical recurrence after RP. It should be noted that median postoperative follow-up duration for their subjects was only about 2 years. On the other hand, Patel et al. [8] reported that DM was associated with a 55 % increase in the risk of biochemical recurrence after RP on multivariate analysis. Also, Abdollah et al. [15] observed from a European cohort that PCa patients with DM had a significantly higher risk of harboring poorly differentiated (pathologic Gleason score ≥ 8) PCa on final pathological examination of RP specimens. Meanwhile, a study of Shared Equal-Access Regional Cancer Hospital (SEARCH) database has shown that DM was associated with 2.5-fold increased risk of biochemical recurrence and a trend toward shorter PSADT among white obese men while DM was associated with 23 % lower risk of recurrence and longer PSADT among non-obese white and black men [9]. Such findings would suggest that among men with PCa, the association between DM and aggressiveness of PCa may vary by race and obesity. Overall, previously reported studies on the impact of DM on biochemical outcome after RP have shown mixed results. Still, most published data indicate that pre-existing DM may have some influence on the outcome of patients who underwent RP for PCa. In 2010, Snyder et al. [16] reported their results on performing a meta-analysis on the effects of pre-existing DM on PCa prognosis. From their review of the literature, they produced a pooled hazard ratio of 1.57 (95 % CI 1.12–2.20) for long-term, overall mortality in PCa patients with DM. Also, they reported that DM was associated with tumor recurrence and treatment failure among PCa patients, concluding that DM affects the outcome of men with PCa. However, Snyder et al. [16] also noted mixed results from relevant RP series and lack of studies on DM’s role regarding PCa-specific survival.
Unlike aforementioned RP series which were multi-racial, all of our subjects were Koreans, eliminating racial factor in the interpretation of observed findings. Also, as Asian men are known to be generally leaner than Western counterparts, almost all of our subjects had BMI <30 kg/m2. We have previously reported that BMI was not a significant factor regarding biochemical recurrence after RP among Korean PCa patients [17]. Accordingly, it can be suggested that our subjects may offer clearer picture of direct interaction between DM and PCa compared with multi-racial western cohort. Meanwhile, PSADT is widely considered an accurate predictor of PCa metastasis and PCa-specific death among those who experience PSA failure after RP [12]. Although the association of DM and biochemical recurrence-free survival only approached statistical significance in our study, we believe that our observation of pre-existing DM having significant association with PSADT provides telling evidence that DM may well contribute to clinically significant, aggressive recurrence following RP, as also been observed among a subset of subjects in an aforementioned study from SEARCH database [9]. As a proportion of men who experience biochemical recurrence after RP will eventually succumb to PCa, early identification of such patients would be crucial. On such grounds, we believe that our findings would certainly justify further study on the effect of DM on PCa-specific mortality.
It is widely postulated that DM represents a very poor environment lacking growth factors, such as testosterone, insulin, and insulin-like growth factor (IGF), for the initial development of tumor [18]. Such hypothesis has been frequently mentioned to explain the decreased incidence of PCa among diabetics compared with nondiabetics. Although the impact of such poor environment on the progression of already developed PCa is unclear, it can be hypothesized that only aggressive PCa can survive and progress through selection pressure created by DM’s poor environment. On the other hand, it can also be suggested that an environment lacking growth factors may well lead to inhibition of cancer progression. Meanwhile, it has been reported that Whites, Blacks, and Asians have different hormonal milieu. For example, a study has revealed that Asians have systemic IGF-1 levels between those of Whites and Blacks [19]. Therefore, among DM patients of different races, different serum levels of insulin and/or IGF-1 may result in different level of selection pressure toward initiation and/or progression of PCa. Such assumption would, at least partially, contribute to understanding the inconsistent findings from our study and aforementioned RP series. In order to better understand interaction between DM and PCa, detailed large-scale prospective study on the effects of various hormones on PCa development and progression among multi-racial cohort would certainly be needed.
In various cancers other than PCa, pre-existing DM has already been reported to have detrimental effect on the prognosis [20–23]. From the Second National Health and Nutrition Examination Survey (NHANES II) Mortality Study, it was found that cancer patients with co-existing DM had 13 % higher risk of dying from cancer than non-DM counterparts, albeit statistically insignificant [22]. Also from the Cancer Prevention Study II which included a prospective cohort of over 1.2 million American volunteers with 16 years of mortality follow-up, men with DM were found to be associated with elevated relative risk of death from colon, pancreatic, liver, and bladder cancer, independent of BMI [23]. Nevertheless, controversies still exist. For example, debates continue on the association between pre-existing DM and breast cancer-specific mortality due to inconsistencies in relevant data published as with PCa [24]. Although available data have linked DM with higher mortality in cancer patients, no definite causal relationship has been established in many cancers. As aforementioned, further investigations would be needed to elucidate exact mechanism underlying the interaction between DM and PCa.
We have previously observed that DM was independently associated with the detection of high-grade PCa via contemporary multi-core prostate biopsy [25]. Similar observation was made in a study from Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial as Leitzmann et al. [7] reported that DM was positively associated with aggressive PCa in relatively lean men who would be similar in BMI to our Korean subjects of the current study. And from another study, we have reported that the level of glycemic control correlated with aggressiveness pathologic profile of PCa among patients with DM, a finding which was later validated by a study from SEARCH database [26, 27]. We believe that these results from our prior studies can also be considered supportive of our findings in the current study as they all linked DM or poor glycemic control with aggressive PCa. Certainly, our findings from a Korean RP series may not be directly applicable to PCa patients of other race or origin. However, based upon our findings, we believe that significance of DM regarding tumor recurrence or progression should be investigated upon further in PCa patients of various origins. DM should receive further attention to assess potential causal relationship that could be modified to improve the outcomes in PCa patients with DM.
Our study may be limited by relatively small number of subjects with DM. Also, our study shares common problems inherent to the retrospective analysis. Only patients who underwent RP for the treatment of PCa were included in this study. We could not assess exact duration of DM and level of glycemic control as well as medications taken for each subject. And we could not investigate the systemic levels of various hormones that have been linked to PCa, such as insulin and IGFs. Due to the lack of long-term follow-up, more important end points, such as PCa-specific mortality, could not be examined. However, we believe that our results highlight the need for further research regarding the potential impact of pre-existing DM on various different subsets of PCa patients, possibly defined by factors such as race, obesity, glycemic control, and disease risk.
Conclusion
Our results show that pre-existing DM in men with PCa is associated with short PSADT following RP. Such finding suggests that DM may contribute to more aggressive recurrence, affecting disease progression following RP. Further investigation would be needed to elucidate exact biologic interaction between DM and PCa and also assess causal relationships that potentially could be modified to improve long-term outcome in patients with the two diseases.
References
Bonovas S, Filioussi K, Tsantes A (2004) Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia 47(6):1071–10782
Kasper JS, Giovannucci E (2006) A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 15(11):2056–2062
Baradaran N, Ahmadi H, Salem S, Lotfi M, Jahani Y, Mehrsai AR, Pourmand G (2009) The protective effect of diabetes mellitus against prostate cancer: role of sex hormones. Prostate 69(16):1744–1750
Kasper JS, Liu Y, Giovannucci E (2009) Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer 124(6):1398–1403
Chan JM, Latini DM, Cowan J, Duchane J, Carroll PR (2005) History of diabetes, clinical features of prostate cancer, and prostate cancer recurrence-data from CaPSURE (United States). Cancer Causes Control 16(7):789–797
Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, Lippman SM, Platz EA, Pollak MN, Thompson IM, Kristal AR (2006) Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev 15(10):1977–1983
Leitzmann MF, Ahn J, Albanes D, Hsing AW, Schatzkin A, Chang SC, Huang WY, Weiss JM, Danforth KN, Grubb RL 3rd, Andriole GL (2008) Diabetes mellitus and prostate cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Causes Control 19(10):1267–1276
Patel T, Hruby G, Badani K, Abate-Shen C, McKiernan JM (2010) Clinical outcomes after radical prostatectomy in diabetic patients treated with metformin. Urology 76(5):1240–1244
Jayachandran J, Aronson WJ, Terris MK, Presti JC Jr, Amling CL, Kane CJ, Freedland SJ (2010) Diabetes and outcomes after radical prostatectomy: are results affected by obesity and race? Results from the shared equal-access regional cancer hospital database. Cancer Epidemiol Biomarkers Prev 19(1):9–17
D’Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH (2003) Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst 95(18):1376–1383
Valicenti RK, DeSilvio M, Hanks GE, Porter A, Brereton H, Rosenthal SA, Shipley WU, Sandler HM (2006) Posttreatment prostatic-specific antigen doubling time as a surrogate endpoint for prostate cancer-specific survival: an analysis of Radiation Therapy Oncology Group Protocol 92–02. Int J Radiat Oncol Biol Phys 66(4):1064–1071
Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW (2005) Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 294(4):433–439
Teeter AE, Banez LL, Presti JC Jr, Aronson WJ, Terris MK, Kane CJ, Amling CL, Freedland SJ (2008) What are the factors associated with short prostate specific antigen doubling time after radical prostatectomy? A report from the SEARCH database group. J Urol 180(5):1980–1984 discussion 1985
WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies Lancet 363(9403):157–163
Abdollah F, Briganti A, Suardi N, Gallina A, Capitanio U, Salonia A, Cestari A, Guazzoni G, Rigatti P, Montorsi F (2011) Does diabetes mellitus increase the risk of high-grade prostate cancer in patients undergoing radical prostatectomy? Prostate Cancer Prostatic Dis 14(1):74–78
Snyder CF, Stein KB, Barone BB, Peairs KS, Yeh HC, Derr RL, Wolff AC, Carducci MA, Brancati FL (2010) Does pre-existing diabetes affect prostate cancer prognosis? A systematic review. Prostate Cancer Prostatic Dis 13(1):58–64
Lee SE, Lee WK, Jeong MS, Abdullajanov M, Kim DS, Park HZ, Jeong SJ, Yoon CY, Byun SS, Choe G, Hong SK (2011) Is body mass index associated with pathological outcomes after radical prostatectomy in Korean men? BJU Int 107(8):1250–1255
Kasper JS, Liu Y, Pollak MN, Rifai N, Giovannucci E (2008) Hormonal profile of diabetic men and the potential link to prostate cancer. Cancer Causes Control 19(7):703–710
Henderson KD, Goran MI, Kolonel LN, Henderson BE, Le Marchand L (2006) Ethnic disparity in the relationship between obesity and plasma insulin-like growth factors: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev 15(11):2298–2302
Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB 3rd, Fuchs CS (2003) Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol 21(3):433–440
Verlato G, Zoppini G, Bonora E, Muggeo M (2003) Mortality from site-specific malignancies in type 2 diabetic patients from Verona. Diabetes Care 26(4):1047–1051
Saydah SH, Loria CM, Eberhardt MS, Brancati FL (2003) Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol 157(12):1092–1100
Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ (2004) Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 159(12):1160–1167
Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, Brancati FL, Wolff AC (2011) Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol 29(1):40–46
Hong SK, Oh JJ, Byun SS, Hwang SI, Lee HJ, Choe G, Lee SE (2012) Impact of diabetes mellitus on the detection of prostate cancer via contemporary multi (≥ = 12)-core prostate biopsy. Prostate 72(1):51–57
Hong SK, Lee ST, Kim SS, Min KE, Byun SS, Cho SY, Choe G, Lee SE (2009) Significance of preoperative HbA1c level in patients with diabetes mellitus and clinically localized prostate cancer. Prostate 69(8):820–826
Kim HS, Presti JC Jr, Aronson WJ, Terris MK, Kane CJ, Amling CL, Freedland SJ (2010) Glycemic control and prostate cancer progression: results from the SEARCH database. Prostate 70(14):1540–1546
Conflict of interest
All authors have no conflict of interest with any institution or product.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, J.J., Hong, S.K., Lee, S. et al. Diabetes mellitus is associated with short prostate-specific antigen doubling time after radical prostatectomy. Int Urol Nephrol 45, 121–127 (2013). https://doi.org/10.1007/s11255-012-0306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-012-0306-x