Abstract
Purpose
Lower urinary tract symptoms (LUTS) affect countless individuals worldwide with an increased prevalence among those ≥60 years of age. As the world’s population ages, the prevalence of LUTS will continue to increase, diminishing the quality of life of many men and women. For men, alpha-1-adrenergic receptor (α1-AR) blockers are used as first-line therapy to mitigate bothersome LUTS, but for women with LUTS, few treatments have been adequately studied. However, new research has emerged evaluating the use of tamsulosin and other α1-AR blockers in female LUTS. Thus, the purpose of this review is to evaluate clinical trials using tamsulosin for the treatment of LUTS in women to determine if tamsulosin is an appropriate treatment option.
Methods
A comprehensive search of the MEDLINE (1966-May 2012) and EMBASE (1980-May 2012) databases was performed. Additional articles were retrieved by manual review of the references cited in publications from the database search. Five published clinical trials and two abstracts were identified.
Results
All seven trials presented in this review demonstrated statistically significant primary outcomes with use of tamsulosin in female LUTS, especially in women with predominant voiding dysfunction. Such efficacy measures included a reduction in urinary symptoms as well as improvements in quality of life and sleep quality. Tamsulosin was found to be safe and well tolerated in all studies reviewed.
Conclusion
Consistent positive findings across multiple clinical studies suggest that in women with LUTS, particularly those with voiding dysfunction, tamsulosin may be an effective and safe treatment option.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction/background
Lower urinary tract symptoms (LUTS) affect approximately 62.5 % of men and 66.6 % of women with an increased prevalence among those ≥60 years of age according to the largest population-based study to date [1]. As the world’s population ages, the prevalence of LUTS is expected to increase, and thus bothersome urinary symptoms and the associated stigma will continue to impact the quality of life of many men and women as well as lead to an increase in economic burden [2, 3].
Although the overall prevalence of LUTS is similar between men and women, there are differences in the various symptom categories that comprise LUTS. According to the International Continence Society, LUTS can be classified into storage, voiding, and post-micturition symptoms [4]. Storage symptoms occur during the storage phase of the bladder and include increased daytime urinary frequency, nocturia, urgency, and incontinence. Voiding symptoms, on the other hand, occur during the emptying phase of the bladder and include signs of slow or intermittent urinary stream, hesitancy, straining, and terminal dribbling. Lastly, post-micturition symptoms include a feeling of incomplete emptying and involuntary loss of urine following urination. Women experience storage symptoms more frequently than men (women 59.2 %; men 51.3 %), and conversely, men experience both voiding symptoms (men 25.7 %; women 19.5 %) and post-micturition (men 16.9 %; women 14.2 %) symptoms more frequently than women [1]. Despite these small differences, it is apparent that both men and women experience storage and voiding symptoms at a high frequency, which could be indicative of a similar underlying etiology at the receptor level [5].
For women with predominately voiding symptoms, few treatments have been adequately studied, and thus a gold standard for female voiding dysfunction has not been identified. However, for men, alpha-1-adrenergic receptor (α1-AR) blockers are effectively used to mitigate voiding symptoms in those with benign prostatic hyperplasia (BPH) and are considered first-line for men with moderate to severe LUTS [6–8]. By blocking noradrenaline from binding to α1-ARs in the prostate, prostatic urethra, and the bladder neck, α1-AR blockers are thought to increase smooth muscle relaxation and improve urinary flow [5]. While the prostate contains mostly α1-ARs of the subtype 1A and fewer of the subtype 1D, the human detrusor muscle of the bladder and the bladder neck also contain α1-ARs but mostly of the subtype 1D [5, 9, 10]. In the urethra of both men and women, subtype 1A is the most prominent of the α1-ARs. Therefore, an α1-AR blocker selective for both subtype 1A and 1D such as tamsulosin could function to reduce LUTS in both men and women by improving voiding symptoms and storage symptoms [5]. This theory may be the reason behind the emergence of clinical trials evaluating the use of tamsulosin in women with LUTS. Other α1-AR blockers with varying degrees of α1-specificity have also been studied for the treatment of LUTS in women (Table 1) [11–14]. Since recent emphasis has been on the use of a selective α1A and α1D-AR blocker, tamsulosin was chosen as the focus of this review. We evaluated published clinical trials using tamsulosin for the treatment of LUTS in women to determine if tamsulosin is an appropriate treatment option.
Data sources
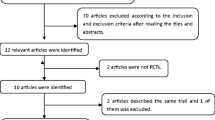
A comprehensive search of the MEDLINE (1966-May 2012) and EMBASE (1980-May 2012) databases was performed using the terms alpha-adrenergic antagonist, alpha 1-adrenergic receptor blocker, urinary incontinence, voiding dysfunction, lower urinary tract symptoms, tamsulosin, women, and female to identify pertinent clinical trials. All results were limited to studies conducted in female human subjects and reported in English. Additional articles were retrieved by manual review of the references cited for each publication identified from the database search. Clinical trials conducted in patients with neurogenic bladder, structural bladder obstruction, overactive bladder, or mixed incontinence and also those trials evaluating combination therapies were excluded. A total of five published clinical trials and two abstracts were selected for review (Table 2) [15–21].
Clinical evidence for tamsulosin for the treatment of LUTS in women
The largest study to date evaluating the efficacy of tamsulosin versus placebo for the treatment of LUTS was conducted by Pummangura et al. [15] between April 2004 and March 2005. In this prospective, randomized double-blind placebo controlled trial, 140 women greater than 20 years of age with International Prostate Symptom Scores (IPSS) ≥8 were enrolled. The IPSS is a validated tool, consisting of four voiding symptom questions and three storage symptom questions. It is widely used for assessing LUTS and stratifying symptoms into mild (1–7), moderate (8–19), and severe (20+) [6]. In this study, the primary outcome was to assess change in IPSS from baseline after 1 month of treatment with tamsulosin. At week 4, a statistically significant difference was found for the mean change in IPSS from baseline between the tamsulosin (n = 70) and placebo (n = 70) groups. Secondary outcomes included improvements in mean and maximum urinary flow rates determined from uroflowmetry recordings. In the tamsulosin group, the mean change in flow rate from baseline was significantly greater than in the placebo group (0.7 vs. −0.5; p = 0.013). However, the mean change in maximum flow rate (Q max) was not significantly greater in the tamsulosin group compared to placebo (1.0 vs. 1.1; p = 0.506). Two patients in the tamsulosin group (2 %) experienced dizziness and asthenia [15].
In a prospective, open-label, multicenter study, a total of 106 female patients with at least 3 months of voiding dysfunction symptoms were initiated on tamsulosin and evaluated after 8 weeks of treatment [16]. Women with moderate to severe LUTS (IPSS ≥ 15) and suspected voiding dysfunction (Q max ≤ 12 mL/sec and/or post-void residual volume (PVR) ≥ 150 mL) were included. After 8 weeks of treatment, mean IPSS were decreased significantly with improvements noted on both storage and voiding symptom scores. In 67.9 % of patients, IPSS declined by more than 5 points. Urodynamic parameters also improved from baseline: Q max increased (10.15 ± 2.79 to 13.47 ± 5.65; p < 0.001) and PVR was reduced (69.13 ± 85.45 to 39.88 ± 48.39; p < 0.001). To capture sexual function and quality of life in addition to symptom improvement, the Bristol Female Lower Urinary Tract Symptoms questionnaire (BFLUTS-SF) was used. The BLUTS-SF total score decreased significantly (21.2 to 16.4; p < 0.001) with significant improvements demonstrated in the voiding, filling, quality of life (p < 0.001), and sexual function (p = 0.021) domains. Incontinence scores, however, did not significantly improve. Overall, 89 patients (84 %) perceived tamsulosin as beneficial treatment. In terms of adverse effects, tamsulosin was associated with dizziness (3 cases), stress incontinence (4 cases), and fatigue (1 case) [16].
In a published abstract, Lee S and colleagues describe their prospective, open-label, multicenter study in which 82 female patients with LUTS were enrolled and started on tamsulosin regardless of their Q max [17]. The majority of patients had moderate LUTS (61 %) or severe LUTS (39 %). After 2 and 4 weeks of treatment, total IPSS as well as voiding and storage scores were significantly reduced. Voiding scores were more significantly reduced than storage scores (35.5 vs. 25.3 %, p < 0.05). In addition, a multivariate analysis demonstrated that a higher voiding symptom score on IPSS prior to treatment was predictive of improvement in LUTS. Significant improvements were also seen on urodynamic parameters—Q max, PVR, day and night urinary frequency, mean voided volume—and on quality of life (QOL) parameters such as the IPSS-QOL score and the Urogenital Distress Inventory (UDI-6). Over the 4 week period, systolic blood pressure did significantly decrease but was not associated with adverse events [17].
In a smaller, open-label study conducted from 1997 to 2002 by Pischedda et al. [18], 18 women suffering from functional bladder neck obstruction were enrolled and started on tamsulosin 0.4 mg daily for 30 days. Using intermittent self-catheterization, PVR was measured daily in women with a PVR ≥ 100 ml (n = 14) and weekly in women with a PVR < 100 ml (n = 4). Statistically significant improvements were seen in symptoms and urodynamic parameters—Q max and PVR—for 10 (56 %) of 18 patients (p < 0.01) [18].
For female patients with LUTS and nocturia, Ryu and colleagues looked at the efficacy of tamsulosin for improving sleep quality in these patients [19]. Their prospective, open-label trial was recently described in abstract form. In this study, 296 women with predominate voiding symptoms (Q max ≤ 15 ml/sec, IPPS ≥ 8) also experiencing nocturia (≥1 void/night) were enrolled from January 2008 to December 2008. Sleep quality was assessed using the Medical Outcomes Study (MOS) sleep scale. Overall, the sleep problem index was significantly decreased with significant changes seen in the following subcategories: sleep disturbance, somnolence, and sleep adequacy (p < 0.05). After 4 weeks of treatment, nocturnal frequency decreased by 1.12 voids; IPSS, Q max, and PVR were also significantly improved compared to baseline [19].
In the only active comparator study, Hajebrahimi and colleagues compared the effects of tamsulosin and prazosin on LUTS in women [20]. Women with voiding dysfunction were included and thus presented with symptoms of hesitancy, low urinary flow, frequency, nocturia, and post-void dribbling with Q max < 12 ml/sec and PVR > 50 mL. In this parallel design, double-blind trial, 40 patients were randomized to 3 months of treatment with tamsulosin (n = 20) or prazosin (n = 20). LUTS were assessed on the American Urological Association Symptom Score (AUASS) at baseline and every month thereafter. In both groups, scores on the AUASS improved; however, a larger decrease in AUASS score was seen in the tamsulosin group compared to the prazosin group, although this difference was not tested for statistical significance. All urodynamic parameters improved in both groups. Adverse effects occurred more frequently in the prazosin group (13 cases) compared to the tamsulosin group (1 case). Such adverse effects included drowsiness in both groups and dizziness, orthostatic hypotension, headache, and blurred vision in the prazosin group [20].
In addition to clinical efficacy and improvement in urodynamic parameters, a reduction in the number of urinary tract infections (UTIs) was included as a primary outcome in the open-label study by Constantini and colleagues [21]. In this study, 63 women with predominate voiding symptoms and functional bladder outlet obstruction were enrolled and given tamsulosin 0.4 mg daily for at least 30 days. After 30 days, women were allowed to continue treatment for 3 months if desired. Following treatment with tamsulosin, voiding symptoms were significantly improved in 45 out of 63 patients overall (71.4 %) with improvement also seen in patients with voiding symptoms alone (79.1 %) and patients with voiding symptoms associated with storage symptoms (66.7 %). In 81 % of patients presenting with a history of recurrent UTIs (21/26 patients), a 50 % reduction in recurrent UTIs was demonstrated with tamsulosin use. In terms of urodynamic parameters, significant improvement in Q max was evident in 57.1 % (36 out of 63 patients; p < 0.0001) of patients and PVR improved in 62.5 % of patients (p = 0.002) and completely resolved in 25 % of patients (p < 0.01). Tamsulosin was found to be well tolerated with adverse effects in only 4 patients: hypotension (n = 3) and urgency (n = 1) [21].
Discussion
For women with overactive bladder or mixed urinary incontinence, anticholinergic agents such as oxybutynin, tolterodine, solifenacin, and darifenacin are recommended as first-line therapy [22]. However, for women with primarily voiding dysfunction, there is a relative paucity of appropriate treatment options. Fortunately, tamsulosin has emerged as a promising agent to fill this void. This assertion is supported by the seven trials presented in this review, all of which demonstrated statistically significant primary outcomes [15–21]. Such efficacy measures included a reduction in urinary symptoms based on both the IPSS [15–17] and AUASS [20] scores as well as improvements in quality of life [16, 17] and sleep quality [19].
The majority of the studies in this review enrolled women specifically with voiding dysfunction. In each of these voiding dysfunction studies, treatment with tamsulosin improved symptom scores and urodynamic parameters compared to baseline [16, 18–20]. In addition, two studies presented in this review enrolled women irrespective of their urodynamic parameters [15, 17]. The first study included women on the basis of IPSS symptoms, age >20 years, and normal urinalysis [15]. However, mean and maximum urinary flow rates were measured as secondary outcomes. In this study, overall improvements in IPSS scores were observed without improvements in maximum urinary flow rate. This finding suggests that symptom improvement with tamsulosin may only be partially due to relieving bladder outlet obstruction [15]. The presence of α1-ARs in the human detrusor muscle of the bladder as well as the bladder neck and urethra supports the possibility of other mechanisms for tamsulosin efficacy [5, 9, 10]. In the second trial, women were enrolled regardless of their Q max. However, investigators soon discovered that a higher voiding symptom score at baseline was significantly associated with improvements in LUTS [17]. This study confirms prior evidence for the efficacy of tamsulosin in women with voiding symptoms and suggests that women with severe voiding symptoms may derive the greatest benefit from tamsulosin [16, 18–20].
Many of the studies reviewed here utilized IPSS as a surrogate outcome for the efficacy of tamsulosin with consistent significant improvements demonstrated [15–17]. This consistent efficacy across studies may offset the low clinical efficacy demonstrated in the only prospective, randomized double-blind placebo controlled trial to date [15]. Pummangura and colleagues did find a statistically significant difference in the change in mean IPSS from baseline between tamsulosin and placebo groups. However, a difference of just 3 points between groups (−5.6 for tamsulosin vs. −2.6 for placebo) at the end of 4 weeks may not be clinically significant. Nevertheless, there is a reasonable explanation for the low clinical efficacy, and this is likely due in part to the study’s eligibility criteria. As mentioned previously, women were not selected on the basis of voiding symptoms or low Q max (at baseline, women in the tamsulosin group had a mean Q max of 18 ± 6.1 ml/sec). Perhaps, if female patients with voiding dysfunction were targeted for this intervention, greater improvements in symptom scores may have been demonstrated.
Regardless of symptom scores, three studies demonstrated improvement in the clinically useful outcomes of quality of life, sleep quality, or both [16, 17, 19]. Using different validated scales—the BFLUTS-SF, IPSS-QOL, and UDI-6—two studies demonstrated statistically significant improvements in quality of life in women treated with tamsulosin [16, 17]. In a similar fashion, Ryu and colleagues translated tamsulosin efficacy into the clinical useful outcome of sleep quality. In female patients with voiding dysfunction, tamsulosin may improve nocturia to the extent that sleep quality is improved [19].
Constantini and colleagues evaluated the effect of tamsulosin on recurrent UTIs—another clinically important outcome that may impact the quality of life in women with voiding dysfunction. Although quality of life was not specifically measured in this study, of the women presenting with a history of recurrent UTIs, 65 % of patients experienced improvements in urodynamic parameters (Q max and PVR), and 81 % of patients had a 50 % reduction in recurrent UTI [21]. As female voiding dysfunction can lead to urinary retention and recurrent UTI, a reduction in UTIs may positively impact the lives of women with voiding dysfunction [23]. However, further studies assessing the role of tamsulosin in reducing recurrent UTIs in female voiding dysfunction is needed.
In all studies reviewed, tamsulosin was found to be a safe and well-tolerated treatment in women with LUTS [15–21]. Compared to other α1-AR blockers, tamsulosin is associated with the fewest blood pressure-related adverse effects [10]. The comparator trial of tamsulosin and prazosin supports this notion [20]. In this study, adverse effects such as dizziness and orthostatic hypotension were more common in the prazosin group. In addition to possessing greater potential for adverse effects, other α1-AR blockers have produced mixed results in women with LUTS. For instance, some trials have demonstrated efficacy [11, 12], while others have not [13, 14]. Coupling its consistent efficacy data with its safety and selective receptor profile, tamsulosin appears to be the ideal α1-AR blocker for female LUTS.
For LUTS associated with female functional bladder outlet obstruction specifically, tamsulosin was found to be effective as evident in the studies conducted by Pischedda et al. and Constantini et al. [18, 21]. Given the role of α1-AR blockers in male bladder outlet obstruction from BPH, this efficacy might be expected. However, in this review, women without functional bladder outlet obstruction also derived benefit from tamsulosin. This is worth mentioning because female bladder outlet obstruction is fairly uncommon with prevalence rates ranging from 2.7 to 29 % in retrospective studies [24]. Therefore, this review supports a broader use of tamsulosin for female LUTS beyond functional bladder outlet obstruction.
However, before tamsulosin should be widely used for the treatment of LUTS in women, several limitations of the present trials must be addressed in future trials. To begin, many studies had relatively small sample sizes [17, 18, 20] or open-label study designs [16–19, 21]. Second, the dose of tamsulosin was not consistent across trials, ranging from 0.2 mg to 0.4 mg daily [15–21]. While 0.4 mg daily is the approved dose of tamsulosin for men with BPH in the United States, smaller 0.2 mg daily doses may be explained by the fact that many trials in this review occurred in Asian countries where 0.2 mg daily is the standard tamsulosin dose for BPH [25–27]. Third, trials were short in duration with the longest trial 3 months and the shortest 4 weeks, likely owing to the recognized rapid onset of α1-AR blockers. However, to overcome placebo effects, longer trials are warranted. In addition, randomized controlled trials of LUTS pharmacotherapy treatment frequently have high placebo response rates [28]. Therefore, the possibility of a placebo effect in this review should be underscored. Of the seven studies included in this review, only one study was placebo-controlled [15]; the remaining studies were either open label [16–19, 21] or compared tamsulosin to prazosin not placebo [20]. In the future, further placebo-controlled studies will be needed to properly elicit placebo response. Despite these limitations, the trials in this review do provide some degree of certainty in regard to who should be treated with tamsulosin. Based on present trials, female patients with voiding dysfunction may derive the greatest benefit from tamsulosin. To confirm this assumption, a large, randomized, placebo-controlled trial comparing tamsulosin to placebo in female patients with voiding dysfunction is needed. Future trials should also utilize 0.4 mg daily to minimize risk of under-dosing and to be comparable to studies using tamsulosin for BPH [25].
Conclusion
The prevalence of LUTS among men and women is expected to increase as the population ages, and those afflicted often suffer great symptomatic and social burden. Fortunately, for women experiencing storage symptoms such as overactive bladder, first-line anticholinergic agents are available for treatment. However, there are no consensus recommendations for women with predominate voiding symptoms [22]. Due to evidence of receptor level similarities between men and women in the lower urinary tract, several recent trials have explored the efficacy of tamsulosin for female LUTS. The consistent positive findings of such studies suggest that in women with LUTS, especially those with voiding dysfunction, tamsulosin may be an effective and safe treatment option for improving urinary symptoms, quality of life, and sleep quality.
References
Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S et al (2006) Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 50:1306–1315
Irwin D, Kopp Z, Agatep B, Milsom I, Abrams P (2011) Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence, and bladder outlet obstruction. BJU Int 108:1132–1139
Milsom I (2009) Lower urinary tract symptoms in women. Curr Opin Urol 19:337–341
Abrams P, Cardozo L, Magnus F, Griffiths D, Rosier P, Ulmsten U et al (2003) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the international continence society. Urology 61:37–49
Fitzpatrick JM (2000) Facts and future lines of research in lower urinary tract symptoms in men and women: an overview of the role of the α1-adrenoreceptor antagonists. BJU Int 85(suppl 2):1–5
Segaran SpeakmanMJ (2011) Management of lower urinary tract symptoms and bladder outlet obstruction. Medicine 39(7):378–383
Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J (2009) Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 181:1779–1787
National Institute for Health and Clinical Excellence (2010) CG97 full guideline. Lower urinary tract symptoms: the management of lower urinary tract symptoms in men. http://www.nice.org.uk/nicemedia/live/12984/48554/48554.pdf. Accessed 26 Feb 2012
Schwinn DA, Michelotti GA (2000) α1-adrenergic receptors in the lower urinary tract and vascular bed: potential role for the α1d subtype in filling symptoms and effects of aging on vascular expression. BJU Int 85(suppl 2):6–11
Lowe F (2004) Role of the newer alpha1-adrenergic-receptor antagonists in the treatment of benign prostatic hyperplasia-related lower urinary tract symptoms. Clin Ther 26:1701–1713
Low BY, Liong M, Yuen K, Chee C, Leong W, Chong W et al (2008) Terazosin therapy for patients with female lower urinary tract symptoms: a randomized, double-blind, placebo controlled trial. J Urol 179:1461–1469
Kessler TM, Studer U, Burkhard FC (2006) The effect of terazosin in functional bladder outlet obstruction in women: a pilot study. J Urol 176:1487–1492
Lepor H, Theune C (1995) Randomized double-blind study comparing the efficacy of terazosin versus placebo in women with prostatism-like symptoms. J Urol 154:116–118
Lee Y, Lee K, Lee HS, Kim JC, Seo JT, Choo M (2011) Efficacy of alpha-blocker for the treatment of voiding dysfunction in women: 8 week, randomized, double blind, placebo-controlled, parallel group study (phase II). Neurourol Urodyn 30(6):1083–1084
Pummangura N, Kochakarn W (2007) Efficacy of tamsulosin in the treatment of lower urinary tract symptoms (LUTS) in women. Asian J Surg 30(2):131
Lee K, Hyun Han D, Lee Y, Choo M, Yoo T, Park H et al (2010) Efficacy and safety of tamsulosin for the treatment of non-neurogenic voiding dysfunction in females: a 8-week prospective study. J Korean Med Sci 25(1):117–122
Lee S, Lee W, Lee S, Kim H, Park C (2011) The effect of tamsulosin in female patients with lower urinary tract symptoms and predictive factors for therapeutic outcome: multicenter, prospective study [abstract]. Int Urogynecol J 22(suppl 3):S1781
Pischedda A, Pirozzi Farina F, Madonia M, Cimino S, Morgia G (2005) Use of alpha1-blockers in female functional bladder neck obstruction. Urol Int 74:256–261
Ryu S, Kim S, Hwang E, Im C, Oh K, Jung S et al (2010) The role of alpha 1(A) adrenoreceptor antagonist tamsulosin for the treatment of patients with lower urinary tract symptoms in women: the effect of nocturia and sleep quality [abstract]. Urology 76(suppl 3A):S61
Hajebrahimi S, Ahmadi Asrbadr Y, Azaripour A, Sadeghi-Bazargani H (2011) Effect of tamsulosin versus prazosin on clinical and urodynamic parameters in women with voiding difficulty: a randomized clinical trial. Int J Gen Med 4:35–39
Constantini E, Lazzeri M, Bini V, Zucchi A, Fioretti F, Frumenzio E et al (2009) Open-label, longitudinal study of tamsulosin for functional bladder outlet obstruction in women. Urol Int 83:311–315
National Institute for Health and Clinical Evidence (2006) CG40 UI: urinary incontinence: the management of urinary incontinence in women. http://www.nice.org.uk/nicemedia/live/10996/30282/30282.pdf. Accessed 26 Feb 2012
Stanton SL, Ozsoy C, Hilton P (1983) Voiding difficulties in the female: prevalence, clinical and urodynamic review. Obstet Gynecol 61:144–147
Patel R, Nitti VW (2001) Bladder outlet obstruction in women: prevalence, recognition, and management. Curr Urol Rep 2:379–387
Flomax [package insert] (2009) Boehringer Ingelheim Pharmaceuticals, Inc, Ridgefield, CT
Chang SJ, Chang IN, Yu HJ (2008) The effectiveness of tamsulosin in treating women with voiding difficulty. Int J Urol 15:981–985
Kaneko T, Matsushima H, Morimoto H, Tsuzaka Y, Homma Y (2010) Efficacy of low dose tamsulosin in medical expulsive therapy for ureteral stones in Japanese male patients: a randomized controlled study. Int J Urol 17:462–465
Schagen van Leeuwen JH, Castro R, Busse M, Bemelmans B (2006) The placebo effect in the pharmacologic treatments of patients with lower urinary tract symptoms. Eur Urol 50:440–453
Conflict of interest
Authors of this manuscript have no reportable conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meyer, L.E., Brown, J.N. Tamsulosin for voiding dysfunction in women. Int Urol Nephrol 44, 1649–1656 (2012). https://doi.org/10.1007/s11255-012-0275-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-012-0275-0