Abstract
Storage lower urinary tract symptoms (LUTS) in men are usually chronic, with a high prevalence and a substantial impact on quality of life; therefore, adequate therapies are desirable and crucial for these men. First line treatment for all patients with storage LUTS should always be behavioral. The gold standard for pharmacological treatment of overactive bladder/storage symptoms is a muscarinic receptor antagonist such as tolterodine. First-marketed antimuscarinics were limited by several adverse events such as dry mouth, constipation, tachycardia, accommodation disorder, and cognitive dysfunction, resulting in poor compliance and early treatment discontinuation in a large number of patients. In order to improve compliance with oral drug treatment, tolterodine was developed, providing a better efficacy/adverse event profile. Tolterodine is available in the following two formulations: the intermediate release (IR) and extended release form (ER). Tolterodine ER 4 mg administered once daily is pharmacokinetically equivalent to tolterodine IR 2 mg twice daily but has a lower incidence of adverse events and increased efficacy. Combination therapy of tolterodine and an alpha-blocker is significantly more efficacious than either monotherapy. Even when compared and added to tamsulosin, tolterodine shows a good safety profile. The incidence of acute urinary retention requiring catheterization and treatment withdrawals due to adverse events are low in all the studies included in the present review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The definition of lower urinary tract symptoms (LUTS) includes a wide group of individual symptoms generally summarized to storage LUTS (urgency, daytime frequency, nocturia, and urinary incontinence), voiding LUTS (slow stream, splitting or spraying, intermittency, hesitancy, straining, and terminal dribble), and post-micturition LUTS (sensation of incomplete emptying and post-micturition dribble) [1]. It was estimated that approximately 90 % of men between 45 and 80 years of age suffer of some type of LUTS [2].
LUTS may result from structural or functional anomalies in one or more parts of the lower urinary tract which comprises the bladder, bladder neck, prostate, distal sphincter mechanism, and urethra. In addition, abnormalities of the peripheral and/or central nervous systems providing neural control to the lower urinary tract can lead to LUTS.
The enlargement of the prostatic gland due to benign hyperplasia of epithelial and/or stromal cells is assumed to be the major cause of LUTS, involving at least two paths such as: (1) direct bladder outlet obstruction (BOO) from enlarged tissue (static component) and (2) increased smooth muscle tone and resistance within the enlarged gland (dynamic component) [3]. Voiding LUTS has often been attributed to the physical presence of BOO. Longstanding BOO and bladder over-distension can cause fibrotic changes of the bladder wall leading to secondary changes in detrusor function and, finally, storage LUTS. Storage LUTS could be caused by primary bladder storage dysfunction (e.g., detrusor overactivity [DO]), primary voiding dysfunction (e.g., detrusor underactivity or benign prostatic obstruction [BPO]), or a combination of both bladder storage and voiding dysfunction [4].
Storage LUTS are usually chronic, with a prevalence ranging from 10 to 26 % [5]. The impact of storage LUTS on quality of life (QoL) is clinically significant. The role of proper therapies is crucial to achieve symptom relief and satisfaction; accordingly, comprehensive counseling of patients is mandatory to evaluate treatment expectations. Patients should be informed that treatments are not always effective and often associated with bothersome adverse events (AEs) that can limit compliance with drugs.
First line treatment for all patients with storage LUTS should be reassurance, education, lifestyle advice, and behavioral modifications. The most commonly prescribed strategies are weight loss, bladder training, bladder control strategies, pelvic floor muscle training (PMFT), and fluid intake restriction. However, the gold standards for the pharmacological treating of overactive bladder/storage symptoms are muscarinic receptor antagonists (antimuscarinics) such as darifenacin, fesoterodine, oxybutynin, propiverine, solifenacin, tolterodine, or trospium chloride [6]. Antimuscarinics act by blocking M2 and M3 cholinoceptors on detrusor smooth muscle cells or on nerves in the urothelium with different selectivity profiles, acting mainly during the urinary storage phase. Antimuscarinics decrease the activity of afferent bladder nerves producing a decrease in urgency and increase bladder capacity. However, other parts of the body, including the brain, heart, gut, salivary glands, and tear ducts, also express muscarinic receptors. For this reason, first-marketed antimuscarinics were limited for the significant incidence of peripheral antimuscarinic adverse events such as dry mouth, constipation, tachycardia, accommodation dysfunction, and central nervous system side effects, resulting in poor compliance and early discontinuation of therapy in a large number of patients [7].
To improve the compliance of patients, tolterodine was developed more than 15 years ago, obtaining a better efficacy/adverse event profile. Due to its lower lipid solubility, tolterodine crosses the blood-brain barrier to a lesser extent than oxybutynin [8]. In addition, even if tolterodine is a non-selective antimuscarinic, it showed a rapid and longer lasting effect on the bladder than on salivary glands [8, 9].
Most of the currently available trials on antimuscarinics have evaluated the effects in the female population. In contrary, only few studies have investigated the effects of antimuscarinic drugs, including tolterodine, in male patients with bladder outlet obstruction and bladder storage symptoms [10••, 11••]. The results of antimuscarinic monotherapy were conflicting, even when storage symptoms were predominant. Nevertheless, antimuscarinics have lately become popular in selected men with moderate-to-severe LUTS and predominant bladder storage symptoms. These studies showed that antimuscarinics in men do not elevate post-void residual urine if the patient was carefully selected and monitored [10••, 11••]. Moreover, the approach of combination therapy with an antimuscarinic and α-blocker has become increasingly popular in men with both storage and voiding LUTS [12]. Tolerability represents a fundamental parameter for the administration of antimuscarinic agents. In this manuscript, we review the overall safety and efficacy of tolterodine in the treatment of male bladder storage LUTS, with a focus on its mechanism of action.
Methods
A systematic literature search, limited to the English language was performed in PubMed and Scopus, without the adoption of temporary limits. The following search terms were included in the systematic search: “LUTS”, “storage LUTS”, “male”, “men”, “BOO”, “OAB”, “antimuscarinic”, “tolterodine”, “tolterodine IR”, “tolterodine ER”. Only articles regarding tolterodine for OAB/LUTS in male population were selected. The validated efficacy outcomes considered were the following: AUA Symptom Score, IPSS, IPSS storage sub-score, and IPSS QoL (International Prostate Symptom Score). Moreover, the numbers of urgency episodes/24 h, urgency incontinence episodes/24 h, incontinence episodes/24 h and pad use were reviewed. In selected studies, the most common adverse events (AEs) reported for tolterodine were also evaluated.
Results
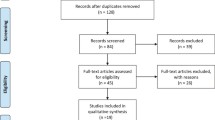
Overall, 257 articles were retrieved in February 2015. After full-text assessment for eligibility, 14 studies were reviewed. The remaining 243 studies were excluded due to low affinity to the topic, missing, or incomplete data, deficiency in methodology (several biases not included), or assessment of clinical outcomes without validated instruments. The flowchart of the literature search and selection is summarized in Fig. 1.
Table 1 reports about the efficacy of tolterodine alone or in combination in terms of mean change in 24-h voiding frequency, mean change of nocturnal voiding frequency and mean change in 24-h urgency episodes; and safety of tolterodine alone or in combination in terms of adverse events rate, withdrawals due to adverse events and acute urinary retention (AUR).
Physiological Rationale of Tolterodine
Detrusor contractions are stimulated by acetylcholine and muscarinic receptors of smooth muscles cells of the bladder. Although all five subtypes of muscarinic receptors can be found in several human tissues, comprising salivary glands, heart muscles, nerve cells of the central or peripheral nervous systems, M2, and M3 subtypes are predominantly expressed in the bladder wall [21].
Tolterodine acts as a non-selective competitive antagonist of acetylcholine at postganglionic muscarinic receptors, demonstrating relative tissue selectivity for the bladder. After oral administration, tolterodine is metabolized by cytochromes P450 (CYP2D6 and CYP3A4) in the liver, with the consequential formation of two main metabolites: 5-hydroxymethyl tolterodine (5-HMT) and N-dealkylated tolterodine [22]. Only the 5-HMT derivative, a major pharmacologically active metabolite, exhibits an antimuscarinic activity similar to that of tolterodine, contributing significantly to the therapeutic effect. Both tolterodine and 5-HMT exhibit a high specificity for muscarinic receptors M2 and M3.
Tolterodine and 5-HMT act through a competitive blockade of the bladder muscarinic receptors in a concentration-dependent manner, as described in in vitro studies in guinea pig detrusor strips and human-isolated urinary bladder [23]. In addition, tolterodine and 5-HMT show functional selectivity for the bladder over the salivary glands in vivo compared to oxybutynin, which showed a distinct affinity profile, exhibiting the highest affinity for muscarinic M3 receptors and the lowest affinity for muscarinic M2 and M5 receptors with a tenfold selectivity for muscarinic M3 over M2. Thus, oxybutynin was significantly more potent in inhibiting salivary secretion than urinary bladder contractions, and the effect of tolterodine on urinary bladder contractions occurred at significantly lower doses than the effect on salivary secretion, showing favorable tissue selectivity. Neither tolterodine nor oxybutynin revealed substantial effects on heart rate [23, 24].
After oral administration, tolterodine immediate release (IR) is rapidly absorbed and metabolized by CYP2D6, reaching the peak serum levels within 1 to 3 h (t max). Tolterodine extended release (ER) reaches its t max slightly later, approximately after 4 h. Elimination half-life depends on the metabolism capacity of the individual and varies for tolterodine IR between 2 and 10 h and for tolterodine ER between 6 and 10 h. The effective exposure to tolterodine is unaffected by CYP2D6 phenotype—the lack or strongly reduced activity of this liver enzyme characterizes poor metabolizers—or simultaneous food intake. Conversely in poor metabolizers, tolterodine clearance was fivefold lower (mean 9.0 L/h) and the elimination half-life was consistently longer (7.5 to 11 h). Moreover, liver cirrhosis or impaired renal function (creatinine clearance 10–30 ml/min [0.6 to 1.8 L/h]) can considerably reduce the clearance of tolterodine [25].
Tolterodine Formulations
Tolterodine is available in two formulations, IR and ER. Tolterodine IR was approved by the FDA in 1998. When Tolterodine IR was compared with placebo, it showed a good tolerability profile; 87 % of subjects in tolterodine arm finished the study compared to 91 % of those who received placebo. Among adverse events, only dry mouth appeared significantly more often in patients treated with tolterodine IR (1 mg bid, 30 %; 2 mg bid, 48 %). Tolterodine 2 mg bid was significantly more effective than placebo in improving the number of urgency incontinence episodes and reducing the number of micturition [26].
To improve patients’ treatment compliance and decrease adverse events, especially dry mouth, the extended release (ER) formulation with a soluble microspheres delivery system was developed and approved by the FDA in 2000. Tolterodine 4 mg once daily is pharmacologically equivalent to tolterodine IR 2 mg twice daily. Moreover, the peak serum concentration of the active metabolites following administration of tolterodine ER was around 75 % of that observed for the IR tablet, whereas the minimum serum concentration was approximately 1.5-fold higher, resulting in less serum drug level fluctuation and sustained drug release over 24 h, with a consequent better tolerability for patients [27]. In addition, multiple daily dosing, a proven factor for poor compliance during medical treatment especially in elderly patients who frequently take concomitant medications, was solved.
In 2001, van Kerrebroeck et al. [28] performed a study comparing tolterodine ER with the IR formulation, resulting in an improved efficacy and tolerability of tolterodine ER compared to tolterodine IR. A significant difference in urgency incontinence episodes reduction was found between these formulations (71 % for tolterodine ER vs. 60 % for tolterodine IR). Additionally, a lower incidence of dry mouth was reported (23 % for tolterodine ER vs. 30 % for tolterodine IR). Otherwise, the incidence of other adverse events such as dizziness constipation and somnolence were similar in frequency and comparable with placebo. These data were confirmed in 2010 by Novara et al. [29] in a meta-analysis of RCTs, demonstrating that patients randomized to tolterodine ER experienced a lower number of micturitions per 24 h (weighted mean difference [WMD], 0.34; p = 0.03) and a higher voided volume per micturition (WMD, 9.12; p = 0.0004) but a similar number of incontinence episodes and pad use per day. Regarding adverse events, patients treated with tolterodine ER formulation had a significantly lower rate of dry mouth (odds ratio [OR], 1.39; p = 0.002) but a higher rate of headache (OR, 0.53; p = 0.004). Withdrawals due to adverse events and especially constipation were similarly prevalent for both formulations.
Discussion
Efficacy Profile of Tolterodine
All clinical studies demonstrated significant reductions in mean change in 24-h voiding frequency, mean change in night voiding frequency, and in mean change in 24-h urgency episodes (Table 1).
In 2003, Athanasopoulos et al. [17] conducted a prospective RCT in men with urodynamically confirmed detrusor overactivity (DO) and concomitant BOO. Patients were first treated with tamsulosin 0.4 mg for 1 week and, afterwards, randomly assigned to continue with tamsulosin alone or tamsulosin + tolterodine 2 mg twice daily. In men treated with tamsulosin plus tolterodine, but not tamsulosin alone, significant improvement was seen in maximum involuntary contraction pressure, maximum detrusor pressure during micturition and HRQL.
Another RCT of symptomatic men with urodynamically confirmed BOO only or confirmed BOO plus DO was published by Lee et al. in 2004 [18]. Patients treated with doxazosin 2 mg for 12 weeks, who did not show improvement (decrease in IPSS of >3 points), were then treated for 8 weeks with doxazosin plus tolterodine 2 mg twice daily. The additional use of tolterodine improved LUTS, also defined as an IPSS decrease of >3 points, in 38 % of patients with BOO only, and in 73 % in subjects with BOO plus DO, who showed no improvement after treatment with doxazosin alone.
In another study, men with LUTS associated with BPH were randomly treated with either terazosin alone or terazosin in combination with tolterodine (2 mg twice daily). After 6 weeks of treatment, there was a significantly greater reduction of LUTS (IPSS) in the combination arm as compared with the terazosin monotherapy arm. The post-void residual volume was reduced in the combination group even if it did not differ significantly between groups [30].
The TIMES study (Tolterodine and tamsulosin In Men with LUTS including OAB: evaluation of Efficacy and Safety) evaluated the efficacy of tolterodine ER 4 mg alone or in combination with tamsulosin 0.4 mg after 12 weeks of treatment in men with both OAB and BPO [19]. The RCT showed that patients treated with combination therapy of tolterodine and tamsulosin had a significant treatment benefit as defined by the patient perception questionnaire. Only combination therapy significantly improved total IPSS and IPSS QoL as well as the IPSS storage sub-score. Tamsulosin, tolterodine, or placebo alone did not reach this goal. However, patients in the tolterodine ER group experienced significant improvement of several bladder diary variables and the IPSS storage score vs. placebo in men with a prostate size or serum-PSA concentration below the study median and significant reductions in urgency urinary incontinence episodes per 24 h, compared with those in the placebo group. A subgroup of patients with prostate volumes ≤29 cm3 or serum-PSA concentrations <1.3 ng/ml demonstrated a significant advantage in favor of tolterodine monotherapy with regard to storage symptom reduction. Conversely, only the combination therapy showed a significant reduction of 24-h voiding frequency in patients with large prostates (prostate volume ≥30 cm3) [16].
A post hoc analysis [31] disclosed that tolterodine ER plus α-blocker was more effective than placebo plus α-blocker in men with baseline serum-PSA levels both above and below the study median, similarly to TIMES.
In another post hoc analysis [14] of data from a 12-week RCT on night-time administration of tolterodine (4 h before bedtime), Kaplan et al. compared tolterodine ER 4 mg with placebo. At week 12, the weekly values for night-time severe OAB micturitions and 24-h and daytime total, OAB, and severe OAB micturitions were significantly reduced in the tolterodine group vs. the placebo group. Tolterodine-treated men also reported a significant reduction in the mean urgency rating vs. placebo.
The efficacy of tolterodine ER 4 mg in a real life setting was evaluated by Höfner et al. [32] in men with OAB who either did not have suspected BOO (Q max >15 mL/s) or had persistent storage symptoms despite α-blocker treatment. This prospective observational 12-week study showed that tolterodine ER significantly improved urgency, urinary frequency, nocturia, incontinence, as well as IPSS total, QoL scores, and scores on all OAB-q subscales and domains.
In another prospective open-label trial, Kaplan et al. [13] also found a significant decrease of daytime frequency, nocturia, and the IPSS storage and voiding sub-scores after 24-week treatment with tolterodine ER in men who had insufficient efficacy or unbearable tolerability with α-blockers.
Another combination therapy with tolterodine ER was with 5α-reductase inhibitors. In particular, Chung et al. [33] demonstrated that in men with persistent OAB symptoms after at least 6 months of treatment with dutasteride, the addition of tolterodine ER allowed to significantly reduce urinary urgency and frequency, such as severe OAB episodes and nocturia. In addition, storage LUTS, as measured with the IPSS questionnaire, were significantly reduced from 9.8 to 4.5.
Safety Profile of Tolterodine
Current pharmacotherapy for OAB/storage symptoms primarily consists of antimuscarinics which also affect the salivary gland, intestine, eye, and CNS producing undesirable adverse events such as dry mouth, constipation, blurred vision, and possibly cognitive impairment in the elderly population. Together with an insufficient response to treatment, these side effects are the major causes for low treatment persistence with antimuscarinics [34, 35].
The theoretical concern about a negative effect on post-void residual urine or even urinary retention has influenced the use of antimuscarinics in elderly men with storage LUTS; regardless studies show no increased risk of urinary tract retention in patients with BOO (Table 1). The safety of tolterodine ER 4 mg was assessed by Kaplan et al. in an open label, prospective study of 2005 with 39 subjects [13]. Four men (9 %) discontinued therapy because of intolerable dry mouth, but there were no reports of urinary retention. In the following year, Kaplan et al. published the results of a post hoc analysis of data from two 12-week RCTs on night-time dosing of tolterodine ER 4 mg in 745 men [14]. Adverse events associated with tolterodine ER were low and comparable to those in the placebo group, with the exception of dry mouth (11 % tolterodine vs. 4 % placebo). Withdrawals because of adverse events were infrequent (3 vs. 4 %). Five men were withdrawn for symptoms suggestive of urinary retention (3 % with tolterodine vs. 2 % with placebo).
Even when compared and added to tamsulosin [19], tolterodine showed a good safety profile. The incidence of acute urinary retention requiring catheterization was low (0.4 % in the tolterodine ER plus tamsulosin study arm and 0.5 % in the tolterodine ER arm vs. 0 % in the tamsulosin and 0 % in the placebo arm). Nine patients reported difficulties, of these six with urinary retention, two with decreased urinary flow, and one with both. Two patients taking placebo, one tolterodine ER, and one tolterodine ER plus tamsulosin discontinued treatment because of urinary retention, decreased urinary flow, or both. In addition, the overall incidence of adverse events was similar among the groups. Only dry mouth was the adverse event most frequently reported by patients receiving active treatment (2 % patients taking placebo; 7 % tolterodine ER; 7 % tamsulosin; 21 % tolterodine ER plus tamsulosin).
The incidence of AUR was also low in ADAM study [36] in which men were randomized to tolterodine ER 4 mg or placebo once daily for 12 weeks while continuing their previously prescribed α-blocker therapy. AUR requiring catheterization occurred in <1 % in either group, two of 323 subjects in the placebo plus α-blocker group and one of 329 patients in the tolterodine plus α-blocker group.
Study withdrawals due to adverse events were low and similar between the groups. No patient developed urinary retention when tolterodine ER was added to dutasteride and dry mouth was experienced by 7.5 % of patients [33].
The efficacy and safety of tolterodine in combination therapies was reviewed by Athanasopoulos et al. in 2011, concluding that combination therapy was effective and the risk of urinary retention was minimal [37•]. When tolterodine ER (4 mg/day) was compared with oxybutynin (10 mg/day) in the OPERA study (Overactive Bladder: Performance of Extended Release Agents) [38], the overall incidence of AEs was similar between the groups but dry mouth, the most common adverse event in each study arm, was statistically significant lower in patients with tolterodine (22.3 %) than in patients with oxybutynin (29.7 %; p = 0.02). The STAR study [34] compared flexible dosing of solifenacin vs. tolterodine 4 mg ER. Solifenacin flexible dosing proved to be slightly superior in reducing the numbers of urgency episodes/24 h (−2.85 vs. −2.42), urgency incontinence episodes/24 h (−1.42 vs. −0-83), incontinence episodes/24 h (−1.60 vs 1–11), and pad use (−1.72 vs −1.19). However, dry mouth and constipation were significantly more common in the solifenacin arm (18.2 vs. 14.5 % and 3.0 vs. 1.2 %, respectively).
Conclusions
Tolterodine is an effective drug for men with moderate-to-severe LUTS who are mainly bothered by bladder storage (OAB) symptoms. Compared with placebo, treatment emergent adverse events appear more frequently after tolterodine administration and include dry mouth, dizziness, and constipation. The overall rate of treatment discontinuation due to adverse events is acceptable, and the risk of acute urinary retention seems negligible. Further RCTs are needed to identify the best candidates for the treatment with tolterodine alone or in combination with α-blocker or 5α-reductase inhibitors used for male LUTS in clinical practice.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49.
McVary K. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(5 Suppl):S122.
Auffenberg GB, Helfand BT, McVary KT. Established medical therapy for benign prostatic hyperplasia. Urol Clin North Am. 2009;36(4):443–59.
Oelke M, Baard J, Wijkstra H, de la Rosette JJ, Jonas U, Höfner K. Age and bladder outlet obstruction are independently associated with detrusor overactivity in patients with benign prostatic hyperplasia. Eur Urol. 2008;54:419–26.
Haab F, Castro-Diaz D. Persistence with antimuscarinic therapy in patients with overactive bladder. Int J Clin Pract. 2005;59:931–7.
Gormley EA, Lightner DJ, Burgio KL, Chai TC, Clemens JQ, Culkin DJ, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6 Suppl):2455–63.
Yarker YE, Goa KL, Fitton A. Oxybutynin: a review of its pharmacodynamics and pharmacokinetic properties, and its therapeutic use in detrusor instability. Drugs Aging. 1995;6:243–6.
Brynne N, Stahl MMS, Hallén BH, Edlund PO, Palmér L, Höglund P, et al. Pharmacokinetics and pharmacodynamics of tolterodine in man: a new drug for the treatment of urinary bladder overactivity. Int J Clin Pharmacol Ther. 1997;35:287–95.
Stahl MMS, Ekström B, Sparf B, Mattiasson A, Andersson KE. Urodynamic and other effects of tolterodine: a novel antimuscarinic drug for the treatment of detrusor overactivity. Neurourol Urodyn. 1995;14:647–55.
Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. Guidelines on the management of male Lower Urinary Tract Symptoms (LUTS) incl. Benign Prostatic Obstruction (BPO). Eur Urol. 2013;64:118–40. The EAU guidelines reported that short-term treatment with antimuscarinic drugs (tolterodine) in men with BOO appears safe, even if not all antimuscarinic agents have been tested in elderly men with LUTS and OAB symptoms.
McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185(5):1793–803. The AUA guideline comprehensively evaluated the published papers, concluding that anticholinergic agents are appropriate and effective treatment alternatives for the management of LUTS secondary to BPH in men without an elevated post void residual (PVR) urine and when LUTS are predominantly irritative.
Chapple CR, Smith D. The pathophysiological changes in the bladder obstructed by benign prostatic hyperplasia. Br J Urol. 1994;73:117–23.
Kaplan SA, Walmsley K, Te AE. Tolterodine extended release attenuates lower urinary tract symptoms in men with benign prostatic hyperplasia. J Urol. 2005;174:2273–6.
Kaplan SA, Roehrborn CG, Dmochowski R, Rovner ES, Wang JT, Guan Z. Tolterodine extended release improves overactive bladder symptoms in men with overactive bladder and nocturia. Urology. 2006;68:328–32.
Abrams P, Kaplan SA, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in Men with bladder outlet obstruction. J Urol. 2006;175(3 Pt 1):999–1004.
Roehrborn CG, Abrams P, Rovner ES, Kaplan SA, Herschorn S, Guan Z. Efficacy and tolerability of tolterodine extended-release in men with overactive bladder and urgency urinary incontinence. BJU Int. 2006;97:1003–6.
Athanasopoulos A, Gyftopoulos K, Giannitsas K, et al. Combination treatment with an alpha-blocker plus an anticholinergic for bladder outlet obstruction: a prospective, randomized, controlled study. J Urol. 2003;169:2253–6.
Lee JY, Kim HW, Lee SJ, et al. Comparison of doxazosin with or without tolterodine in men with symptomatic bladder outlet obstruction and an overactive bladder. BJU Int. 2004;94:817–20.
Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296:2319–28.
Chapple C, Herschorn S, Abrams P, Sun F, Brodsky M, Guan Z. Tolterodine treatment improves storage symptoms suggestive of overactive bladder in men treated with alpha-blockers. Eur Urol. 2009;56(3):534–41.
Postlind H, Danielson A, Lindgren A, Andersson SH. Tolterodine, a new muscarinic receptor antagonist, is metabolized by cytochromes P450 2D6 and 3A in human liver microsomes. Drug Metab Dispos. 1998;26(4):289–93.
Larsson G, Hallén B, Nilvebrant L. Tolterodine in the treatment of overactive bladder: analysis of the pooled phase II efficacy and safety data. Urology. 1999;53(5):990–8.
Nilvebrant L, Andersson KE, Gillberg PG, Stahl M, Sparf B. Tolterodine: a new bladder-selective antimuscarinic agent. Eur J Pharmacol. 1997;327(2–3):195–207.
Olsson B, Szamosi J. Multiple dose pharmacokinetics of a new once daily extended release tolterodine formulation vs. immediate release tolterodine clinical pharmacokinetics. Clin Pharmacokinet. 2001;40(3):227–35.
Clemett D, Jarvis B. Tolterodine. Drugs Aging. 2001;18(4):277–304.
Malone-Lee JG, Walsh JB, Maugourd MF. Tolterodine: a safe and effective treatment for older patients with overactive bladder. J Am Geriatr Soc. 2001;49(6):700–5.
Chung DE, Te AE. Tolterodine extended-release for overactive bladder. Expert Opin Pharmacother. 2009;10(13):2181–94.
Van Kerrebroeck P, Kreder K, Jonas U, Zinner N, Wein A, Tolterodine Study Group. Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology. 2001;57(3):414–21.
Novara G, Galfano A, Secco S, D’Elia C, Cavalleri S, Ficarra V, et al. A systematic review and meta-analysis of randomized controlled trials with antimuscarinic drugs for overactive bladder. Eur Urol. 2008;54:740–63.
Yang Y, Zhao XF, Li HZ, Wang W, Zhang Y, Xiao H, et al. Efficacy and safety of combined therapy with terazosin and tolterodine for patients with lower urinary tract symptoms associated with benign prostatic hyperplasia: a prospective study. Chin Med J (Engl). 2007;120:370–4.
Chapple CR, Herschorn S, Abrams P, et al. Efficacy and safety of tolterodine extended-release in men with overactive bladder symptoms treated with an alpha-blocker: effect of baseline prostatespecific antigen concentration. BJU Int. 2010;106:1332–8.
Höfner K, Burkart M, Jacob G, Jonas U. Safety and efficacy of tolterodine extended release in men with overactive bladder symptoms and presumed nonobstructive benign prostatic hyperplasia. World J Urol. 2007;25:627–33.
Chung DE, Te AE, Staskin DR, Kaplan SA. Efficacy and safety of tolterodine extended release and dutasteride in male overactive bladder patients with prostates >30 grams. Urology. 2010;75(5):1144–8.
Chapple CR, Martinez-Garcia R, Selvaggi L, et al. A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial. Eur Urol. 2005;48:464–70.
Wagg A, Compion G, Fahey A, Siddiqui E. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int. 2012;110(11):1767–74.
Chapple C, Herschorn S, Abrams P, et al. Tolterodine treatment improves storage symptoms suggestive of overactive bladder in men treated with a-blockers. Eur Urol. 2009;56:534–43.
Athanasopoulos A, Chapple C, Fowler C, Gratzke C, Kaplan SA, Stief C, et al. The role of antimuscarinics in the management of men with symptoms of overactive bladder associated with concomitant bladder outlet obstruction: an update. Eur Urol. 2011;60(1):94–105. The good review of Athanasopoulos et al. focused on the contemporary role of antimuscarinics in the management of men with symptoms of bladder outlet obstruction and concomitant overactive bladder. They confirmed the safety of antimuscarinics for these patients and concluded that the addition of an antimuscarinic seems to offer an amelioration of the symptoms and a moderate improvement in quality of life.
Diokno AC, Appell RA, Sand PK, et al. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003;78:687–95.
Compliance with Ethics Guidelines
Conflict of Interest
Mauro Gacci has received support for travel to meetings for the study, manuscript preparation or other purposes, and payment for lectures from GSK, Eli-Lilly, Menarini, Pfizer, and Bayer.
Giacomo Novara has been an advisory board member or speaker for Astellas, GlaxoSmithKleine, Lilly, Menarini, Nycomed, Pfizer Inc., Pierre Fabre, and Recordati.
Matthias Oelke has worked on the advisory board for Eli-Lilly and Company and has received payment for lectures from Eli-Lilly and Company, Pfizer and Bayer.
Stavros Gravas has been advisory board member or speaker for Astellas, GlaxoSmithKleine, Pierre Fabre, and Angelini Pharma Hellas.
Arcangelo Sebastianelli, Matteo Salvi, Riccardo Schiavina, Eugenio Brunocilla, Cosimo De Nunzio, Andrea Tubaro, Marco Carini, Sergio Serni each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Benign Prostatic Hyperplasia
Rights and permissions
About this article
Cite this article
Gacci, M., Sebastianelli, A., Salvi, M. et al. Tolterodine in the Treatment of Male LUTS. Curr Urol Rep 16, 60 (2015). https://doi.org/10.1007/s11934-015-0531-9
Published:
DOI: https://doi.org/10.1007/s11934-015-0531-9