Abstract
Tamsulosin has been used for the off-label treatment of lower urinary tract symptoms (LUTS) in women. Over the past few years, several randomized controlled trials (RCTs) have reported the clinical effectiveness and safety of tamsulosin for LUTS in women. Therefore, the aim of the present study was to perform a meta-analysis to evaluate the safety and efficacy of tamsulosin in treating LUTS in women, which may resolve some of the current controversies over use of the drug and provide more reliable evidence for the use of tamsulosin. A literature review was performed to identify all published RCTs of tamsulosin for the treatment of LUTS in women. The search included the following databases: PUBMED, EMBASE, the Cochrane Controlled Trail Register of Controlled Trials, Chinese Biomedical Literature Database, China National Knowledge Infrastructure, Chinese Science and Technique Journals Database (VIP) and Wanfang Database. A systematic review and meta-analysis were conducted. Six RCTs studies involving 764 female participants were included in the analysis. Four out of the six RCTs compared tamsulosin with placebo, one RCT compared tamsulosin with prazosin and the other study compared tamsulosin with tamsulosin combined with tolterodine. Two RCTs evaluated total International Prostate Symptom Score (IPSS) and improved total IPSS compared with the placebo (standardized mean difference=−4.08, 95% confidence interval=−5.93 to −2.23, P<0.00001). IPSS (storage symptom score), IPSS (voiding symptom score) and quality-of-life score also showed the similar effects. In addition, tamsulosin improved the Overactive Bladder Questionnaire score when compared with placebo in only one RCT. For urodynamic parameters, tamsulosin improved the average flow rate and the post-void residual volume when compared with prazosin and tolterodine combined with tamsulosin, respectively. Beyond that, the other parameters showed no significant difference between the treatment and control groups. On the basis of the present evidence, tamsulosin is an effective treatment for the relief of LUTS in women when compared with placebo. However, the safety of the tamsulosin remains unknown. Further, well-conducted trials that examine long-term outcomes are required.
Similar content being viewed by others
Introduction
Lower urinary tract symptoms (LUTS) is often overlooked and underdiagnosed in women. The overall prevalence is estimated to be as high as 19%.1
LUTS comprise storage, voiding and post-micturition symptoms affecting the lower urinary tract. Voiding symptoms mainly bladder outlet obstruction include weak or intermittent urinary stream, straining, hesitancy, terminal dribbling and incomplete emptying.2 Storage symptoms include urge urinary incontinence and overactive bladder featured by urgency, frequency, urgency incontinence and nocturia.3 The major post-micturition symptom is post-micturition dribbling, which is common and bothersome.
There are many possible causes of LUTS such as abnormalities or abnormal function of the prostate, urethra, bladder or sphincters.2 In men, the most common cause is benign prostate enlargement, which obstructs the bladder outlet.2 The treatment options include lifestyle modifications, behavioral therapy, pelvic floor muscle training, bladder training and drug therapy.4 Muscarinic receptor antagonists are the first-line drugs of use. However, they are associated with the typical anticholinergic side effects of dry mouth, somnolence, constipation and blurred vision, and thus compliance with therapy is often poor.5
The adrenergic receptors found at the bladder neck are α1-adrenergic and three subtypes have been identified: α1A, α1B and α1D. Those receptors present in the bladder are predominantly α1A and α1D, whereas α1B receptors are found in the vasculature and are involved in blood pressure control.6 Consequently, α1-adrenergic-blocking agents with sub-selectivity for α1A and α1D might be most useful in the management of lower urinary tract dysfunction, and it was speculated that the α1D receptor might mediate the overactive symptoms of overactive bladder,6 whereas the α1A receptor subtype mediates the obstructive symptoms.7 α-Adrenergic receptor antagonists currently used in men with LUTS is proved effectively. A selective α1 adrenoceptor antagonist that is known to have greater specificity for α1A and α1D receptors than for α1B might have a role in the management of LUTS in women. Thus, α-adrenergic blockers, especially those that are specific to α-1a in the smooth muscle of the genitourinary tract, bladder neck, and various regions of the pelvic floor (for example, tamsulosin) have been implicated and used for the off-label treatment of LUTS associated with urinary dysfunction in women.8
In the past few years, several randomized controlled trials (RCTs) have reported the clinical effectiveness and safety of tamsulosin for LUTS in women. To date, however, no systematic reviews or meta-analyses that including RCTs to determine the effectiveness and safety of tamsulosin for LUTS. Therefore, the aim of the present study was to perform a meta-analysis to evaluate the safety and efficacy of tamsulosin in treating LUTS in women, which may resolve some of the current controversies over use of the drug and provide more reliable evidence for the use of tamsulosin.
Materials and methods
Inclusion and exclusion criteria
Types of studies
RCTs that evaluated tamsulosin in treating LUTS in women were included.
Trials were excluded if the data could not be obtained even though we attempted to contact the original study investigators.
Participants
Female patients with a clinical diagnosis of LUTS were included.
Interventions
All studies that administered tamsulosin used either alone or as an add on to an approved treatment for LUTS were included. The controls were:
-
1
Tamsulosin vs placebo only.
-
2
Tamsulosin plus approved treatments vs approved treatments.
-
3
Tamsulosin vs approved treatments.
Outcome measurements
Primary outcome measurements should comprise indicators in accordance with storage and voiding symptoms in LUTS patients. Storage symptoms include urinary urgency, frequency, urgency incontinence and nocturia. These outcomes use one of the following scales or methods: International Prostate Symptom Score (IPSS), quality-of-life (QoL) score, Overactive Bladder Questionnaire (OAB-q) or voiding diary. Voiding symptoms measure outcomes include maximum urinary flow rate (Qmax), average flow rate, the post-void residual volume (PVR), urethral closure pressure and pressure at Qmax.
When evaluating LUTS, symptom score was an important indicator. The severity scores include IPSS, American Urological Association symptom index, bother score, Kings Health Questionnaire and Bristol Female Lower Urinary Tract Symptoms questionnaire. IPSS, developed by Barry et al.,9 was initially used to assess the symptom severity of benign prostatic hyperplasia.10 Subsequently, it has been noted that the IPSS is neither sex-specific nor disease-specific for benign prostatic hyperplasia.11, 12 Many scholars have already used IPSS for daily practice and/or epidemiologic survey of female LUTS.13, 14 It was demonstrated that IPSS was a good indicator of the degree of bother and effect on QoL.10
Search strategy
PubMed, EMBASE, the Cochrane Library, Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure, Chinese Science and Technique Journals Database (VIP) and Wanfang Database (http://www.wanfangdata.com/) were searched to identify RCTs that referred to the effects of tamsulosin treatment for LUTS in women. All the data were searched from inception of the database to October 2015. Additional articles from relevant reference citations were retrieved and reviewed. The following search terms were used: ‘female’, ‘women’, ‘tamsulosin’, ‘lower urinary tract symptoms’, ‘LUTS’, ‘overactive bladder’, ‘OAB’, ‘bladder outlet obstruction’, ‘BOO’ and ‘urinary incontinence’. The language of publications was restricted to English or Chinese.
Selection of studies and data extraction
Two reviewers (HZ and ZH) independently screened the titles and abstracts of every record. Full articles were obtained when either information given in the title or abstracts conformed to the selection criteria outlined previously, or could not be ascertained due to limited information. To include studies, data were extracted independently by each reviewer and entered into a standardized form. Discrepancies were resolved by consensus.
Quality assessment
Two reviewers (HZ and ZH) independently evaluated the methodological quality of identified studies. The ‘risk of bias tool’ referred to the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 was used to assess methodological quality.15 In terms of the assessment criteria, each study was rated and assigned to one of the three following quality categories: (A) if all quality criteria were adequately met, the study was deemed to have a low risk of bias; (B) if one or more of the quality criteria was only partially met or was unclear, the study was deemed to have a moderate risk of bias; or (C) if one or more of the criteria were not met, or not included, the study was deemed to have a high risk of bias.16
Statistical method
Dichotomous outcomes were expressed as risk ratios with 95% confidence interval, and continuous outcomes were expressed as mean difference (if the same scale for each trial was available) or standardized mean difference (if different scales were used).17 Heterogeneity among the included studies was evaluated by the I2 test. A value >50% to indicate substantial heterogeneity and sought the potential sources of heterogeneity (clinical heterogeneity and methodological heterogeneity).17 Regardless of the size of heterogeneity, the random-effects model was used for statistical analysis. The meta-analysis of comparable data was carried out using Review Manager 5.1.0 (The Cochrane Collaboration, England, UK). If the results of the studies could not combine using meta-analysis (due to significant clinical heterogeneity and unconventional methods used in the analysis of studies), they were just only presented individually.
Results
Results of the literature search
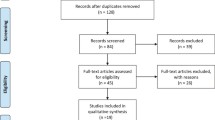
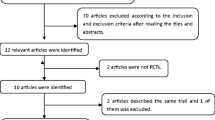
The database search found 45 articles that could potentially have been included in our meta-analysis. After removing duplicate articles, 39 studies were eligible. On the basis of the inclusion and exclusion criteria, 30 articles were excluded after a simple reading of the titles and abstracts of the articles. Two articles were excluded because they were incomplete text and one article was excluded owing to it is written in other language. Thus, a total of 6 RCTs studies18, 19, 20, 21, 22, 23 written in English were included in this review (Figure 1).
Characteristics of included studies
In all, six RCTs studies involving 764 female participants were included in the analysis. Four out of the six RCTs compared tamsulosin with placebo, one RCT compared tamsulosin with prazosin and the other study compared tamsulosin with tamsulosin combined with tolterodine. The trials in these articles had been conducted in Korea, Sweden, Thailand, the Netherlands, Egypt and Taiwan. Sample size in all of the studies was ranged from 30 to 273 cases. The baseline characteristics of the studies included in our meta-analysis are listed in Table 1.
Quality assessment
The outcomes were shown in Table 2. Among the studies included in the analysis, three described the randomization processes that they had employed.18, 19, 22 As well as blinding, sealed envelope technique for allocation concealment was applied in four studies.18, 19, 20, 21 All of the participants in the six studies had performed the follow-up. Only one of the included studies encompassed the information of intention-to-treat analysis.18 Besides, all studies did not have selective reporting bias. The level of quality of each identified study ranged from A to C.
Efficacy analysis
We used total IPSS improvement, IPSS voiding improvement, IPSS storage improvement, QoL score, as well as urodynamic parameters including average flow rate change, PVR changes, Qmax changes and urethral pressure improvement to evaluate the efficacy of tamsulosin. Furthermore, voiding diary changes, comprising the number of urgency, frequency, nocturia urgency incontinence and volume, were also used to evaluate the effect of tamsulosin. We also compared tamsulosin with a placebo and approved treatments medicines (solifenacin and tolterodine).
Total IPSS improvement
When compared with placebo, two of the RCTs20, 22 enrolled 191 participants with 92 patients assigned to the tamsulosin group and the other 99 patients assigned to the control group. The test for heterogeneity of two studies demonstrated significant heterogeneity (P=0.13; I2=57%), and the randomized effect model was performed. On the basis of our analysis, the pooled estimate of standardized mean difference was −4.08, and the 95% confidence interval was −5.93 to −2.23. (P<0.00001). The result suggested that tamsulosin was significantly effective in improving total IPSS compared with the placebo (Figure 2).
When compared with solifenacin, only one study22 enrolled 50 subjects was included. Likewise, one trail23 containing 181 participants was included when compared with tolterodine combined with tamsulosin. The results revealed that patients treated with tamsulosin showed no significant difference no matter which methods were used compared to patients treated with either solifenacin or tolterodine combined with tamsulosin (Figure 2).
IPSS (storage symptom score)
Two of the RCTs18, 22 included the IPSS storage improvement data representing a cohort of 88 participants (41 in the tamsulosin group and 47 placebo controls). The test for heterogeneity of two studies revealed significant heterogeneity (P<0.0001; I2=94%), and the randomized effect model was conducted. The pooled estimate of standardized mean difference was −3.16 and the 95% confidence interval was −4.47 to −1.85. The result showed that tamsulosin was superior to placebo in terms of IPSS storage improvement (Figure 3).
Similarly, the patients treated with tamsulosin showed no significant difference when compared with solifenacin or tolterodine combined with tamsulosin (Figure 3).
IPSS (voiding symptom score)
One of the RCTs22 included the IPSS voiding improvement data representing a cohort of 58 participants (27 in the tamsulosin group and 31 placebo controls). The result showed that tamsulosin was better than placebo in terms of IPSS voiding improvement (Figure 4). However, when it was compared with solifenacin or tolterodine combined with tamsulosin, no difference was shown (Figure 4).
QoL score
Just like the IPSS storage, QoL score included two same RCTs20, 22 comprising participants (41 in the tamsulosin group and 47 placebo controls). The test for heterogeneity of two studies revealed significant heterogeneity (P=0.01; I2=84%), and the randomized effect model was conducted. The pooled estimate of standardized mean difference was −2.1 and the 95% confidence interval was −2.67 to −1.71. The result indicated that tamsulosin was superior to placebo in terms of QoL score improvement (Figure 5).
Similarly, the patients treated with tamsulosin showed no significant difference when compared with solifenacin or tolterodine combined with tamsulosin (Figure 5).
OAB-q score
The OAB-q was developed by Coyne24 in the United States. It consists of 33 self-administered questions, eight that involve a symptom-bother scale and 25 involving health-related QoL scores that constitute four subscales measuring coping, concern, sleep and social interaction.25 The OAB-q questions are simple and easy to answer. In this review, only one RCT22 mentioned to this outcome. The result was shown in the Figure 6. As we can see from the chart, tamsulosin was more effective than placebo in terms of OAB-q score improvement. When compared with solifenacin, tamsulosin did not show the advantage.
Voiding diary
Voiding diary including urgency, frequency, incontinence, nocturia and volume. In this review, only one RCT19 mentioned to these outcomes. Apart from volume voided, all the other outcomes have no significant difference between the tamsulosin and placebo group (Figure 7).
Urodynamic parameters
Urodynamic parameters including average flow rate change, PVR, Qmax and urethral pressure mainly connected with voiding dysfunction. All or part of the urodynamic parameters were including in three RCTs18, 21, 23 that compared tamsulosin with prazosin, placebo and tamsulosin combined with tolterodine, respectively. When compared with prazosin, tamsulosin has boosted the average flow rate (Figure 8). Meanwhile, it also improved the PVR when compared with tamsulosin combined with tolterodine (Figure 9). Beyond that, the other urodynamic parameters showed no significant different between the treatment and control group (Figures 8, 9, 10, 11).
Discussion
This systematic review collated studies and provided general information on the efficacy of tamsulosin for LUTS in women.
Although tamsulosin showed no significant difference in IPSS score (including total IPSS, IPSS voiding, IPSS storage and QoL) when compared with solifenacin or tolterodine combined with tamsulosin. However, it revealed profound effective in improving IPSS score (P<0.05) than placebo. In addition, tamsulosin also showed the excellent ability in OAB-q score and volume voided improvement when compared to the placebo group.
Urodynamic parameters including average flow rate change, PVR, Qmax and urethral pressure mainly connected with voiding dysfunction. Even though tamsulosin has boosted the average flow rate compared with prazosin, it also improved the PVR compared with tamsulosin combined with tolterodine. Yet, it was not more effective in the improvement of average flow rate and Qmax when compared with placebo. One possible explanation is that seldom studies on this topic were reported, and more high-quality trails with larger samples are proposed to learn more about the efficacy and safety of the agent.
Several reviews6, 26, 27 on the alpha-blockers in the treatment of female LUTS have been published in the past few years. Although the majority of evidence suggest that these agents may have a place in therapy for female LUTS, data are conflicting. Compared with these review, some differences in our meta-analysis should be noted. First, these previous reviews are only the qualitative analysis. In comparison, our present meta-analysis included not only qualitative but also the quantitative analysis, which is the statistical combination of results from two or more separate studies. Potential advantages of meta-analyses include an increase in power, an improvement in precision, the ability to answer questions not posed by individual studies. In addition, all the eligible studies in our meta-analysis was the RCT, the gold standard of scientific evidence.
As well as the efficacy, the safety was one of the important factors to evaluate the drugs. Generally, tamsulosin was fairly well tolerated and showed no acute urinary retention or serious adverse events. The most commonly reported adverse events were dry mouth, constipation, nausea, abdominal pain, dyspepsia, headache, orthostatic hypotension, asthenia and dizziness.4, 18, 19, 21 All these were common clinical types of adverse drug reactions and the incidence of adverse reactions was low. Thus, tamsulosin was considered to be safe for the treatment of LUTS in women.
In this meta-analysis, there are some limitations. First is the methodologic quality. In these trials, three studies20, 21, 23 did not describe the randomization processes that they had employed. One of the trials22 had no descriptions the ways of allocation concealment and blinding. Apart from this, allocation concealment and blinding were not mentioned at all in another study.23 Some researchers believe that allocation concealment, rather than a perfect test and a non-hidden distribution plan or distribution plan to hide imperfections test, often exaggerated treatment effect of 30−41%.28 Other limitations of this study included that the sample sizes were not large and all the retrieved literature were English. In addition, unpublished studies were not included in the analysis. These factors may have resulted in bias.
Conclusion
In conclusion, on the basis of the present evidence, tamsulosin is an effective treatment for the relief of LUTS in women when compared with placebo. However, some limitations of the study may have resulted in bias, and the results should be interpreted cautiously. Hence, high-quality and adequately powered RCTs are required.
References
Moller LA, Lose G, Jorgensen T . The prevalence and bothersomeness of lower urinary tract symptoms in women 40-60 years of age. Acta Obstet Gynecol Scand 2000; 79: 298–305.
Jones C, Hill J, Chapple C Guideline Development Group. Management of lower urinary tract symptoms in men: summary of NICE guidance. BMJ 2010; 340: c2354.
Robinson D, Giarenis I, Cardozo L . New developments in the medical management of overactive bladder. Maturitas 2013; 76: 225–229.
Kim KH . Ischemic tolerance induced by cerebral preconditioning: new urologic research area. Int Neurourol J 2010; 14: 201–202.
Robinson D, Cardozo L . Pharmacotherapy of overactive bladder syndrome. Expert Rev Clin Pharmacol 2008; 1: 163–175.
Boyd K, Hilas O . alpha-adrenergic blockers for the treatment of lower-urinary-tract symptoms and dysfunction in women. Ann Pharmacother 2014; 48: 711–722.
Schwinn D . Novel role for α1‐adrenergic receptor subtypes in lower urinary tract symptoms. BJU Int 2000; 86: 11–22.
Groutz A, Blaivas JG . Non-neurogenic female voiding dysfunction. Curr Opin Urol 2002; 12: 311–316.
Barry MJ, Fowler FJ Jr., O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148: 1549–1557, discussion 1564.
Okamura K, Nojiri Y, Osuga Y, Tange C . Psychometric analysis of international prostate symptom score for female lower urinary tract symptoms. Urology 2009; 73: 1199–1202.
Chai TC, Belville WD, McGuire EJ, Nyquist L . Specificity of the American Urological Association voiding symptom index: comparison of unselected and selected samples of both sexes. J Urol 1993; 150 (5 Pt 2): 1710–1713.
Chancellor MB, Rivas DA . American Urological Association symptom index for women with voiding symptoms: lack of index specificity for benign prostate hyperplasia. J Urol 1993; 150 (5 Pt 2): 1706–1708, discussion 1708-1709.
Boyle P, Robertson C, Mazzetta C, Keech M, Hobbs FD, Fourcade R et al. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int 2003; 92: 409–414.
Terai A, Matsui Y, Ichioka K, Ohara H, Terada N, Yoshimura K . Comparative analysis of lower urinary tract symptoms and bother in both sexes. Urology 2004; 63: 487–491.
Higgins JP, Green S . Cochrane handbook for systematic reviews of interventions version 5.1.0. Available at (http://handbook.cochrane.org/, 2011.
Cui Y, Zong H, Zhang Y . The efficacy and safety of silodosin in treating BPH: a systematic review and meta-analysis. Int Urol Nephrol 2012; 44: 1601–1609.
Yang CS, Huang H, Zhang LL, Zhu CR, Guo Q . Aripiprazole for the treatment of tic disorders in children: a systematic review and meta-analysis. BMC Psychiatry 2015; 15: 179.
Pummangura N, Kochakarn W . Efficacy of tamsulosin in the treatment of lower urinary tract symptoms (LUTS) in women. Asian J Surg 2007; 30: 131–137.
Robinson D, Cardozo L, Terpstra G, Bolodeoku J Tamsulosin Study Group.. A randomized double-blind placebo-controlled multicentre study to explore the efficacy and safety of tamsulosin and tolterodine in women with overactive bladder syndrome. BJU Int 2007; 100: 840–845.
Wang CJ, Huang SW, Chang CH . Effects of tamsulosin on lower urinary tract symptoms due to double-J stent: a prospective study. Urol Int 2009; 83: 66–69.
Hajebrahimi S, Asrbadr YA, Azaripour A, Sadeghi-Bazargani H . Effect of tamsulosin versus prazosin on clinical and urodynamic parameters in women with voiding difficulty: a randomized clinical trial. Int J Gen Med 2011; 4: 35–39.
Shalaby E, Ahmed AF, Maarouf A, Yahia I, Ali M, Ghobish A . Randomized controlled trial to compare the safety and efficacy of tamsulosin, solifenacin, and combination of both in treatment of double-j stent-related lower urinary symptoms. Adv Urol 2013; 2013: 752382.
Kim SO, Hwang EC, Oh KJ, Kwon D, Park K, Ryu SB . Efficacy and safety of combined therapy with tamsulosin and tolterodine in female patients with a maximal flow rate less than 12ml/s. Int Urogynecol J 2011; 22: 1287–1291.
Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res 2002; 11: 563–574.
Oh SJ, Son H, Kim SW, Lee KS, Choo MS, Kim SO et al. Psychometric Properties of the Korean Version of the Overactive Bladder Questionnaire (OAB-q) in a Korean Population. Int Neurourol J 2012; 16: 77–85.
Cipullo LM, Cosimato C, Filippelli A, Conti V, Izzo V, Zullo F et al. Pharmacological approach to overactive bladder and urge urinary incontinence in women: an overview. Eur J Obst Gynecol Reprod Biol 2014; 174: 27–34.
Lo T-S . Alpha blockers in the clinical treatment of female lower urinary tract dysfunction. Incont Pelvic Floor Dysfunct 2010; 4: 69–71.
Wei J, Song Y, Sun L, Lv C . Comparison of artificial total disc replacement versus fusion for lumbar degenerative disc disease: a meta-analysis of randomized controlled trials. Int Orthop 2013; 37: 1315–1325.
Acknowledgements
This project was financially supported by the National Natural Science Foundation of China (no. 81460569) and the Guangxi Zhuang Autonomous Region Health Department of Traditional Chinese Medicine Science and Technology projects, Guangxi Province, China (nos GZZJ13-17 and gzzc1233).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, H., Huang, Z., Qiu, Y. et al. Tamsulosin for treatment of lower urinary tract symptoms in women: a systematic review and meta-analysis. Int J Impot Res 29, 148–156 (2017). https://doi.org/10.1038/ijir.2017.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijir.2017.12
- Springer Nature Limited
This article is cited by
-
Outcomes of bladder neck botulinum toxin injection for female primary bladder neck obstruction—does subjective improvement correlate with an objective assessment?
International Urogynecology Journal (2023)
-
The effect of tamsulosin in postoperative urinary retention: a meta-analysis of randomized controlled trials
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
The resonance® metallic ureteral stent in the treatment of malignant ureteral obstruction: a prospective observational study
BMC Urology (2019)
-
Effects of urine alkalinization with sodium bicarbonate orally on lower urinary tract symptoms in female patients: a pilot study
International Urogynecology Journal (2018)