Abstract
Time of peak bat activity during the night differs among bat species due to temperature, prey availability, habitat availability, and/or interactions between species. Habitat availability is altered in urban areas, which may affect insect prey availability and interspecies interactions. Our objectives were to use mobile acoustic monitoring to determine when bat species were active in a single night in urban and nonurban sites and if nightly bat activity patterns differed in urban versus nonurban sites. Bat echolocation call sequences were recorded using Anabat acoustic detectors while driving transects through the night at five sites (three “urban” and two “nonurban”) located in the Piedmont region of north-central North Carolina from May through August 2016. Transects were driven three times per night starting 45 min, 180 min, and 300 min after sunset. Recorded echolocation call sequences were analyzed manually using AnalookW and automatically using Bat Call Identification and Echoclass software. Total bat activity was not different between urban and nonurban sites. However, total bat activity was lower later in the night in urban sites, but stayed the same in nonurban sites. Species specifically, there were more Eptesicus fuscus, Lasionycteris noctivagans, and Tadarida brasiliensis call sequences and fewer Lasiurus borealis, Nycticeius humeralis, and Perimyotis subflavus call sequences in urban sites than nonurban sites. There were also fewer E. fuscus, L. noctivagans, and N. humeralis call sequences later in the night in both urban and nonurban sites. Only Lasiurus borealis activity in urban sites later in the night reduced and L. borealis activity in nonurban sites remained at the same. These results suggest that bats in urban areas partition time differently, which is important to consider for urban conservation efforts and planning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Competition is an important factor in determining which species are present in communities by excluding species that use environmental resources in the same way (May and MacArthur 1972). Species may be separated into niches along environmental gradients to avoid competitors, resulting in niche partitioning (Schoener 1974). Niches include space, food, and time, which can be partitioned among coexisting species when these species alter their habitat space, diets, or behaviors (Schoener 1974; Jachowski et al. 2014). Temporal partitioning strategies are usually adaptive, with mechanisms that include avoiding predators, energetic limitations, diet quality, and interspecific competition (Bennie et al. 2014).
Time of peak bat activity in a single night (nightly bat activity pattern) can differ among species, which may correspond to prey, habitat availability, and interactions between species (Kunz 1973; Kalcounis et al. 1999; Agosta et al. 2005; Razgour et al. 2011; Jachowski et al. 2014). While temporal partitioning is less common than habitat or dietary partitioning, species in some bat communities partition timing of peak activity and habitat space to reduce competition among sympatric species (Kunz 1973; Kronfeld-Schor and Dayan 2003; Adams and Thibault 2006).
Overall, insectivorous bats emerge during or after peak aerial insect abundance and forage while insect abundance is decreasing (Rydell et al. 1996). Nightly activity patterns can vary among sympatric species, which may be a result of preference and selection for different habitat types or for specific insect groups and sizes as insect activities vary temporally (Kunz 1973; Agosta et al. 2005). For example, peak bat activity has been shown to vary vertically in mature boreal forests, with uniform intra-night activity within and above the canopy and early peak activity below the canopy (Kalcounis et al. 1999). This variation in peak activity may be a result of species-specific foraging differences in the habitat types above and below the canopy. Sympatric bat species also partition foraging periods spatially and temporally in locations where water is scarce (Razgour et al. 2011).
Natural and anthropogenic environmental changes affect bat species distributions and behavior, which can lead to foraging strategy changes in individuals (Jung and Kalko 2010). For example, White-Nose Syndrome (WNS) caused species specific mortality in many bat species, allowing Lasionycteris noctivagans to have earlier peak activity and occupy empty niches after this rapid decline (Jachowski et al. 2014). In a similar mechanism, urbanization alters bat communities through changes in composition and spatial distribution of foraging habitat along with altered prey and roost availability (Avila-Flores and Fenton 2005; Li and Wilkins 2014; Russo and Ancillotto 2015; Rodríguez-Aguilar et al. 2017). Insect prey populations are reduced with increased impervious surfaces in highly urbanized sites, which negatively affects bat foraging activity (Threlfall et al. 2011; Russo and Ancillotto 2015). Responses to urbanization may also depend on roost preferences because some species are able to use roosts that become available in man-made structures when natural roosts are lost (Kunz 1982; Duchamp et al. 2004; Neubaum et al. 2007; Li and Wilkins 2015).
As with other taxonomic groups, only a few generalist species with certain morphological characteristics and foraging strategies may be able to overcome spatial barriers in urban areas (Fabianek et al. 2011; Threlfall et al. 2012; Krauel and LeBuhn 2016). Generalist bat species may be less affected by urbanization than specialized ones because they can take advantage of altered habitats and changes in insect prey in some landscape types (Gehrt and Chelsvig 2004; Shochat et al. 2006; Threlfall et al. 2012; Luck et al. 2013; Russo and Ancillotto 2015). For example, open space adapted bat species have more uniform distribution in urbanized areas and can take advantage of foraging area around streetlights, unlike cluttered space adapted species (Fabianek et al. 2011; Luck et al. 2013; Stone et al. 2015).
Urbanization has an overall negative effect on bat diversity and abundance, but species specific responses to urbanization can be complex (Kurta and Teramino 1992; Gehrt and Chelsvig 2004; Li and Wilkins 2014). Fewer opportunities may lead to more widespread, generalist species, which are recorded more frequently, but because bats are able to move from patch to patch, fragmented landscapes can still serve as habitat for several bat species (Mendes et al. 2014; Li and Wilkins 2014; Nunes et al. 2017). On the other hand, fragmented landscapes may lead to fewer opportunities, so bats have to use habitats that are less optimal (Gehrt and Chelsvig 2003; Mendes et al. 2014).
Examining the implications of habitat conversion and fragmentation on behavior and conservation of bats should be a priority, including investigating activity pattern changes in urban areas (Schoener 1974; Kronfeld-Schor and Dayan 2003). There is evidence that spatial foraging strategies are altered in urban areas, which affects species distributions along urban gradients (Jung and Kalko 2010; Threlfall et al. 2011; Li and Wilkins 2014). It is not known if changes in species distributions from land use changes also affect bat activity peaks in a night. Many bat species have overlapping activity peak periods. If urbanization causes a rapid decline in species that are not able to adapt to urban environments, the remaining species may be able to shift their activity to occupy empty niches and forage at a different time of night in urban versus nonurban areas.
The objective of this study was to investigate overall and species specific nightly bat activity in urban versus nonurban sites. We hypothesized that patterns of nightly bat activity would differ in urban versus nonurban sites. We predicted that patterns of nightly bat activity in urban versus nonurban sites, would differ among species based whether species are urban adaptors. Species that are adapted to urban foraging can adjust their nightly activity patterns to potentially occupy empty niches in urban sites.
Methods
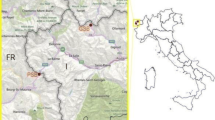
Bat activity was monitored at 5 different sites in the Piedmont region of north-central North Carolina, USA: Burlington (BL; city in Alamance and Guilford Counties; 36°02′37.0”N 79°29′07.9”W), North Greensboro (NG; city in Guilford County; 36°09′31.6”N 79°49′57.6”W), Pine Hall (PH; unincorporated community in Stokes County; 36°25′15.0”N 80°05′00.2”W), Siler City (SC; town in Chatham County; 35°37′41.3”N 79°24′00.5”W), and West Greensboro (WG; city in Guilford County; 36°09′31.6”N 79°49′57.6”W; Fig. 1). All sites are at least 10 km away from each other, a distance that is larger than common species activity ranges (Barclay 1985; Kunz and Fenton 2006).
Locations of driving transects for mobile acoustic monitoring in Burlington (BL), North Greensboro (NG), Pine Hall (PH), Siler City (SC), and West Greensboro (WG) in the Piedmont area of North Carolina (shown as the star in the US map). Developed lands in National Land Cover Database 2011(Homer et al. 2015) are in darker shades of gray. Lighter shades of gray represent forests or farm lands
The acoustic monitoring sites used in the study varied in urban intensity. The urban intensity of study sites was determined using developed land categories from National Land Cover Database 2011 (NLCD 2011, Homer et al. 2015; also see Li and Kalcounis-Rueppell 2018 for examples of developed lands in North Carolina). We used ArcMap (v10.2.2, Esri, Redlands, CA) to create a 1-km radius buffer around each transect and used FRAGSTATS (v4, McGarigal et al. 2012) to calculate the total percentage of developed land cover classes surrounding transects in each site. The total percentage of developed land cover surrounding transects in WG, NG, and BL was 99.8%, 76.0%, and 31.2% respectively. The total percentage of developed land cover surrounding transects in PH and SC was 5.8% and 6.8% respectively (Fig. 1, see electronic supplementary material Online Resource 1 for the colored map with the NLCD 2011 original color code). Using these land cover percentages, we defined that WG, NG, and BL as urban and PH and SC as nonurban. Additionally, the North Greensboro, West Greensboro, and Burlington sites were urban sites in residential/industrial areas with surrounding public parks and other greenspaces. We did not have a site in downtown area with high rise buildings. The Pine Hall and Siler City sites were nonurban sites in areas surrounded by agriculture, pastureland, and forest patches.
Field methods
Anabat acoustic detectors (Anabat SD2, Titley Scientific, Australia) were used for mobile acoustic monitoring with driving transects. The Anabat microphone was mounted on the roof of the vehicle, while the detector itself remained inside the vehicle (Loeb et al. 2015). The microphone was pointed straight up (90 degrees) from the roof to maximize sampling potential (Britzke and Herzog 2009). A Global Positioning Satellite (GPS) unit (Mouse, Titley Scientific, Australia) was used to record transect routes. The data division ratio and audio division ratio used for the Anabat were 8 and 16, respectively. Microphone sensitivity was set between 4 and 5, a level that has been known suitable for species in the study area. All recordings were saved on a compact flash card (SanDisk, USA) that was downloaded and backed up after each night of sampling.
All driving transects were driven in one direction along roads at approximately 32 km per hour. This speed was chosen because it is approximately the upper limit of how fast bats can fly and may help reduce the probability of individual bats being detected multiple times (Britzke and Herzog 2009; Loeb et al. 2015). All transects were driven from a set start point to a set end point while following traffic rules and without making any extra stops. The Anabat detector was set to only record along transect routes and was turned off between transects to ensure that bat were recorded only along these routes (Loeb et al. 2015). Transects were driven only when weather conditions were appropriate (no rain or strong wind, Britzke and Herzog 2009).
Each driving transect was about 3.2 km long with predetermined start and end points at street intersections. Six transects were used at each site to maximize the time spent recording bat call sequences during each time period (see transect details in Online Resource 2). There was minimal overlap of transects within sites. All transects were within approximately a 4 km radius (Fig. 1, black circles). Transect length was shorter than suggested by the North American Bat Monitoring Protocol so that we could sample in urban areas with high road density (see Online Resource 2, NG, WG; Loeb et al. 2015). Transects of this length were chosen to minimize travel time between transects and avoid high traffic areas while being able drive safely at 32 km per hour, even in urban areas. All six transects were driven back to back during one time period in a predetermined randomized order. Within each night, transects were driven at three different time periods to establish bat nightly activity patterns. The first time period (time period 1) was 45 min after sunset, the second (time period 2) was 180 min after sunset, and the last (time period 3) was 300 min after sunset. Each site was sampled during each time period for two nights within about one week (as suggested by Loeb et al. 2015; Wright et al. 2016) during each month of May, June, July and August of 2016.
Acoustic analyses
Both automated and manual approaches were used to analyze bat call recordings from transects. First, AnalookW (version 4.1 t; Titley Scientific, Australia) was used to manually screen for analyzable bat call sequences. An analyzable bat call sequence should include at least three search phase bat echolocation calls in less than 0.5 s (O’Farrell et al. 1999; Kunz and Parsons 2009; Morris et al. 2009). Second, automated identification programs Bat Call Identification (BCID version 2.7c; Bat Call Identification, Inc., Missouri, USA) and EchoClass (version 3.1; U.S Army Engineer Research and Development Center, Mississippi, USA) were used to initially identify the species for analyzable call sequences. Finally, each call sequence was further examined using AnalookW to verify the automated identification of species. Analyzable bat call sequences were manually identified to species based on frequencies that correspond to specific species or groups of species and other species specific call characteristics (such as high frequency, low frequency, characteristic frequency, slopes, duration, and gap; O’Farrell et al. 1999; Kunz and Parsons 2009). The output from the automated identification programs were used as a reference for manual identification. Bat call sequences that could not be completely identified to one species were labeled “NOID” (No Identification). Target species for the sampled region were: Eptesicus fuscus (big brown bat), Lasionycteris noctivagans (silver haired bat), Lasiurus borealis (red bat), Lasiurus cinereus (hoary bat), Myotis lucifugus (little brown bat), Myotis septentrionalis (northern long eared bat), Nycticeius humeralis (evening bat), and Perimyotis subflavus (tricolored bat). Due to the quality of recordings, we did not differentiate M. lucifugus and M. septentrionalis and grouped them together as Myotis group (Myotis spp).
Statistical analyses
The 6 driving transects driven at one site within one time period on one night were treated as a sample in all analyses. Each sample had a total number of bat call sequences from each species (including NOID) and a corresponding temperature. This temperature was calculated using an average of the temperature recorded at the beginning of the first transect driven and the end of the last transect driven in each time period. Temperatures were recorded at the study site using the closest recording station in the Weather Underground mobile app (www.wunderground.com). Statistical differences in temperature between time periods and sites were tested using a series of individual analysis of variance tests (ANOVA). The bat call sequences from 2 nights (time periods driven in the same site and same time of night but on different nights within roughly the same week) were kept separate, as each sample has a unique temperature value. Samples for each time period were taken 2 nights per month over a 4-month sampling period in 5 sites resulting in 120 total samples in analyses.
Generalized linear models were used to analyze the effects of time of night (time period 1, 2, 3) and urban intensity (urban/nonurban) and the interaction of time of night and urban intensity on bat activity (number of call sequences per site) with temperature (°C) as a covariate. There were one model for total bat activity (number of call sequences from all species including NOID) and additional separate models for each species (except Myotis spp. and L. cinereus). For individual species models without a significant interaction term, results were presented from models without the interaction term included. No individual species models were run for call sequences from Myotis spp. and L. cinereus due to the very low recorded call sequences and inability to distinguish the species specific differences in the Myotis spp. call sequences. All models were run on untransformed data. A Poisson distribution was used for all generalized linear models because bat call sequences on each transect were recorded as count data during a fixed time period and each sample was independent. Program R 3.1.1 (R Development Core Team, Vienna, Austria) was used for all statistical analyses.
Results
We recorded a total of 4145 files, with 2618 analyzable bat call sequences over the entire study (Table 1). We identified 1250 (47.7% of analyzable call sequences) to 8 species or species group (abbreviations used in tables and figures): Eptesicus fuscus (EPFU), Lasiurus borealis (LABO), Lasiurus cinereus (LACI), Lasionycteris noctivagans (LANO), Myotis spp. (MYspp), Nycticeius humeralis (NYHU), Perimyotis subflavus (PESU), and Tadarida brasiliensis (TABR). The remaining 1368 call sequences (52.3%) were not identifiable to species (NOID). The number of bat call sequences from all 8 species, including bat call sequences that were not able to be identified to species (NOID), were used to represent total bat activity. We did not include L. cinereus and Myotis spp. for any further individual species analysis. The average number of bat call sequences per sample (including all 8 species and NOID) was 21.82 (SD 13.95).
The mean temperature ± SD for time period 1, 2, and 3 was 22.3 ± 4.5 °C, 20.7 ± 4.6 °C, and 19.9 ± 4.6 °C respectively. We found no significant difference in temperature between time period 1 and time period 2 (df = 2, p = 0.120) and between time period 2 and time period 3 (df = 2, p = 0.462). But there was a significant difference in temperature between time period 1 and time period 3 (df = 2, p = 0.023). The coldest sample was 12.2 °C and the hottest sample was 27.5 °C. Total bat activity was dependent on temperature, with more call sequences recorded on warmer samples (estimate = 0.093, p < 0.001; Table 2).
We found no significant difference in total bat activity in urban sites compared to nonurban sites (estimate = 0.052, p = 0.405; Table 2). There was also no significant decrease in total bat activity later in the night at all sites (estimate = 0.044, p = 0.542 for time period 2 and estimate = 0.090, p = 0.217 for time period 3; Table 3). However, the time period and urban site interaction was significant. We found a significant decrease in total bat activity later in the night in urban sites than earlier but not in nonurban sites (estimate = −0.366, p < 0.001 for urban and time period 2; estimate = −0.410, p < 0.001 for urban and time period 3; Table 3 and Fig. 2), indicating different total bat activity patterns in urban versus nonurban sites.
Total bat activity and species specific bat activity (number of call sequences/sample) for each time period in urban and nonurban sites in the Piedmont area of North Carolina from summer 2016. Time period 1 was 45 min after sunset, time period 2 was 180 min after sunset, and time period 3 was 300 min after sunset. Bat species abbreviations refer to Eptesicus fuscus (EPFU), Lasiurus borealis (LABO), Lasionycteris noctivagans (LANO), Nycticeius humeralis (NYHU), Perimyotis subflavus (PESU), and Tadarida brasiliensis (TABR). Error bars represent ±1 standard deviation
Species specifically, the number of bat call sequences recorded per species increased with an increase in temperature (Table 3) for E. fuscus (estimate = 0.167, p < 0.001), L. borealis (estimate = 0.043, p < 0.001), L. noctivagans (estimate = 0.142, p < 0.001), P. subflavus (estimate = 0.055, p = 0.045), and T. brasiliensis (estimate = 0.095, p = 0.015). A significant increase in call sequences was not observed in N. humeralis as temperatures increased (estimate = −0.010, p = 0.623, Table 3).
We recorded more E. fuscus, L. noctivagans, and T. brasiliensis call sequences in urban than nonurban sites in all three time periods (estimate = 1.136, p < 0.001 for E. fuscus; estimate = 1.694; p < 0.001 for L. noctivagans; estimate = 1.591, p < 0.001 for T. brasiliensis; Table 3 and Fig. 2). We recorded fewer L. borealis, N. humeralis, and P. subflavus call sequences in urban than nonurban sites in all three time periods except for N.humeralis in time period 1 (estimate = −0.666, p < 0.001 for L. borealis; estimate = −0.763, p < 0.001 for N. humeralis; estimate = −1.985, p < 0.001 for P. subflavus; Table 3 and Fig. 2).
We recorded fewer E. fuscus call sequences in both later time periods versus time period 1 (coefficient estimate = −0.421, p = 0.008 for time period 2; coefficient estimate = −0.745, p < 0.001 for time period 3; Table 3 and Fig. 2). We recorded fewer L. noctivagans and N. humeralis call sequences in time period 3 versus time period 1 (coefficient estimate = −0.452, p = 0.009 for L. noctivagans; coefficient estimate = −0.649, p = 0.016 for N. humeralis; Table 3 and Fig. 2). We found a significant interaction term of time period and urban site in L. borealis. We recorded fewer L. borealis call sequences later in the night in urban sites, with a significant decrease during time period 3 and a trend of decrease during time period 2 whereas no such change was found in nonurban sites (estimate = −0.485, p = 0.023 for urban and time period 3; estimate = −0.418, p = 0.051 for urban and time period 2; Table 3 and Fig. 2), indicating nightly activity patterns of L. borealis differed in urban versus nonurban sites. P. subflavus and T. brasiliensis activities did not differ throughout three time periods.
Discussion
We found that urbanization affects total bat activity because total bat activity in nonurban sites showed no difference between time periods, while total bat activity in urban sites decreased later in the night. Lower total bat activity later in the night in urban sites might be due to urban associated species roosting in urban areas and traveling to nonurban areas to forage. For example, E. fuscus readily uses buildings for roosts and can cross large areas of urban habitat to reach preferred foraging habitat in forested and/or agricultural areas (Geggie and Fenton 1985; Kunz and Racey 1998; Duchamp et al. 2004). Similarly, T. brasiliensis also uses buildings for roosts and is known for traveling long distances nightly to forage (Best et al. 2003; Li and Wilkins 2015). It is also possible that bats might travel to other parts of the city (such as city parks or city lakes) as bats could recognize the heterogeneity of the city (Li and Wilkins 2014). In other words, higher total activity in urban areas in time period 1 may be a result of large numbers of bats emerging from dense roosting sites in our study sites (such as homes and buildings) before dispersing to outside of the city or other parts of the city. Interestingly, we only found a similar pattern in L. borealis but not in other species, which suggests more L. borealis urban roosting behavior studies are needed.
Species specifically, our results were consistent with other studies that examined bat community in urban versus nonurban sites. Previous studies have found evidence of E. fuscus and T. brasiliensis adapting to urban areas to roost (Neubaum et al. 2007; Li and Wilkins 2015). This is consistent with the higher activity of E. fuscus and T. brasiliensis that we recorded in urban sites than in nonurban sites. We also recorded higher number of L. noctivagans call sequences in urban areas, which is consistent with what Gehrt and Chelsvig (2004) found near Chicago, IL, USA. L. borealis and P. subflavus are mostly forest associated species and prefer to forage in agricultural land or forest edges or larger forest patches (Walters et al. 2007; Fabianek et al. 2011; Dixon 2011). N. humeralis prefers roosting in tree cavities and foraging in areas that are less developed (Duchamp et al. 2004). These are consistent with the higher activity of L. borealis, P. subflavus, and N. humeralis that we recorded in nonurban sites.
We also found species specific nightly bat activity patterns. A reduction in E. fuscus and L. noctivagans activity later in the night, likely due to a reduction in insect prey activity, was verified by our study (Kunz 1973; Agosta et al. 2005). Specifically, the reduction in activity for L. noctivagans for transects driven 5 h after sunset was consistent with a predicted reduction in activity between two activity peaks around 2–4 h and 6–8 h after sunset (Kunz 1973). There also was a reduction in activity for N. humeralis later in the night which has never been reported by any previous study. However, none of these nightly activity patterns differed in urban versus nonurban sites, suggesting urbanization did not cause an activity shift in these bats in urban sites.
We found a significant decrease in L. borealis call sequences later in the night in urban areas, whereas L. borealis call sequences were consistent throughout the night in nonurban sites. This nightly activity pattern difference in L. borealis might suggest an empty niche in urban areas later in the night. However, L. borealis nightly activity pattern in nonurban sites was different from what previous literature suggested. We expected to observe a decrease in L. borealis call sequences later in the night in nonurban areas because L. borealis has been shown to maximize foraging effort in the first 2 h after sunset and would return to roosts after the initial foraging period (Kunz 1973). In contrast, only what we observed in urban sites was consistent with the expected L. borealis nightly activity pattern. As our results showed that both total bat activity and L. borealis was more active in nonurban sites than in urban sites, it is possible that more L. borealis concentrated in nonurban sites, which increased competition and forced individuals to extend activity to later in the night.
In urban adapted species, the lack of difference in nightly activity patterns between urban and nonurban sites is not consistent with our prediction that species that are adapted to urban foraging would adjust their nightly activity in the absence of species that avoid urban areas. None of these urban adapted species (E. fuscus, L. noctivagans, and T. brasiliensis) had significantly different nightly activity patterns in urban versus nonurban sites. We did not record activity peak shifts in urban sites as compared to nonurban sites, which would have suggested urban adapted species occupying empty niches in urban areas (Jachowski et al. 2014).
In this study, we recorded a very low number of call sequences from genus Myotis. Previous studies have shown that Myotis species could adapt to urban habitats and be benefited from urbanization (Ethier and Fahrig 2011; Coleman and Barclay 2012). Due to the prevalence of White-Nose Syndrome, Myotis species have experienced drastic declines in the US (Dzal et al. 2011; Jachowski et al. 2014; Frick et al. 2015). We suspected that the effect of White-Nose Syndrome on niche partitioning (Jachowski et al. 2014) could be more significant than the effect of urbanization in our study area, which might also explain the lack of difference in nightly activity patterns between urban and nonurban sites. Additionally, our survey method only allowed sampling at certain time periods at night. Future studies should consider methods for continuous monitoring throughout a night for better resolution of bat activity patterns. We also suggest that future studies should sample multiple seasons/years as bat activity is highly related to weather conditions and annual differences are possible.
Overall, our results show that there are species specific nightly bat activity pattern differences in urban versus nonurban areas. While the total amount of call sequences did not change throughout the night in nonurban sites, there was a different activity pattern through the night for total bat activity and L. borealis in urban sites. These results suggest that bats in urban areas might partition time differently, which is important to consider in urban conservation efforts and planning. For example, the decrease of bat activity in urban areas might indicate bats tend to roost in the city and forage more outside of the city. Therefore, it is important to maintain high quality foraging habitats near the city and avoid extensive suburban development. It is also important to consider the potential isolation effects of constructing highway loops surrounding the city, as bats tend to avoid highways (Berthinussen and Altringham 2012; Bennett et al. 2013) and these highway loops can potentially block the commuting routes.
References
Adams RA, Thibault KM (2006) Temporal resource partitioning by bats at water holes. J Zool 270:466–472. https://doi.org/10.1111/j.1469-7998.2006.00152.x
Agosta SJ, Morton D, Marsh BD, Kuhn KM (2005) Nightly, seasonal, and yearly patterns of bat activity at night roosts in the central Appalachians. J Mammal 86:1210–1219
Avila-Flores R, Fenton MB (2005) Use of spatial features by foraging insectivorous bats in a large urban landscape. J Mammal 86:1193–1204. https://doi.org/10.1644/04-MAMM-A-085R1.1
Barclay RMR (1985) Long- versus short-range foraging strategies of hoary (Lasiurus cinereus) and silver-haired (Lasionycteris noctivagans) bats and the consequences for prey selection. Can J Zool 63:2507–2515. https://doi.org/10.1139/z85-371
Bennett VJ, Sparks DW, Zollner PA (2013) Modeling the indirect effects of road networks on the foraging activities of bats. Landsc Ecol 28:979–991. https://doi.org/10.1007/s10980-013-9874-0
Bennie JJ, Duffy JP, Inger R, Gaston KJ (2014) Biogeography of time partitioning in mammals. Proc Natl Acad Sci U S A 111:13727–13732. https://doi.org/10.1073/pnas.1216063110
Berthinussen A, Altringham J (2012) The effect of a major road on bat activity and diversity. J Appl Ecol 49:82–89. https://doi.org/10.1111/j.1365-2664.2011.02068.x
Best TL, Geluso KN, Ammerman LK (2003) Summer foraging range of Mexican free-tailed bats (Tadarida brasiliensis mexicana) from Carlsbad cavern, New Mexico. Southwest Nat 48:590–596. https://doi.org/10.1894/0038-4909(2003)048<0590:SFROMF>2.0.CO;2
Britzke ER, Herzog C (2009) Using acoustic surveys to monitor population trends in bats. U.S. Army Engineer Research and Development Center, Vicksburg
Coleman JL, Barclay RMR (2012) Urbanization and the abundance and diversity of prairie bats. Urban Ecosyst 15:87–102. https://doi.org/10.1007/s11252-011-0181-8
Dixon MD (2011) Relationship between land cover and insectivorous bat activity in an urban landscape. Urban Ecosyst 15:683–695. https://doi.org/10.1007/s11252-011-0219-y
Duchamp JE, Sparks DW, Whitaker JO (2004) Foraging-habitat selection by bats at an urban-rural interface: comparison between a successful and a less successful species. Can J Zool 82:1157–1164
Dzal Y, McGuire LP, Veselka N, Fenton MB (2011) Going, going, gone: the impact of white-nose syndrome on the summer activity of the little brown bat (Myotis lucifugus). Biol Lett 7:392–394. https://doi.org/10.1098/rsbl.2010.0859
Ethier K, Fahrig L (2011) Positive effects of forest fragmentation, independent of forest amount, on bat abundance in eastern Ontario, Canada. Landsc Ecol 26:865–876. https://doi.org/10.1007/s10980-011-9614-2
Fabianek F, Gagnon D, Delorme M (2011) Bat distribution and activity in Montreal Island green spaces: responses to multi-scale habitat effects in a densely urbanized area. Ecoscience -QUEBEC 18:9–17
Frick WF, Puechmaille SJ, Hoyt JR, Nickel BA, Langwig KE, Foster JT, Barlow KE, Bartonička T, Feller D, Haarsma AJ, Herzog C, Horáček I, van der Kooij J, Mulkens B, Petrov B, Reynolds R, Rodrigues L, Stihler CW, Turner GG, Kilpatrick AM (2015) Disease alters macroecological patterns of north American bats. Glob Ecol Biogeogr 24:741–749
Geggie JF, Fenton MB (1985) A comparison of foraging by Eptesicus fuscus (Chiroptera: Vespertilionidae) in urban and rural environments. Can J Zool 63:263–266. https://doi.org/10.1139/z85-040
Gehrt SD, Chelsvig JE (2003) Bat activity in an urban landscape: patterns at the landscape and microhabitat scale. Ecol Appl 13:939–950
Gehrt SD, Chelsvig JE (2004) Species-specific patterns of bat activity in an urban landscape. Ecol Appl 14:625–635. https://doi.org/10.1890/03-5013
Homer CG, Dewitz JA, Yang L et al (2015) Completion of the 2011 National Land Cover Database for the conterminous United States-representing a decade of land cover change information. Photogramm Eng Remote Sens 81:345–354
Jachowski DS, Dobony CA, Coleman LS, Ford WM, Britzke ER, Rodrigue JL (2014) Disease and community structure: white-nose syndrome alters spatial and temporal niche partitioning in sympatric bat species. Divers Distrib 20:1002–1015. https://doi.org/10.1111/ddi.12192
Jung K, Kalko EKV (2010) Where forest meets urbanization: foraging plasticity of aerial insectivorous bats in an anthropogenically altered environment. J Mammal 91:144–153
Kalcounis MC, Hobson KA, Brigham RM, Hecker KR (1999) Bat activity in the boreal forest: importance of stand type and vertical strata. J Mammal 80:673–682. https://doi.org/10.2307/1383311
Krauel JJ, LeBuhn G (2016) Patterns of bat distribution and foraging activity in a highly urbanized temperate environment. PLoS One 11:e0168927. https://doi.org/10.1371/journal.pone.0168927
Kronfeld-Schor N, Dayan T (2003) Partitioning of time as an ecological resource. Annu Rev Ecol Evol Syst 34:153–181. https://doi.org/10.1146/annurev.ecolsys.34.011802.132435
Kunz TH (1973) Resource utilization: temporal and spatial components of bat activity in central Iowa. J Mammal 54:14–32. https://doi.org/10.2307/1378869
Kunz TH (1982) Ecology of bats. Springer, New York
Kunz TH, Fenton MB (2006) Bat ecology. University of Chicago Press, Chicago
Kunz TH, Parsons S (2009) Ecological and behavioral methods for the study of bats. Johns Hopkins University Press, Baltimore
Kunz TH, Racey PA (1998) Bat biology and conservation. Smithsonian Institution Press, Washington, D.C.
Kurta A, Teramino JA (1992) Bat community structure in an urban park. Ecography 15:257–261
Li H, Kalcounis-Rueppell M (2018) Separating the effects of water quality and urbanization on temperate insectivorous bats at the landscape scale. Ecol Evol 8:667–678. https://doi.org/10.1002/ece3.3693
Li H, Wilkins KT (2014) Patch or mosaic: bat activity responds to fine-scale urban heterogeneity in a medium-sized city in the United States. Urban Ecosyst Salzbg 17:1013–1031. https://doi.org/10.1007/s11252-014-0369-9
Li H, Wilkins KT (2015) Selection of building roosts by Mexican free-tailed bats (Tadarida brasiliensis) in an urban area. Acta Chiropterologica 17:321–330. https://doi.org/10.3161/15081109ACC2015.17.2.007
Loeb SC, Rodhouse JT et al (2015) A plan for the North American bat monitoring program (NABat). Gen Tech Rep SRS-208 Asheville NC US Dep Agric For Serv South Res Stn 208:1–100
Luck GW, Smallbone L, Threlfall C, Law B (2013) Patterns in bat functional guilds across multiple urban centres in south-eastern Australia. Landsc Ecol 28:455–469. https://doi.org/10.1007/s10980-012-9842-0
May RM, MacArthur RH (1972) Niche overlap as a function of environmental variability. Proc Natl Acad Sci U S A 69:1109–1113
McGarigal K, Cushman SA, Ene E (2012) FRAGSTATS v4: Spatial pattern analysis program for categorical and continuous maps. Computer software program produced by the authors at the University of Massachusetts, Amherst. http://www.umass.edu/landeco/research/fragstats/fragstats.html. Accessed 27 Jan 2016
Mendes ES, Pereira MJR, Marques SF, Fonseca C (2014) A mosaic of opportunities? Spatio-temporal patterns of bat diversity and activity in a strongly humanized Mediterranean wetland. Eur J Wildl Res 60:651–664
Morris AD, Miller DA, Kalcounis-Rueppell MC (2009) Use of forest edges by bats in a managed pine forest landscape. J Wildl Manag 74:26–34. https://doi.org/10.2193/2008-471
Neubaum DJ, Wilson KR, O’shea TJ (2007) Urban maternity-roost selection by big brown bats in Colorado. J Wildl Manag 71:728–736. https://doi.org/10.2193/2005-684
Nunes H, Rocha FL, Cordeiro-Estrela P (2017) Bats in urban areas of Brazil: roosts, food resources and parasites in disturbed environments. Urban Ecosyst 20:953–969. https://doi.org/10.1007/s11252-016-0632-3
O’Farrell MJ, Miller BW, Gannon WL (1999) Qualitative identification of free-flying bats using the AnaBat detector. J Mammal 80:11–23. https://doi.org/10.2307/1383203
Razgour O, Korine C, Saltz D (2011) Does interspecific competition drive patterns of habitat use in desert bat communities? Oecologia 167:493–502
Rodríguez-Aguilar G, Orozco-Lugo CL, Vleut I, Vazquez L-B (2017) Influence of urbanization on the occurrence and activity of aerial insectivorous bats. Urban Ecosyst 20:477–488. https://doi.org/10.1007/s11252-016-0608-3
Russo D, Ancillotto L (2015) Sensitivity of bats to urbanization: a review. Mamm Biol - Z Für Säugetierkd 80:205–212. https://doi.org/10.1016/j.mambio.2014.10.003
Rydell J, Entwistle A, Racey PA (1996) Timing of foraging flights of three species of bats in relation to insect activity and predation risk. Oikos 76:243–252. https://doi.org/10.2307/3546196
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39
Shochat E, Warren PS, Faeth SH, et al (2006) From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–191. doi: https://doi.org/10.1016/j.tree.2005.11.019
Stone EL, Harris S, Jones G (2015) Impacts of artificial lighting on bats: a review of challenges and solutions. Mamm Biol - Z Für Säugetierkd 80:213–219. https://doi.org/10.1016/j.mambio.2015.02.004
Threlfall C, Law B, Penman T, Banks PB (2011) Ecological processes in urban landscapes: mechanisms influencing the distribution and activity of insectivorous bats. Ecography 34:814–826. https://doi.org/10.1111/j.1600-0587.2010.06939.x
Threlfall CG, Law B, Banks PB (2012) Sensitivity of insectivorous bats to urbanization: implications for suburban conservation planning. Biol Conserv 146:41–52. https://doi.org/10.1016/j.biocon.2011.11.026
Walters BL, Ritzi CM, Sparks DW, Whitaker JO (2007) Foraging behavior of eastern red bats (Lasiurus Borealis) at an urban-rural interface. Am Midl Nat 157:365–373
Wright WJ, Irvine KM, Rodhouse TJ (2016) A goodness-of-fit test for occupancy models with correlated within-season revisits. Ecol Evol 6:5404–5415. https://doi.org/10.1002/ece3.2292
Acknowledgements
Funding was provided by the University of North Carolina at Greensboro Department of Biology, the North Carolina Academy of Science. Equipment was provided by U.S. Fish and Wildlife Service Competitive State Wildlife Grant Program [grant number SC-U2-F14AP00958]. Feedback during project development, data analysis, and the writing process was provided by S. Faeth, G. Wasserberg, A. Larsen, R. Petric, K. Parker, and A. Matteson. Assistance with data collection was provided by A. Wagoner, A. Campos, C. Wicker, K. Corson, R. Gameros, C. Melendez, S. Singleton, B. Wysong, S. Schimpp, and T. Schimpp, which was greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No conflict of interest is declared.
Electronic supplementary material
ESM 1
Locations of driving transects for mobile acoustic monitoring in Burlington (BL), North Greensboro (NG), Pine Hall (PH), Siler City (SC), and West Greensboro (WG) in the Piedmont area of North Carolina. Urban intensity of the study sites is shown with developed land categories from National Land Cover Database 2011. Color coded land cover types can be found in Homer et al. (2015). (PDF 32447 kb)
ESM 2
Detailed transects (6 transects as a set) at each sampling site, Burlington (BL), North Greensboro (NG), Pine Hall (PH), Siler City (SC), and West Greensboro (WG) in the Piedmont area of North Carolina. Urban intensity of the study sites is shown with developed land categories from National Land Cover Database 2011. Color coded land cover types can be found in Homer et al. (2015). (PDF 7703 kb)
Rights and permissions
About this article
Cite this article
Schimpp, S.A., Li, H. & Kalcounis-Rueppell, M.C. Determining species specific nightly bat activity in sites with varying urban intensity. Urban Ecosyst 21, 541–550 (2018). https://doi.org/10.1007/s11252-018-0737-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-018-0737-y