Abstract
Activity and species-specific responses of insectivorous bats in different urban-forest conditions provides a general perspective on the adaptability and vulnerability of bat species towards urbanization intensity. Here we evaluated species richness and activity patterns of aerial insectivorous bats across an urbanized landscape in the highlands of Chiapas, in Mexico. Acoustic monitoring of echolocation calls was conducted for 27 nights over a period of four months. Species richness and relative activity of insectivorous bats were estimated in a landscape with different conditions of urbanization intensity: urban areas, non-urban and forest areas. We identified a total of 14 bat species and three phonotypes. Bat species richness and relative activity was similar (X2 = 0.568, gl = 2, p > 0.05), but species composition differed among conditions. We observed a significant higher occurrence of Bauerus dubiaquercus, Eptesicus brasiliensis and Myotis californicus in forest sites. Urban sites presented higher occurrence of Molossus rufus and phonotype Molossidae 2, while non-urban sites presented a higher occurrence of Eptesicus furinalis and phonotype Molossidae 2. We were able to identify bat species according to their relative activity in relation todifferent landscape conditions. Species of the Molossidae family presented the highest activity in urban sites, which was positively affected by the number of streetlights, while species of the Vespertilionidae presented the highest activity in forest sites, which was positively related totree density. While urbanization tends to diminish native biodiversity and alter faunal communities, our results show a similar richness and relative activity of aerial insectivorous bats along the urban ecosystem. The effect of urbanization intensity becomes more apparent in species-specific bat activity; the response of species towards particular habitat conditions depends on local habitat quality and characteristics (i.e., presence of streetlights, vegetation cover and tree density).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization is an important and complex factor that promotes the fragmentation and complete transformation of natural habitats, directly affecting the structure and composition of species assemblages through abiotic and biotic changes (Alberti 2005; Luniak 2004; Grimm et al. 2008; Voigt and Kingston 2016). As with all fragmentation processes, urbanization implies a reduction in forest cover and patch sizes and can be correlated with shifts in animal density and richness values (McKinney 2002). An important portion of natural ecosystems worldwide has been urbanized rapidly over the last five decades; urban population analysis trends have estimated that the a global urban population of around 70 % of the total world human population will be urban population by 2050 with ca. 6.3 billion urban dwellers living in cities (UN 2014). Most of this growth will occur in cities with less that one million people (i.e small and medium-sized cities), not in megacities (Fragkias et al. 2013; Montgomery 2008). Even there is no common agreement about what urban means, in general an urban area is consider as such if >50 % of impermeable surface (grey area) and more than 10 people/ha is present. However, there is a significant variation in the criteria for defining what is urban (for a discussion see Seto et al. 2013).
Many countries are facing rapid development and growth: over the last 20 years, many countries such as Mexico, have experienced important demographic transitions with migration taking place from rural to urban locations (Seto et al. 2011). Today in Latin America, around 80 % lives in urban settlements and a significant further increase is expected over the next 30 years, projections for 2050 show an increase of urban-dwellers in this region to reach 90 % of the total population (UN 2014. This urban expansion will impact heavily on natural resources, especially on the quality and availability of water and on biodiversity, as well as affecting human health. This will be particularly true in areas with low economic income and high social vulnerability (i.e. developing countries, Lyytimäki et al. 2008).
Research on the effect of urbanization is increasing; however, more detailed information is required in order to adequately assess the role of wild and domestic species in urban ecosystems (Beninde et al. 2015) and to understand how urbanization influences wild species distribution and its provision of ecosystem services (MacGregor-Fors and Ortega-Álvarez 2013; Pauchard and Barbosa 2015). Generating such information is key forguiding cities, and their surrounding areas, towards appropriate urban planning and sustainable policies (SCBD 2012).
Among other flying vertebrates, bat and its populations are expected to be affecting (at both global and local scales) by the process of urbanization (IUCN 2015). They can be especially susceptible to human-driven perturbations (i.e., land-use and land-cover change), some usually persisting only in areas of low human presence and unperturbed ecosystems (Jung and Kalko 2011). However, many species can coexist in urban ecosystems (McKinney 2006, 2008). Many previous studies examining urban bat species have principally focused on habitat structure, showing that bats assemblages are more diverse in urban ecosystems with larger vegetation patches (Gehrt and Chelsvig 2003; Threlfall et al. 2016), at local scale usually bats communities are beneficial by presence of large trees and abundant nocturnal invertebrates (Avila-Flores and Fenton 2005).
Studies have revealed that, while urbanization could be in general unfavorable to aerial insectivorous bat diversity and abundance (Pierson 1998; Geggie and Fenton 1985; Kurta and Teramino 1992), the capacity to adapt to the urban ecosystems is probably species-specific (Russo and Ancillotto 2015). Certain aerial insectivorous bat species have been found to use buildings forroosting and to feed on insects attracted by the light emitted from streetlights, these species made extensive use of urban habitats (Rydell 1992; Avila-Flores and Fenton 2005; Ellison et al. 2007; Jung and Kalko 2010). In contrast, studies have reported higher species diversity in patches within the urban matrix than patches outside the city (see Gehrt and Chelsvig 2004). In tropical cities, bat diversity and activity has been recorded as significant higher in low urban construction areas (Hourigan et al. 2010), natural forest sites and the forest-town interface (Jung and Kalko 2011).
In the Neotropical region, urban or residential areas usually present lower species richness and relative activity in comparison to forest areas; however certain bat species are positively affected by the presence of streetlights and actually increase their relative activity in urban areas (Avila-Flores and Fenton 2005; Jung and Kalko 2010, 2011; Threlfall et al. 2013). Insectivorous bat species of the Molossidae family have shown a positive association with urban areas while species of the Vespertilionidae family have been shown negatively related to cities and recorded mostly in natural areas (Avila-Flores and Fenton 2005; Jung and Kalko 2010).
Other studies conducted on urban ecosystems have generalized that bat activities declines as urban components increase, also that some species could take advantages of streetlights but most of bats species will not, and finally urban ecosystems containing natural and semi-natural areas could support more bat activities (Dixon 2012).
The advances in acoustic monitoring has caused an increase in the studies of aerial insectivorous bats in urban ecosystems (Avila-Flores and Fenton 2005; Hourigan et al. 2006; Kalko et al. 2007; Jung and Kalko 2010, 2011), but detailed information on why and how certain insectivorous bat species use urban ecosystems is still needed.
Our primary objective was to further expand our understanding of the effect of urbanization intensity on aerial insectivorous bat species in the Southwestern region of Mexico under a rapid urban growth process. Using acoustic sampling, we assessed insectivorous bat species composition, richness and relative activity at different levels of urbanization and evaluated how these are associated with local habitat factors and urban variables. We expected that (i) the species richness and relative activity of insectivorous bats would were lowest in urban areas and highest in forested areas, and (ii) the response of insectivorous bats at selected urbanization levels would be species-specific, with higher activity of Molossidae bats in urban areas due to the attraction of insects to the street lights and higher activity of Vespertilionidae bat species in the forested areas.
Materials and methods
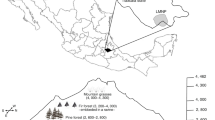
This study was conducted in the region known as the highlands of Chiapas (HLCH), Mexico (Fig. 1a). The study region (ca. 1144.39 sq. km) is increasingly marked by the growing influence of urban areas, with cities expanding in both number and size. The HLCH is home to ca. one million residents (INEGI 2011), ~75 % of these people is actually living in cities; some projections forecast a change of this proportion (INEGI 2010), showing an increase of urban-dwellers in this region to over 90 % by 2050. The HLCH region is immersed in a complex socio-ecological region and is recognized as one of the most important cultural and natural centers in Mexico (INEGI 2010). Moreover, the region has a high biodiversity and endemism and provides a broad suite of ecosystem services (Vázquez and Gaston 2006). The landscape consists of mountains and valleys and altitude ranges from 1200 to 2700 masl (INEGI 2011). The region has experienced a significant increase in the human population (50 %) overthe last 20 years, mainly in urban areas (INEGI 2010).
a Study area and location of study sites in the southeastern state of Chiapas, Mexico. b Aerial images of study sites, A San Cristóbal, B Teopisca, C Betania, D Bochilte, E Corazon de Maria, F Amatenango, G Huitepc, H Rancho Nuevo, I Chichihuixtan. A-C represents urban areas; D-F non-urban areas; and G-I- forest areas
Landscape and environmental variables
Landscape was categorized based on the level of urbanization and native remnant vegetation (forest) cover using Arc Map (ESRI, Redlands, California, USA, version 10.0) and GIS layers SPOT 5 (multispectral with resolution of 10 m per pixel with four bands and a panchromatic with a spatial resolution of 2.5 m per pixel). Landscape was categorized as: urban (>20 dwellings/ha, 10 % vegetation cover and >50 % build cover); non-urban, i.e. shrublands, agricultural patches, and crop-lands (5–20 dwellings/ha, 5–40 % vegetation cover and 3–50 % build cover); and forest (<5 dwellings/ha, >40 % vegetation cover, and <3 % build cover). Nine study sites (3 site for each category) were assigned to one of three categories based on urbanization level and environmental conditions: urban, non urban and forest areas (see Table 1, Fig. 1b). At each site, one quadrant was randomly selected for quantifying the proportion of area covered by urban infrastructure. We also recorded human population density, percentage of impermeable surface (asphalt and constructions that prevent the infiltration of water), housing with a basic provision of electricity, water supply, drainage and paved streets (data from, INEGI 2011) and forest cover (%). All sampling sites were selected based on two SPOT 5 satellite images from 2010 and imported to Arc Gis 10.0 software.
In order to characterize urban areas in each site, we recorded some environmental variables including human population density (Pop. Den), percentage of asphalt (Per.asph), building density (Buil.den), percentage of area with no tree cover (Per.no.vegcov), tree density (Tree.den) percentage of native vegetation (% nat.veg) and number of streetlights (Num.st.lights; Table 1, Cook 2002; MacGregor-Fors 2011; MacGregor-Fors and Schondube 2011). Population density and building density values were both obtained from a population census (INEGI 2011), while the percentage of asphalt and areas with no tree cover were estimated using Google Earth Pro, the streetlights were quantified in situ and tree density was estimated in one quadrant of 50 × 20 m per site.
Bat data
We conducted acoustic sampling monthly between April and July of 2012. Sampling night consisted of four hours of sampling divided in two periods each. First starting at two hours after astronomical twilight and a second period starting two hours before astronomical dawn and finish it before sunrise, which are the two periods of highest insectivorous bat activity (Rydell et al. 1996; Hayes 1997; Meyer et al. 2004). Bats are affected by cold and rainy conditions (Voigt et al. 2011) and we therefore did not survey on cold (< 0 °C) or extreme rainy days (> 100 mm) in order to minimize the probability that weather induced changes in activity influenced our activity estimates. Acoustic surveys are the most efficient methods on the study of aerial insectivorous bats in Neotropical regions (Meyer et al. 2011). However, there are some factors affecting detection of echolocation calls: environmental conditions, vegetation structure and calls intensity (Grindal and Brigham 1999; Limpens 2002). Here, in order to minimize possible bias of these factors an active-sampling survey approach was used for recording echolocation calls, walking along pre-established routes of 2 km in length at a speed of 3.2 km/h (Orozco-Lugo 2007).
Echolocation calls were recorded in real-time with a Ultramic 250Kmicrophone (Dodotronic) at a sample rate of 250,000 Hz, 16 bits, in WAV format (Waveform audio file format) connected via USB port to a laptop (Samsung NP300V4A Electronics) using the SeaWave 2.0 (G. Pavan/CIBRA, 1998–2011) software and a sampling frequency range of between 0 and 125 kHz. It should be noted that there is a tendency in bat detector surveys to overestimate the abundance of louder species and underestimate the activity of quieter species. Loud species are more likely to be present in open (urban or non-urban) sites while quiet species may be more likely to be found in close (forest) sites. This is a likely source of bias, and therefore the activity and diversity of bats in open areas has probably been overestimated compared to those of more closed areas.
Sound analysis and species identification
To determine the occurrence of bat individuals, we considered search-phase calls of sufficient intensity (i.e good quality sound) and every recording was registered as a pass, regardlessof the presence of feeding buzzes (complete sequence of echo pulses). Various studies have documented quantitative and qualitative parameters of the echolocation calls of different bat species that were also used for identification of their recorded echolocation-calls (e.g. Fenton and Bell 1981; O′Farrell et al. 1999; Orozco-Lugo 2007; Rizo-Aguilar 2008).
The parameters that were measured and compared with previously described echolocation data included: maximum amplitude frequency, initial (Fi) and final (Ff) frequency, pulse duration (ms), duration of the interval between pulses (ms), bandwidth (difference between Fi and Ff) and qualitative parameters of the pulse form, including frequency-modulated (FM), constant frequency (CF) or quasi-constant frequency (QCF; Schnitzeler and Kalko 2001). These parameters were analyzed in the program Bat Sound 3.2 (Pettersson Elektronics, Uppsala, Sweden), with a Hanning window type and a Fast Fourier Transformation of 1024, identical to a frequency resolution of 244.141 Hz. To identify bat species, we compared all of the parameters measured per sound call with the parameters described in previous published echolocation data (e.g. O′Farrell and Miller 1999; Ochoa et al. 2000; Granados-Herrera 2001; Siemers et al. 2001; Ratcliffe et al. 2004; Rodriguez and Mora 2006; Orozco-Lugo 2007; MacSwiney et al. 2008; Rizo-Aguilar 2008; Fuentes-Moreno 2010; Kraker 2010; Orozco-Lugo et al. 2011).
Data analyses
We standardized acoustic sampling to a sampling effort of 154 min of recording per night (i.e. 77 min for both sunset and dawn recordings). We estimated species richness, considering the presence and absence of species for each sampling site and calculated a relative activity index by recording the occurrence of species in one-minute intervals, divided by the total nightly sampling effort of 154 min (Miller 2001). In order to evaluate the representativeness of the sampling effort, we generated species accumulation curves for each of three conditions based on the CHAO2 species richness estimator using EstimateS (Colwell 2006), recommended for small-grained sample sizes (Hortal et al. 2006). In addition, we made rank curves of relative activity of species to show the composition of the species assemblage in each condition and a relative activity index by species (Magurran 2004). The curves were divided into ranks of 5, representing the first range of 0 to 5 species with a higher rate of relative activity and so on until the last 30 ranges.
Normality of relative activity values was subjected to a Shapiro-Wilk test, but proved non-normal even after anattempt to normalize the data using logarithm transformations. Differences in relative activity among conditions were subsequently analyzed with a Kruskal-Wallis non-parametric test. Jaccard index was estimated to verify species similarity among the three conditions. We also conducted a canonical correspondence analysis (CCA) to analyze the relationship between the relative activity of bat species and the environmental variables. This analysis permits exploration of the composition and community structure of insectivorous bats through out an urban ecosystem (TerBraak 1986; Sherry and Henson 2005). A Pearson correlation test was used to explore correlation among the independent environmental variables. All analyses were carried out with the software Past version 2.16 (Hammer et al. 2001), except for the CCA, which was conducted using the CANOCO package (TerBraak and Smilauer 2002).
Results
From a total of 27 sampling periods we achieved a total of 4158 min of acoustic sampling were with three sites per condition. From that sampling effort we identified a total of 14 species and three phonotypes of insectivorous bats. These species and phonotypes belong to three insectivorous bat families: Mormoopidae (1 species), Molossidae (4 species) and Vespertilionidae (9 species) (Fig. 2 and Table 2). Each phonotype includes a mix of species whose echolocation calls are to similar to be assigned to a particular species but easily assigned to a particular family of bat species. The species accumulation curves approached an asymptote, indicating that sampling effort was sufficient to encounter the majority of the potential bat species present in the study area (Fig. 3).
Spectrogram with search calls of the aerial insectivorous bat species recorded in the study area. The species Molossus rufus and M. sinaloae presented high and low frequency pulses, represented by a and b, and Tadarida brasiliensis presented two different searching pulses. The complete species names are presented in the Table 3
Bat species richness was similar among conditions; we recorded 15 species (including two phonotypes) in each of the three conditions. According to Jaccard similarity index the three conditions recorded a 0.76 similarity, with 13 share species and four exclusive in each condition. However, we found differences in bat community compositions among the different conditions. Bauerus dubiaquercus (Van Gelder’s bat) and Eptesicus brasiliensis (Brazilian Brown bat) were not recorded in urban areas, while Mormoops megalophylla (Ghost-Faced bat) and Myotis californicus (California Myotis) were absent from the non-urban areas and there was no detected occurrence of the phonotypes Molossidae 1 and Vespertilionidae 1 in the forests.
Overall, the relative activity of bat species was similar among conditions (X2 = 0.568, gl = 2, p > 0.05); however, the rank relative activity curves showed differences in bat species activity per condition (Table 3). Molossus rufus (Black Mastiff bat) and the phonotype Molossidae 2 presented the highest relative activity in urban sites, while three species showed higher relative activity in the forests: Myotis velifer (Cave Myotis) Eptesicus furinalis (Argentinian Brown bat) and Lasiurus intermedius (Northern Yellow bat). Non-urban sites presented the highest activity of Eptesicus furinalis, Molossus rufus and phonotype Molossidae 2 (Fig. 4).
Curves ranges relative activity of aerial insectivorous bats in a the three conditions; urban areas, forests and non-urban areas and separated for b urban areas, c forests and d non-urban areas. The point value of each species represents the sum of the relative activity index for each condition. See bat species names abbreviations on Table 3
Building density, human population density and asphalt percentage were significantly correlated and were therefore excluded from the CCA. The number of streetlights also presented a significant correlation with human population density (Table 4); however, we decided not to exclude this variable because of its importance in explaining the occurrence and activity of insectivorous species (Rydell 1992; Jung and Kalko 2010). Tree density, percentage of tree cover and number of streetlights, ordered bat species according to their specific relative activity per condition (CCA: test of significance for all canonical axes of the canonical correspondence analysis: trace =0.623, F = 2.781, P = 0.005 with 1000 unrestricted permutations). Number of streetlights was the most important factor in terms of separating bat species and was significantly correlated with the 1st canonical axis (correlation ratio = 0.87, eigenvalue =0.417, F = 3.589, P = 0.016; 1000 unrestricted permutations). Molossid bat species showed a tendency of increase along with the number of streetlights, while the relative activity of Vespertilionid and Mormoopid species increased with increasing tree density (Fig. 5).
Canonical correspondence analysis (CCA) ordination illustrating the relative activity of aerial insectivorous bats and environmental variables based on acoustic sampling in three sites per condition; urban areas (triangles), non-urban areas (diamonds) and forests (crosses). The vectors length indicates the explanatory importance of the environmental variables in separating bat species according to their relative activity. Bat species are plotted as circles and the abbreviations represent the following names: Bdub, Bauerus dubiaquercus; Ebra, Eptesicus brasiliensis; Efur, E. furinalis; Efus, E. fuscus; Eund, Eumops underwoodi; Lcin, Lasiurus cinereus; Lint, L. intermedius; Mcal, Myotis californicus; Mnig, M. nigricans; Mvel, M. velifer; Mmeg, Mormoops megalophylla; Mruf, Molossus rufus; Msin, M. sinaloae; Mol1, Molossidae 1; Mol2, Molossidae 2; Tbra, Tadarida brasiliensis; Vesp, Vespertiliondae
Discussion
Our study showed a similar richness among three different urban conditions, even though we had expected to find a lower species richness of aerial insectivorous bats in areas of higher urbanization intensity. Same assemblage composition has been previously found in other Neotropical urban areas (see, Avila-Flores and Fenton 2005; Jung and Kalko 2010, 2011). The proximity of forested mountain areas surrounding the urban matrix increases the landscape heterogeneity and can reduce the impact of urbanization on bat species by providing a large variety of roosts and foraging habitats (Gehrt and Chelsvig 2004; Threlfall et al. 2012a). This would partially explain the occurrence, although relatively low, of several species that can forage in urban areas, but prefer to seek shelter in the surrounding forests and caves (Jung and Threlfall 2016), and clear example of this are Lasiurus cinereus and Myotis californicus using trees for roosting (Brigham et al. 1997; Willis and Brigham 2005).
Contrary to expectation, the relative activity of insectivorous bat species did not differ significantly among the three conditions. This indicates that each of these conditions provide recourses that can maintain the activity of insectivorous bats. For example, non-urban and urban sites provide open-space foraging habitat for certain bat species, while buildings in the urban sites offer roosting opportunities and streetlights could attract insect prey (Rydell 1992; Avila-Flores and Fenton 2005; Ellison et al. 2007). Forest areas provide shelter in the foliage, tree cavities or exfoliated bark, and foraging areas for many close-space aerial insectivorous bat species (Grindal and Brigham 1999; Kunz and Lumsden 2003; Gehrt and Chelsvig 2003).
However, the effect of urbanization on insectivorous bats becomes more visible when looking at the composition of bat species per condition (Threlfall et al. 2012b). At the species-specific level, absence of the bat species Bauerus dubiaquercus and Eptesicus brasiliensis and low activity of Myotis californicus in urban areas suggests that these species are negatively affected by the changes in habitat structure produced by increasing urbanization intensity. The ecomorphological and echolocation characteristics of these species are more suited to foraging in a closed environment, rather than in the more open urban and rural areas (Norberg and Rayner 1987; Schnitzeler and Kalko 2001). In contrast, the species Molossus rufus, Lasiurus cienereus and L. intermedius were recorded in all three conditions, which demonstrates, in addition totheir wide plasticity of foraging, the capacity of these species to adapt to the structural changes of the habitat throughout the urban ecosystem. The urban sites presented the highest occurrence of M. rufus and phonotype Molossidae-2 and these can therefore be considered tolerant towards increasing urbanization intensity. Molossus rufus normally roosts in hollow trees, but has also been encountered in roofs and attics (Esbérard et al. 2005; Carvalho et al. 2011). Most bat species constantly return to their roosting site, but the absence of sufficient hollow trees caused by deforestation practices could force M. rufus individuals to find shelter in urban areas.
From the rank abundance curves of relative activity and the CCA ordination graph, it was possible to identify assemblages of species with the highest activity for each condition, related to several environmental variables. This pattern of assemblages has been documented in other studies in the Neotropics, where relative activity is concentrated in groups of insectivorous bat species according to their similarity in sensitivity towards urbanization (Avila-Flores and Fenton 2005; Jung and Kalko 2011), or reorganized by ecomorphological and echolocation characteristics at family level.
As expected, species of the Molossidae family were mostly active in the urban areas. The elongated narrow wings of Molossid bats permit high velocities and reduced energy costs of flight and their echolocation characteristics permit the location of fast flying prey from a relatively long distance. These ecomorphological attributes have favored foraging in open areas or those with low percentage of tree cover (Norberg and Rayner 1987; Jones 1999; Altringham 2011). The forested areas presented the expected highest relative activity of species of the Vespertilionidae family. These bats are characterized by high maneuverability and produce short broadband FM calls, which enable them to forage in closed spaced areas such as the interior of forests (Norberg and Rayner 1987; Kalko 1998).
The number of streetlights was a component closely associated to urban areas, and this factor possibly defined species assembly in these sites through its influence on food resources. However, artificially increased light could have effects on behavior and foraging efficiency (Lewanzik and Voigt 2014) since these illuminated areas offer a greater availability of food within a relatively small area. Some studies have reported that both distribution and abundance of nocturnal prey insects are mainly influenced by housing densities and artificial streetlights (Caryl et al. 2016; Russo and Ancillotto 2015). In addition, it is possible that artificially lit areas have direct impacts on the behavior of some bats species, which is reflected in a higher or lower energy costs during foraging.
In summary, study of the richness, activity and species-specific responses of insectivorous bats in different urban-forest conditions provides a general perspective on the adaptability and vulnerability of bat species towards urbanization intensity. Only two bat species were not encountered in urban areas, which implies that most insectivorous bats are able to adapt to anthropologically modified landscapes. One of these bat species, B. dubiaquercus is listed as nearly threatened (Miller and Medina 2008) and is mostly restricted to forested areas (Engstrom et al. 1987; Reid 1997; Miller and Medina 2008). Although this species is still widely distributed, populations along its distribution range have been in decline (Miller and Medina 2008) and increasing human population density and consequent urban expansion could have future negative consequences for the conservation of this species. We propose that future studies on the impact of urbanization on insectivorous bats should identify the threatened species and focus on studying their specific roosting and foraging behavior in order to gather detailed information on their ecology and design appropriate species-specific conservation plans.
References
Alberti M (2005) The effects of urban patterns on ecosystem function. Int Reg Sci Rev 28:168–192
Altringham JD (2011) Bats from evolution to conservation. Oxford University Press, Oxford, 368 pp
Avila-Flores R, Fenton M (2005) Use of spatial features by foraging insectivorous bats in a large urban landscape. J Mammal 86:1193–1204
Beninde J, Veith M, Hochkirch A, Haddad N (2015) Biodiversity in cities needs space: a meta-analysis of factors determining intra-urban biodiversity variation. Ecology Letters 18(6):581–592
Brigham RM, Vonhof MJ, Barclay RM, Gwilliam JC (1997) Roosting behavior and roost-site preferences of forest-dwelling California bats (Myotis Californicus). J Mammal 78:1231–1239
Carvalho CD, Gonçales JF, Franco R, Casagrande DKA, Pedro WA, Queiroz LH (2011) Bat (Chiroptera, Mammalia) survey and rabies virus occurrence in northwest region of São Paulo state, Brasil. Veterinária e Zootecnia 18:490–503
Caryl FM, Lumsden LF, van der Ree R, Wintle BA (2016) Functional responses of insectivorous bats to increasing housing density support ´land-sparing´ rather than ´land-sharing´ urban growth strategies. J Appl Ecol 53:191–201
Colwell RK (2006) EstimateS: statistical estimation of species richness and shared species from samples, Version 8.2.0. Available from http://viceroy.eeb.uconn.edu/EstimateS.
Cook E (2002) Landscape structure indices for assessing urban ecological networks. Landsc Urban Plan 58:269–280
Dixon MD (2012) Relationship between land cover and insectivorous bat activity in an urban landscape. Urban Ecosyst 15:683–695
Ellison L, O′sheda T, Neubaum D, Bowen R (2007) Factors influencing movement probabilities of big Brown bats (Eptesicus fuscus) in buildings. Ecol Appl 17:620–627
Engstrom MD, Lee TE, Wilson DE (1987) Bauerus dubiaquercus. Mammalian Species 282:1-3
Esbérard CEL, Jesus AC, Motta AG, Bergallo HG, Gettinger D (2005) Hesperoctenes fumarius (Hemiptera: Polyctenidae) infesting Molossus rufus (Chiroptera: Molossidae) in Southeastern Brazil. J Parasitol 91(2):465-46
Fenton B, Bell GP (1981) Recognition of species of insectivorous bats by their echolocation calls. J Mammal 62:233–243
Fragkias M, Güneralp B, Seto KC, Goodness J (2013) A synthesis of global urbanization projections. In: Elmqvist T et al (eds) Urbanization, biodiversity and ecosystem services: Challenges and opportunities. Springer, pp 409–436
Fuentes-Moreno H (2010) Estructura del ensamble de murciélagos de la Venta, Oaxaca, México. Tesis de Maestría. Instituto Politécnico Nacional, Oaxaca, 63 pp
Geggie JF, Fenton MB (1985) A comparison of foraging by Eptesicu sfuscus (Chiroptera: Vespertilionidae) in urban and rural environments. Can J Zool 63:263–267
Gehrt S, Chelsvig J (2003) Bat activity in an urban landscape: patterns at the landscape and microhabitat scale. Ecol Appl 13:939–950
Gehrt S, Chelsvig J (2004) Species-specific patterns of bat activity in an urban landscape. Ecol Appl 14:625–635
Granados-Herrera J (2001) Caracterización de los llamados de ecolocalización de los murciélagos insectívoros del estado de Yucatán, México. Dissertation, Mexico, Distrito Federal, pp 72
Grimm NB, Faeth SH, Golibiewski NE, Redman CL, Wu J, Bai X, Briggs JM (2008) Global change and the ecology of cities. Science 319:756–760
Grindal S, Brigham R (1999) Impacts of forest harvesting on habitat use by foraging insectivorous bats at different spatial scales. Ecoscience 6:25–34
Hammer O, Harper DA, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Hayes J (1997) Temporal variation in activity of bats and the design of echolocation-monitoring studies. J Mammal 78:514–524
Hortal J, Borges P, Gaspar C (2006) Evaluating the performance of species richness estimators: sensitivity to sample grain size. J Anim Ecol 75:274–287
Hourigan C, Johnson C, Robson S (2006) The structure of a micro-bat community in relation to gradients of environmental variation in a tropical urban area. Urban Ecosys 9:67–82
Hourigan CL, Catterall CP, Jones D, Rhodes M (2010) The diversity of insectivorous bat assemblages among habitats within a subtropical urban landscape. Austral Ecology 35(8):849–857
INEGI (2010) Cuentame. Consulted 10th of Jan 2016, in http://cuentame.inegi.org.mx/poblacion/rur_urb.aspx?tema=P
INEGI (2011) México en cifras. Consulted 10th of Jan 2016, in http://www3.inegi.org.mx/sistemas/mexicocifras/default.aspx?e=07
IUCN (2015) The IUCN Red List of Threatened Species. Version 2015-3. http://www.iucnredlist.org. Downloaded on 8 September 2016
Jones G (1999) Scaling of echolocation call parameters in bats. J Exp Biol 202:3359–3367
Jung K, Kalko EK (2010) Where forest meets urbanization: foraging plasticity of aerial insectivorous bats in an anthropogenically altered environment. J Mammal 91:144–153
Jung K, Kalko EK (2011) Adaptability and vulnerability of high flying Neotropical aerial insectivorous bats to urbanization. Divers Distrib 17:262–274
Jung J, Threlfall CG (2016) Urbanisation and its effects on bats – a global meta-analysis. In: Voigt et al (eds) Bats in the Antrropocene: conservation of bats in a changing world. Springer. doi:10.1007/978-3-319-25220-9_2
Kalko EK (1998) Organisation and diversity of tropical bat communities through space and time. Zoology 101:281–297
Kalko EKV, Villegas SE, Schmidt M, Wegmann M, Meyer CFJ (2007) Flying high--assessing the use of the aerosphere by bats. Integr Comp Biol 48(1):60-73
Kraker C (2010) Análisis de la alimentación de murciélagos insectívoros en cultivos de café del departamento de Sacatepéquez. Informe final Proyecto No. 12–2008, Fodecyt, Guatemala, pp 86
Kunz TH, Lumsden LF (2003) Ecology of cavity and foliage roosting bats. Pp. 3–69, in Ecology of bats (Kunz TH, Fenton MB eds.). The University of Chicago Press, Chicago and London, pp 798
Kurta A, Teramino JA (1992) Bat community structure in an urban park. Ecography 15:257–261
Lewanzik D, Voigt C (2014) Artificial light puts ecosystem services of frugivorous bats at risk. J Appl Ecol 51:388–394
Limpens HJGA (2002) Field identification: using bat detectors to identify species. In: Brigham RM, Kalko EKV, Jones G, Parsons S, Limpens HJGA (eds) Bat Echolocation research: tools, techniques, and analysis. Bat Conservation International, Austin, TX, pp. 46–58
Luniak M (2004) Synurbization - adaptation of animal wildlife to urban development. In: Shaw et al. (2004) (eds) Proceedings 4th International Urban Wildlife Symposium
Lyytimäki J, Petersen LK, Normander B, BezÁk P (2008) Nature as a nuisance? Ecosystem services anddisservices to urban lifestyle. Environ Sci 5:161–172
MacGregor-Fors I (2011) Misconceptions or misunderstandings? On the standardization of basic terms and definitions in urban ecology. Landsc Urban Plan 100:347–349
MacGregor-Fors I, Ortega-Álvarez R (2013) Ecología urbana: Experiencias en América Latina. www1.inecol.edu.mx/libro_ecologia_urbana
MacGregor-Fors I, Schondube JE (2011) Gray vs. green urbanization: relative importance of urban features for urban bird communities. Basic Appl Ecol 12:382–381
Macswiney MC, Clarke FM, Racey PA (2008) What you see is not what you get: the role of ultrasonic detector in increasing inventory completeness in Neotropical bat assemblages. J Appl Ecol 45:1364–1371
Magurran AE (2004) Ecological diversity and its measurement. Wiley-Blackwell, p 264
Mckinney M (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
Mckinney M (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260
Mckinney M (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosys 11:161–176
Meyer CFJ, Schwarz CJ, Fahr J (2004) Activity patterns and habitat preferences of insectivorous bats in a west African forest–savanna mosaic. J Trop Ecol 20:397–407
Meyer CFJ, Aguiar LMS, Aguirre LF, Baumgarten J, Clarke FM, Cosson J-F, Villegas SE, Fahr J, Faria D, Furey N, Henry M, Hodgkison R, Jenkins RKB, Jung KG, Kingston T, Kunz TH, Gonzalez MCM, Moya I, Patterson BD, Pons J-M, Racey PA, Rex K, Sampaio EM, Solari S, Stoner KE, Voigt CC, von Staden D, Weise CD, Kalko EKV (2011) Accounting for detectability improves estimates of species richness in tropical bat surveys. J Appl Ecol 48(3):777–787
Miller B (2001) A methods for determining relative activity of free flying bats using a new activity index for acoustic monitoring. Acta Chiropterol 3:93–105
Miller B, Medina A (2008) Bauerus dubiaquercus. The IUCN Red List of Threatened Species. Version 2014.3. <www.iucnredlist.org>. Downloaded on 12 September 2016
Montgomery MR (2008) The urban transformation of the developing world. Science 319:761–764
Norberg U, Rayner J (1987) Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philos Trans R Soc Lond 316:335–427
O′Farrell MJ, Miller B (1999) Use of vocal signatures for the inventory of free-flying Neotropical bats. Biotropica 31:507–516
O′Farrell MJ, Miller B, Gannon WL (1999) Qualitative identification of free-flying bats using the anabat detector. J Mammal 89:11–23
Ochoa J, O′Farrel MJ, Miller B (2000) Contribution of acoustic methods to the study of insectivorous bat in protected area from northern Venezuela. Acta Chiropterol 2:171–183
Orozco-Lugo CL (2007) Efecto de la perturbación del hábitat en la comunidad de murciélagos insectívoros de la selva baja caducifolia. Dissertation, Universidad Nacional Autónoma de México, Distrito Federal, 118 pp
Orozco-Lugo CL, Cuaron A, Valenzuela D, Vazquez E (2011) Nuevos registros de murciélagos para la isla de Cozumel y observaciones sobre sus abundancias. III Congreso Mexicano de Ecología, Sociedad Científica Mexicana de Ecología, A.C., Boca del Río
Pauchard A, Barbosa O (2015) Regional assessment of Latin America: rapid urban development and social economic inequity threaten biodiversity hotspots. In: Elmqvist T et al (eds) Urbanization, biodiversity and ecosystem services: Challenges and opportunities. Springer, pp. 589–608
Pierson ED (1998) Tall trees, deep holes, and scarred landscapes: conservation biology of North American bats. In: Kunz TH, Racey PA (eds) Bat biology and conservation. Smithsonian Institution Press, Washington, pp. 309–325
Ratcliffe JM, TerHofstede HM, Avila-Flores R et al (2004) Conespecific influence call design in the Brazilian free-tailed bat, Tadarida brasiliensis. Can J Zool 82:966–971
Reid F (1997) A field guide to the mammals of Central America and Southeast Mexico. Oxford University Press, New York, p 346
Rizo-Aguilar A (2008) Descripción y análisis de los pulsos de ecolocación de 14 especies de murciélagos insectívoros aéreos del Estado de Morelos. Dissertation, Instituto de Ecología, A. C., Xalapa, pp. 101
Rodriguez A, Mora E (2006) The echolocation repertoire of Eptesicus fuscus (Chiroptera: Vespertilionidae) in Cuba. Caribb J Sci 42:121–128
Russo D, Ancillotto L (2015) Sensitivity of bats to urbanization: a review. Mammalian Biology-Zeitschrift für Säugetierkund 80:205–212
Rydell J (1992) Exploitation of insects around streetlamps by bats in Sweden. Funct Ecol 6:744–750
Rydell J, Entwistle A, Racey P (1996) Timing of foraging flights of three species of bats in relation to insect activity and predation risk. Oikos 76:243–252
SCBD-Secretariat of the Convention on Biological Diversity (2012) Cities and Biodiversity Outlook. SCBD, Montreal, pp 64
Schnitzeler H, Kalko EK (2001) Echolocation by insect-eating bats. Bioscience 51:557–569
Seto KC, Fragkias M, Güneralp B, Reilly MK (2011) A meta-analysis of global urban land expansion. PloSOne 6(8):e23777. doi:10.1371/Citation
Seto KC, Parnell S, Thomas E (2013) A Global Outlook on Urbanization. In: Elmqvist T et al (eds) Urbanization, biodiversity and ecosystem services: challenges and opportunities. Springer, pp 1–12
Sherry A, Henson RH (2005) Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. J Pers Assess 84:37–48
Siemers BM, Kalko EK, Schnitzeler H (2001) Echolocation behavior and signal plasticity in the Neotropical bat Myotisnigricans (Schinz, 1821) (Vespertilionidae): a convergent case with European species of Pipistrellus? Behav Ecol Sociobiol 50:317–328
TerBraak JF (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179
TerBraak CJF, Smilauer P (2002) CANOCO Reference manual and Canodraw for Windows user’s guide: Software for Canonical Community Ordination (version 4.5). Microcomputer Power, Ithaca
Threlfall CG, Law B, Banks PB (2012a) Influence of landscape structure and human modifications on insect biomass and bat foraging activity in an urban landscape. PLoS One 7(6):e38800
Threlfall CG, Law B, Banks PB (2012b) Sensitivity of insectivorous bats to urbanization. Biol Conserv 146:41–52
Threlfall CG, Law B, Banks PB (2013) The urban matrix and artificial light restricts the nightly ranging behaviour of gould's long-eared bat (nyctophilus gouldi). Austral Ecology 38:921–930
Threlfall CG, Williams NSF, Hahs AK, Livesley SJ (2016) Approaches to urban vegetation management and the impacts on urban bird assemblages. Landsc Urban Plan 153:28c39
UN-United Nations (2014) World urbanization prospects: the 2014 revision. United Nations, New York
Vázquez L-B, Gaston KJ, (2006) People and mammals in Mexico: conservation conflicts at a national scale. Biodivers Conserv 15(8):2397–2414
Voigt CC, Kingston T (2016) Bats in the Anthropocene. Pages 1–9. In: Voigt CC, Kingston T (eds) Bats in the Anthropocene: conservation of bats in a changing world . doi:10.1007/978-3-319-25220-9_1Springer Open
Voigt CC, Schneeberger K, Voigt-Heucke SL, Lewanzik D (2011) Rain increases the energy cost of bats flight. Biol Lett-UK 7:793–795
Willis CK, Brigham RM (2005) Physiological and ecological aspects of roost selection by reproductive female hoary bats (Lasiurus cinereus). J Mammal 86:85–94
Acknowledgments
This research was partially funded by a master scholarship awarded to the first author by CONACyT-Mexico No. 255206. We are grateful to the authorities of the different local communities for access to the study sites, to ECOSUR for the use of facilities and equipment and to David Douterlungne for statistical assistance. We also thank anonymous reviewers who greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez-Aguilar, G., Orozco-Lugo, C.L., Vleut, I. et al. Influence of urbanization on the occurrence and activity of aerial insectivorous bats. Urban Ecosyst 20, 477–488 (2017). https://doi.org/10.1007/s11252-016-0608-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-016-0608-3