Abstract
Information about bat migration routes across the Alps is generally scarce and there is no existing data available for the Italian part of the chain. Through acoustic surveys, we explored the possibility that even a region characterized by high Alpine mountains may be crossed by migrant bats. Data were recorded in August–September 2016 at two sites located near mountain passes in the Aosta Valley (NW Italy), respectively for 29 and 53 entire nights. Activity of different species/acoustic groups of species was associated with period and weather variables, the most important of which was wind speed (negatively related), followed by temperature (positively related). Only the acoustic group N. leisleri/N. noctula/V. murinus/E. serotinus, at both sites, showed a significant increase in activity in the period 31 August–14 September. Additional elements suggesting the occurrence of a late-summer migratory flow involving this group were the fact that it mainly consists of migratory species; the attribution to N. leisleri of the sequences that could be identified at the species level; and the timing of activity through the night (generally later than the other bats) and some characteristics of the recorded calls. Contacts with B. barbastellus were recorded at both study sites, possibly due to migrating individuals or, as an alternative, to resident bats using open environments located far from woods during the summer. The occurrence of P. kuhlii was ascertained at the highest elevation so far reported for this species in the Alps (2208 m a.s.l.).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migration is a complex natural phenomenon which profoundly influences the life cycles of many species. It is a solution to avoid seasonal unfavorable conditions, but it also involves major threats: migrants are vulnerable to habitat changes that may occur along their migration routes, affecting their orientation, reducing the availability of stepping stones or exposing them to direct mortality factors (Hardesty-Moore et al. 2018). In recent years, the widespread development of wind farms has proved to be a significant threat to migratory birds and bats (e.g., Rydell et al., 2017; Thaxter et al., 2017). The importance of understanding and mitigating the impact of such infrastructures has become a goal of ecological studies, which in turn have acted as an impetus for the implementation of legislation, making it particularly important to collect accurate information on migratory routes. However, in contrast to birds, for which many detailed studies have been carried out on this topic, there is only partial information about the routes through which bats choose to migrate and the strategies they adopt to cross or avoid ecological barriers. Some studies have suggested that they may use large rivers as landmarks for orientation and that valley bottoms are migration flyways (Strelkov 1969; Serra-Cobo et al. 2000; Furmankiewicz and Kucharska 2009). Bats have also been found to migrate along coasts (Jarzembowski 2003; Petersons 2004; Strelkov 1969) and to cross open sea (Ahlén 1997; Ahlén et al. 2007; Strelkov 1969; Walter et al. 2005; Hüppop and Hill 2016). Migrating bats can also move through mountain areas, the Alpine chain included (Bontadina et al. 2014; Widerin and Reiter 2017). In the Western Alps, migrating bat flows have been documented at Col de Jaman (1512 m a.s.l.) and at Col de Bretolet (1923 m a.s.l.), between Switzerland and France (Oppliger 2004). More recently, transits of bats, including long-distance migratory species, have been reported for a site located at 3460 m a.s.l. in the Swiss Alps, suggesting that bat migration can occur even at very high elevation (Zingg and Bontadina 2016).
At present, the routes followed by migratory bats in Italy are unknown (Hutterer et al. 2005). Here, we explored the possibility that the Aosta Valley—the northwesternmost region of Italy—may be used by migrating bats despite being characterized by mountains that are among the highest in Europe. High elevation areas are usually considered of marginal importance to bats, but their role may be overlooked. Understanding if they are used by migrating bats could help to address the management of these areas more correctly. This may prove particularly relevant in the event of the expansion of wind farming at high altitude due to growing energy demand (although projects of this type are not currently planned in the Aosta Valley).
Typically, data on bat migration are collected by capturing animals. This method has undoubted advantages, for example, the identification of each species, but, in particular at high altitude, fixed stations with mistnets (mainly used by ornithologists to ring birds) are scarce. For this reason, we based our research on acoustic methods.
In August–September 2016, we performed acoustic surveys at two alpine passes in the Aosta Valley. Our aims were to provide a preliminary characterization of bat activity at the two sites and to verify the possible occurrence of a bat migratory flow across them. To the latter purpose, we considered two aspects: (I) the presence of species known to migrate, and particularly of long-distance migrants (i.e., Pipistrellus nathusii, Nyctalus leisleri, Nyctalus noctula, and Vespertilio murinus) and (II) the occurrence of activity peaks that could be considered indicatory of migration for being constrained to the migratory period and (compared with the activity peaks of resident species) more conditioned by the period itself than by the meteorological variables.
Materials and methods
Study area
Data were collected close to Gran San Bernardo and Piccolo San Bernardo passes.
Gran San Bernardo pass lies at an elevation of 2472 m and is the lowest pass situated along the northern boundary of Aosta Valley, which separates Italy from Switzerland. Bat activity data were recorded in the Italian territory, at about 1 km from the pass (Fig. 1). The recording site, located at 45.861330° N, 7.159638° E, is identified hereafter as GSB. It coincides with “Casa don A. Carioni,” a building situated at 2208 m a.s.l. (264 m below the pass), in an open alpine landscape dominated by pastures, prairies, and rocks. The site is within the SAC IT1205020, which has been designated to protect typical alpine flora and fauna species (Bocca et al. 2016).
The Piccolo San Bernardo pass has an elevation of 2188 m. It is located on the western border of Aosta Valley, between Italy and France, and is the lowest alpine pass of the northwestern Alps. Compared with the Gran San Bernardo pass, it lies further west: therefore, it is likely to be the most favorable site to cross the mountains for migrating bats engaged in NE-SW flights, i.e., in the main direction followed in Europe by long-distance migratory bats (Hutterer et al. 2005). Bat activity data were recorded at 45.674900° N, 6.880534° E, inside the Chanousia Alpine botanical garden (Fig. 1). The site, hereafter termed PSB, is in France, 670 m from the pass, at an elevation of 2177 m (11 m below the pass). The small botanical garden (about 1 ha) is surrounded by alpine pastures and prairies.
Both the study areas feature harsh climatic conditions: minimum temperatures fall below zero roughly from the beginning of October to the end of May, strong winds are frequent, and snowfalls particularly abundant (Mercalli et al. 2003).
Migrant birds of many species are regularly observed at the two passes, mainly during autumn migration, but no systematic study of the migration has been carried out up to now, neither at Gran San Bernardo and Piccolo San Bernardo sites nor elsewhere in the Aosta Valley. However, available data suggest that the region is crossed by minor bird migration flows (Bocca and Maffei 1997).
Surveys on the migration of noctuid moths, carried out at the Gran San Bernardo pass, proved that the area is crossed by migratory transits of these insects. While resident species are more abundant from June to the middle of August, peaks of migratory moths can occur (during periods of several nights or on single nights) both in the early and in the late summer/autumn, but they seem more important in the latter period, when flight direction is south-oriented (Hachler et al. 2002).
Data recording
Bat activity was surveyed using two Wildlife Acoustics Inc. (Maynard, MA) bat detectors: an SM2BAT+ equipped with an SMX-UT microphone at GSB and an SM4BAT FS with an SMM-U1 ultrasonic microphone at PSB. These instruments have a similar sensitivity to bat calls and it has been demonstrated that SM2 performs similarly to Batcorder 2.0 (Adams et al., 2012). Both bat detectors were positioned on the outside of isolated buildings, at about 5 m from the ground, with the microphones oriented towards each alpine pass. They were set to record automatically from about 30 min before sunset to sunrise, with a sampling rate of 384 kHz, trigger level 12 dB, trigger window 2 s, and maximum length of recordings set to 8 s. Further settings were Dig HPF fs/48 (8 kHz) and Dig LPF off (SM2BAT+); Min Trig Freq 10 kHz and 16 k Hight Filter off (SM4BAT FS).
Surveys started on 1 August 2016 and were carried out until the middle of September at GSB and until the end of September at PSB. Although the equipment was checked and the batteries replaced about every fortnight, some operating interruptions occurred, probably caused by premature exhaustion of some batteries due to low temperatures. Excluding interruptions, at GSB, the survey lasted 29 nights: from 1 to 7 August (6 nights) and from 17 August to 9 September (23 nights). At PSB, recording was performed continuously for 53 nights: from 1 August to 23 September.
Weather data were obtained from the nearest meteorological stations.
In the case of Gran San Bernardo, air temperature and precipitation data, measured hourly, were collected a short distance below the pass (Col Grand-Saint-Bernard, 2360 m a.s.l., weather station managed by Regione Autonoma Valle d’Aosta), at a linear distance of about 1 km from site GSB. Wind data were collected every 10 min at a site located about 2.3 km from GSB (Grand-Saint-Bernard Mont-Botsalet, 2500 m a.s.l., weather station managed by Regione Autonoma Valle d’Aosta); the anemometer was situated at a height of 10 m above ground level.
At Piccolo San Bernardo, all the data were collected every 30 min close to the pass (Col Petit-Saint-Bernard, 2188 m a.s.l., weather station managed by Osservatorio meteorologico Piccolo San Bernardo), at a distance of 900 m from PSB; the anemometer was at a height of 8 m above ground level.
Sound analysis
Sound analysis was performed in two steps.
Search-phase echolocation calls were first analyzed using the software BatSound version 4.03 (Petterson Elektronik AB, Uppsala, S). A 512-pt Hamming window (98% overlap) was applied. We identified the echolocation sequences that could be attributed to genera or species according to the criteria presented in Appendix I.
The second step of analysis was performed using the software SonoBat 3.11P (DNDesign, Arcata, CA) to measure the calls, and the identification tool iBatsID (https://sites.google.com/site/ibatsresources/iBatsID; Walters et al., 2012) to identify the species/acoustic groups. The procedure was applied to the sequences of echolocation calls that were not identifiable according to the criteria followed in the first step of analysis. A highly conservative approach was adopted: for each sequence, several search-phase calls were examined (average 5.7) and identifications suggested by iBatsID were accepted when concordant (i.e., calls extracted from the same sequence were classified in the same way) and associated with a probability of correct classification ≥ 95% for most of the calls.
In many cases, the identification procedure did not allow to definitively identify species, and was therefore interrupted at earlier stages of identification (due to groups of species with similar echolocation calls). This was the case for almost all the sequences attributed to the group composed by N. leisleri, N. noctula, V. murinus, and E. serotinus. Considering the poor performance of iBatsID in the identification of V. murinus, we assigned the few sequences that had been attributed to this species to the same group. In some cases, we referred to an even larger species assemblage, adding to the former group a fifth species, E. nilssonii; this was done when some calls of the sequences were attributed to E. nilssonii and others to the species of the group. Finally, several sequences of poor quality QCF or FMQCF calls (not measurable by SonoBat) were attributed to N. leisleri/N. noctula/V. murinus/E. serotinus or to N. leisleri/N. noctula/V. murinus/E. serotinus/E. nilssonii on the basis of their shape, frequencies, and bandwidth.
We did not try to identify species of the genus Myotis, considering both the limits of the identification tool and the scarce relevance of reaching a specific identification for bats that do not include long-distance migrants. Similarly, the identification procedure was stopped at the genus level for Plecotus spp., since iBatsID does not consider P. macrobullaris, which is known to occur in the Aosta Valley.
Some echolocation sequences of poor quality, mainly represented by very faint recordings, were attributed to “chiroptera not otherwise identified”.
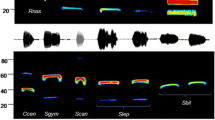
When present, social calls were used for specific identification, referring to descriptions reported by Russo and Jones (1999 and 2000), Pfalzer (2002 and 2007), Pfalzer and Kusch (2003), Skiba (2009), Barataud (2012), and Middleton et al. (2014).
Quantification of bat activity
Bat activity was quantified by an activity index (AI) based upon the presence/absence of defined acoustic categories during 1-min time intervals and consisting of the sum of minutes with the presence of a given category. This method was firstly proposed by Miller (2001), with reference to the species level. Compared with “bat pass” counts, it is time-saving, more objective, and less inflated by the behavior of those bats that repeatedly fly within the range of the detector (a problem even more relevant if the goal of the survey is to detect transit of migrants). Furthermore, it minimizes the discrepancies that may arise from the use of different bat detectors.
However, relying on this methodological approach also poses some problems because the current methods of acoustic analysis do not allow all the acoustic sequences to be identified to the species level which can cause problems in quantifying total bat activity. When quantifying general bat activity, in order to avoid this problem, we considered the active minute with respect to the order (Chiroptera), regardless of the number of species occurring during that minute.
A second consequence of the problems encountered in call identification is that results usually comprise some categories that are not mutually exclusive (for example, in a given minute, some of the recorded sequences may be attributed to P. kuhlii and others to the species pair P. kuhlii/P. nathusii). There were therefore definitely identified calls (“certain” minutes), and those for which identification was less certain (“possibly additional” minutes). The previous example would result in one active minute for P. kuhlii and one “possibly additional” minute for P. nathusii. In these cases, the analysis of data is more complicated and makes it necessary to apply one of the following solutions: pooling non-mutually exclusive categories (in the example, this means considering together the species pair P. kuhlii/nathusii, counting only one active minute referred to this acoustic group); examining different possibilities (case A: only 1 min for P. kuhlii; case B: 1 min for P. kuhlii and 1 for P. nathusii); considering only the certain case (1 min for P. kuhlii), while being aware of the limits this may have. We adopted the first solution (i.e., pooling data), discussing the other possibilities if relevant.
Statistical analysis
We analyzed the effect of local weather variables and period on the activity of the species/acoustic groups.
In order to select the weather data relevant to the nightly activity phase of bats, we firstly examined the acoustic recordings. Bat activity preceding sunset was never observed in the surveyed period; the earliest acoustic contact was recorded 16 min after sunset and the latest 37 min before sunrise (Appendix II). Based on these results, we selected the weather data collected between 1 h after sunset and 1 h before sunrise.
The local weather variables considered were total precipitation (mm), mean wind speed (m/s), and mean temperature (°C). A preliminary analysis showed a high correlation between minimum, maximum, and mean temperature values; thus, we selected mean temperature as representative of the entire night conditions since it was derived from multiple measurements per night.
We partitioned the data according to periods of 15 days, forming groups larger than those usually applied in the works on bird migration (5–10 days). This was decided a priori, to compensate for the relatively small sample size and to allow comparison with the few published studies which have used periods of either months or half-month periods.
In order to determine variations in species/acoustic group activity in relation to weather conditions and period, a statistical hypothesis testing framework was adopted, with the null hypothesis that bats showed a random activity in relation to weather variables and period. Univariate Generalized Linear Models (GLMs) were used. Quadratic terms for weather variables were included in the models following visual assessment of scatterplots (following Zuur et al. 2009). To reduce correlation among variables, we first examined all pairwise correlations to identify strongly correlated pairs (r > |0.7|). The result of this preliminary analysis showed that none of the above weather variables was highly collinear. To model the distribution of activity index, count (i.e., activity index values for each species/acoustic group) was initially modelled specifying a Poisson error distribution and a log link function. Since these preliminary models showed over-dispersion, a negative binomial error distribution was specified in subsequent models. The number of days of sampling per period was log-transformed and used as an offset in the model to account for the differences in the number of days sampled per period. We performed two different models, one for PSB and one for GSB, due to the difference in the sampling periods between the two sites. Data analysis was performed with R 3.5.1 (R Core Team 2018) and MASS package (Venables and Ripley 2002).
Results
Taxa recorded
We identified the same taxa/acoustic groups in the two survey sites, except for the group P. pipistrellus/M. schreibersii/P. pygmaeus, recorded only at GSB (Table 1).
P. pipistrellus was the species most contacted at both sites, followed by E. nilssonii, the group N. leisleri/N. noctula/V. murinus/E. serotinus, and H. savii at GSB, and by the genus Myotis and the group N. leisleri/N. noctula/V. murinus/E. serotinus at PSB.
Among the species of the group N. leisleri/N. noctula/V. murinus/E. serotinus, only N. leisleri could be clearly identified. Sequences attributed univocally by iBatsID to this species, and sequences characterized by alternated call types and frequency of maximum energy of the lower calls > 22 kHz, which can be associated with high probability to N. leisleri, were recorded both at GSB and PSB. We never recorded alternated calls with peak frequency of the lower calls in the range 18–20 kHz, i.e., attributable to N. noctula.
With respect to specific identification, it must be noted that most of the echolocation sequences of this acoustic group that were recorded during the period 31 August–14 September were sequences of QCF calls, both at GSB (86% of the sequences of the group) and at PSB (73%). QCF calls are rarely used by E. serotinus, but they are commonly emitted by the other three species of the group.
Within the genus Pipistrellus, species other than P. pipistrellus were comparatively marginally represented in the recordings.
A social call of P. kuhlii was recorded at GSB on 27 August, proving with certainty the occurrence of this species at the site. Social calls of P. nathusii were never recorded at GSB and PSB; yet, on 14 September, we recorded an advertisement song surely emitted by this species a little below the GSB site (at Saint-Rhémy-En-Bosses, Grand Rocher hamlet, 45.823380 lat. N; 7.178397 long. E, m 1582 a.s.l.).
Several echolocation sequences were attributed to the acoustic groups P. kuhlii/P. nathusii and P. nathusii/P. pipistrellus. Some of them were sequences of QCF calls, whose identification is discussed in Appendix I. Based on the overall results of acoustic analysis (social and echolocation calls), we consider certain the presence, at least occasionally, of P. kuhlii both at GSB and PSB, and probable, although not confirmed with absolute certainty, the presence of P. nathusii.
We did not record calls certainly attributable to P. pygmaeus, but at GSB, we recorded several sequences conservatively attributed to the group P. pipistrellus/M. schreibersii/P. pygmaeus. Among these, there was one sequence of QCF calls with peak energy frequency of 52.3–52.4 kHz which was recorded on 30 August and which looks likely to have been emitted by P. pygmaeus, since the occurrence of M. schreibersii at the site is considered unlikely (for the reasons discussed in Appendix I).
Some marginal activity could be attributed to genus Plecotus and B. barbastellus at both the sites. We never recorded, instead, Tadarida teniotis, whose loud low frequency calls are easily detectable by the instruments and the settings used.
Influence of weather and period on bat activity
Because of the problems in the specific identification based on echolocation calls, it was impossible to quantify the activity of P. kuhlii, P. nathusii and, regarding only GSB, P. pygmaeus (at PSB, we did not detect any activity possibly attributable to this species). The analysis of the activity of the acoustic groups including these species (Table 1) was avoided, because of no clear biological meaning (being referred to mixed resident and migratory species and, moreover, to small sample sizes).
We took into consideration the identified species/acoustic groups (Table 1) that belonged to mutually exclusive categories (Myotis spp., H. savii, Plecotus spp., B. barbastellus) or that showed an activity almost entirely attributable to mutually exclusive categories (P. pipistrellus, E. nilssonii, and the group N. leisleri/N. noctula/V. murinus/E. serotinus, also including in the latter the activity more precisely attributed to N. leisleri).
The models for Plecotus genus and B. barbastellus did not converge, probably due to the limited sample size, and thus, results for those taxa are not presented. Nevertheless, the presence of the barbastelle at the two study sites is noteworthy because of the unusual habitat where it was detected (extensively characterized by alpine pastures, prairies, and rocks, the nearest woods being located at 1.5 km at GSB and 3 km at PSB), and the recording period, compatible with migration (first detection took place on 13 August and all the other contacts were recorded from 25 August onwards).
The following results relate to the remaining species/acoustic groups and to the general bat activity (Chiroptera activity).
During the nights surveyed, air temperature, wind speed, and precipitation varied as summarized in Table 2. Bat activity showed a positive relationship with temperature and a negative one with wind speed and precipitation (Appendix III).
More precisely, the positive relationship with temperature was significant in all the examined models, except for H. savii and E nilssonii at GSB (Appendix IV, Fig. 2). For PSB, where the highest mean temperatures were around 13 °C, the results of GLMs show a linear relationship for all the considered species/acoustic groups, with the exception of the group N. leisleri/N. noctula/V. murinus/E. serotinus, to which a quadratic relationship provided a better fit. At GSB, the highest mean temperatures reached about 14–15 °C, and quadratic terms fitted the data better in most cases, suggesting that bat activity reached a peak at a mean temperature of 10–12 °C; in the case of the group N. leisleri/N. noctula/V. murinus/E. serotinus, there was an evident decrease in activity above the same temperature threshold (see below).
Visualization of univariate GLMs of the activity index of CHIROPTERA (whatever the species), P. pipistrellus, Myotis spp., H. savii, E. nilssonii, and N. leisleri/N. noctula/V. murinus/E. serotinus group (Nl/Nn/Vm/Es) in relation to weather variables for GSB and PSB. Only significant models are shown
Wind speed was even more frequently correlated with bat activity: with the only exception of E. nilssonii at PSB (whose activity did not relate significantly with the variable); all the other species/acoustic groups showed a negative correlation with mean wind speed (Appendix IV, Fig. 2). In particular, sharp decreases in activity were observed when mean wind speeds exceeded 6 m/s, despite some marginal activity, mainly due to the group N. leisleri/N. noctula/V. murinus/E. serotinus, which was recorded up to a mean wind speed of 14 m/s.
During the nights of the survey, precipitation at the two sites was scarce and this probably resulted in a limited association between this variable and bat activity: a significant and negative relationship emerged only for general activity and P. pipistrellus at GSB and for the group N. leisleri/N. noctula/V. murinus/E. serotinus at PSB (Appendix IV, Fig. 2).
Bat activity was influenced by period both at GSB and PSB (Appendix IV, Fig. 3).
Visualization of univariate GLMs of the activity index of CHIROPTERA (whatever the species), P. pipistrellus, Myotis spp., H. savii, E. nilssonii, and N. leisleri/N. noctula/V. murinus/E. serotinus group (Nl/Nn/Vm/Es) in relation to the period of sampling (P1 = 1 Aug–15 Aug; P2 = 16 Aug–30 Aug; P3 = 31 Aug–14 Sep; P4 = 15 Sep–29 Sep) for GSB and PSB. Only significant models are shown
For the most part, significant peaks of activity were recorded in the second half of August (P2). At PSB, this was the case for P. pipistrellus, H. savii, and of Chiroptera activity; Myotis showed maximum activity in the same period both at GSB and PSB, but at GSB, the peak was not significant and at PSB, the value was similar to that observed in the first part of August (P1), differing significantly only from the values recorded from 31 August throughout September (P3 and P4). At GSB, activity values recorded during the second half of August (P2) for P. pipistrellus, H. savii, and Chiroptera activity were higher than those of the first part of the month (P1), but did not differ significantly from September values (P3). As for E. nilssonii, at both sites the activity values were higher in August (P1 and P2) than later (P3), but the difference was significant only for GSB.
The group N. leisleri/N. noctula/V. murinus/E. serotinus showed different results both at GSB and PSB: the activity of this group peaked in the period 31 August–14 September (P3), differing significantly from the activity values recorded in any other period.
In order to determine which covariates were more important in explaining the patterns of activity observed for this group, we ran a model combining the significant variables from univariate models.
The final model discarded non-significant variables according to Zuur et al. (2009).
At GSB, there was a significant effect of period, wind speed, and its quadratic component (Table 3).
Temperature was not significant. According to the univariate analysis, temperature showed an overall positive correlation with the activity of the group, but the data suggested an inverse relationship at (relatively) higher temperatures (Fig. 2). Checking the data set, it emerged that the latter result was influenced by the exceptionally high values of activity of the acoustic group recorded during the nights of 6/7 and 7/8 September, characterized by mean temperatures (7.4 °C) below the average of the period (8.8 °C) and, on the contrary, by the low activity recorded on 23/24 and 24/25 August, when the highest mean temperatures were recorded (around 14 °C) (Fig. 4). We conclude that the multivariate model corrects what looks like an anomalous result (the decrease in activity at higher temperatures), indicating that activity was mainly conditioned by period and wind speed rather than temperature.
For PSB, the model indicated precipitation (negatively related), temperature and its quadratic component, and period as factors affecting the activity of the acoustic group (Table 3).
Therefore, at both sites, there was significant variation in activity according to period and the timing of the activity peaks distinguished this acoustic group from the other bats, which showed different activity patterns. Moreover, during the nights of the peak period, the proportion of activity of N. leisleri/N. noctula/V. murinus/E. serotinus recorded in the central part of the night was comparatively higher than that observed for the other bats; in the case of GSB, the difference was significant (Appendix V). Finally, only 3 feeding buzzes were observed in the echolocation sequences of the acoustic group during the entire period at the two sites.
Discussion
A full understanding of bat migration behavior and the identification of migration routes are difficult yet important goals for bat conservation, particularly regarding the aim of preventing negative effects caused by the creation of wind farms and, more generally, for the implementation of the EUROBATS agreement. Here, we show evidence of bat migration through the Western Italian Alps.
According to the literature (Fleming and Eby 2003; Hutterer et al. 2005; Dietz and Kiefer 2014), most bat species detected at GSB and PSB are sedentary or partly sedentary, but at least one species recorded, N. leisleri, is known to carry out long migrations across Europe (see Appendix VI for a more detailed discussion).
At GSB and PSB, significant variations in bat activity were negatively related to wind speed, positively related to air temperature and, to a lesser extent, negatively related to precipitation. Similar relationships between bat activity and meteorological variables are well documented in the literature (e.g., Erkert 1982; Erickson and West 2002; Reynolds 2006; Cryan and Brown 2007; Horn et al. 2008; Arnett et al. 2008; Baerwald and Barclay 2011).
Bats markedly lowered their activity in the presence of wind speeds higher than 6.0 m/s, as previously reported in case studies at wind farms (e.g., Reynolds 2006; Arnett et al. 2008; Horn et al. 2008; Korner-Nievergelt et al. 2013; Voigt et al. 2015).
Bat activity increased linearly for mean temperatures below 10–12 °C, while between the same threshold and the highest values of mean temperature recorded during the survey (13 °C at PSB, 14–15 °C at GSB), a tendency to interrupt the increase in activity was observed for P. pipistrellus at GSB and for N. leisleri/N. noctula/V. murinus/E. serotinus both at PSB and GSB.
Such observations are largely consistent with published knowledge on bat physiology and the availability of their prey in cold climates: low temperatures raise the energetic costs of bat activity (Racey and Speakman 1987) and, at the same time, they negatively influence the availability of insect-prey (Cockbain 1961; Williams 1961; Taylor 1963; Lewis and Taylor 1965; Alma 1970; Jones et al. 1995). In northern Scandinavia, the availability of aerial insects sharply declined below 14 °C, while they were found to be constantly abundant for temperatures ≥ 14 °C (Speakman et al. 2000). Likewise, increases in bat activity should be expected to stop when an optimal (relative to the area) value of temperature is reached.
The data recorded at GSB for N. leisleri/N. noctula/V. murinus/E. serotinus appear to be partially in contrast to these considerations, suggesting a decrease in the activity of the group at increasingly higher temperatures, but further analysis demonstrated that this result was a consequence of the pre-eminent role of other factors, in particular the period, in explaining the activity of the group.
In both sites, the activity peaks of the identified species/acoustic groups mainly occurred in the second half of August. Only the group N. leisleri/N. noctula/V. murinus/E. serotinus showed a maximum of activity, significantly different from the values recorded in all the other periods, from 31 August to 14 September. Taking into account the period, the fact that the echolocation sequences of the group that could be identified at species level were attributed to N. leisleri (a long-distance migratory bat), the activity peak of the group in the central part of the night (tendentially later than observed for the other bats) and the characteristics of the calls (clear predominance of QCF calls, almost total absence of feeding buzzes), we conclude that a late-summer migratory flow passes through GSB and PSB and that at least one species—N. leisleri—is involved.
Present knowledge concerning the migratory movements of this species across Italy is very limited. The species is present in the country throughout the entire year and some evidence of its reproduction has been reported for northeast (Niederfriniger 2001) and central Italy (Ancillotto and Russo 2015); most records, however, are collected in migratory periods or in winter.
Females are reported to reach a mating area located in the Northern Apennines (central Italy) in late August/early September (Vergari and Dondini 2011); a female banded at the same site was later recaptured within a nursery in Poland, proving the occurrence of migration between the two areas (Dondini et al. 2012).
In Piedmont, in the plain area of La Mandria Park, lesser noctules, comprising a few females, are already present in June, but their number (monitored thanks to the use of bat boxes) increases from the middle of August to reach a maximum in September (P. Debernardi and S. Lacaria, unpublished data). Similar data are reported in Switzerland for Canton Ticino, where the first females are observed in bat boxes during the second half of August (Zambelli et al. 2008).
According to long-term mist-netting results, at Col de Bretolet (1923 m a.s.l.; canton of Valais), male and female lesser noctules transit, with few exceptions, from the middle of August to the end of September, with a peak at the beginning of September; at Col de Jaman (1512 m; Vaud Prealps), the passage begins around the middle of August, has its maximum at the end of August to beginning of September, and, probably thanks to the low elevation, continues until the end of October (Oppliger 2004).
Temporal variations in the activity of the N. leisleri/N. noctula/V. murinus/E. serotinus group at the two study sites are in agreement with the above data, although they did not allow the determination of the end of migratory flow. The data recorded in the second half of September, available only for PSB, showed a general decrease in the activity of the group. Nevertheless, it is impossible to say if the migration came to an end, or if it was just temporarily interrupted, since the period was characterized by an abrupt decrease in temperatures coincidental with a decrease in general bat activity.
Another unanswered question regards the increase in the activity of the acoustic group observed at GSB at the beginning of September, larger than that recorded at PSB: it might indicate that some bats that entered the region remained to winter in the Aosta Valley, or that they continued the migration along different routes; the available data are in any case too scarce to draw any conclusions on the question.
Nevertheless, the data recorded add evidence to previous records suggesting that N. leisleri can migrate across the Alps, transiting at high elevation (Zingg and Bontadina 2016; Widerin and Reiter 2017; Patriarca et al. 2018), which includes the finding of a dead individual of the species on an alpine glacier at 2600 m a.s.l. (Aellen 1962).
Because of identification problems, it is not possible to draw conclusions concerning the other species of the same acoustic group that are known to migrate over long distances, i.e., N. noctula and V. murinus. However, the occurrence of the latter seems more likely because the species, contrary to N. noctula, has already been recorded in the Aosta Valley (Patriarca and Debernardi 2014).
Some social calls of P. nathusii, the other long-distance migrating bat species, were recorded during the survey a little below GSB, proving without any doubt the presence of this species in the Aosta Valley, which was previously only conjectured (Patriarca and Debernardi 2014). It is not possible to draw conclusions about the presence of P. nathusii at GSB and PSB because of the problems in identification of its echolocation calls, whose characteristics largely overlap with those of the calls emitted by P. pipistrellus and P. kuhlii. The latter species, despite being primarily a low altitude bat (Lanza 2012; Dietz and Kiefer 2014), was recorded at both study sites, reaching the highest elevation so far ascertained in Italy at GSB (2208 m a.s.l.).
Nevertheless, at GSB and PSB, the activity referable to all the acoustic groups possibly comprising P. nathusii was limited, suggesting that if a migration flow of this species does exist at the two passes, it is either marginal or takes place later in the season, in periods that were not considered in the survey. At Col de Bretolet, P. nathusii mainly transits from the end of August to the end of September, and at Col de Jaman, the passage of this species, likely favored by the lower elevation, peaks about 1 month later and is much more intense (Oppliger 2004).
The presence of P. pygmaeus, rarely recorded in the Aosta Valley (Debernardi and Patriarca 2008; Patriarca and Debernardi 2014) and possibly a migratory bat, was not detected at PSB, while at GSB, some acoustic sequences probably emitted by individuals of this species were conservatively attributed to the group P. pipistrellus/M. schreibersii/P. pygmaeus.
Finally, concerning the species that are usually considered of stationary habits, it remains to be ascertained if the presence of B. barbastellus, recorded at both study sites, represents a case of ecological adaptation of this primarily forest species to open habitat types (as demonstrated for a coastal habitat in Central Italy: Ancillotto et al., 2012), or whether it is the result of migratory movements, which may pass through unfavorable habitat types, like sea areas. Widerin and Reiter (2017) recorded this species at two high elevation sites (2250 and 2315 m) in the Central Alps and one barbastelle was captured in the Orco Valley (Gran Paradiso National Park, Western Alps) in conditions similar to those recorded in this study with regard to the environment (open alpine habitat), the elevation (2292 m), and the period (middle of August) (Patriarca et al. 2018). According to the review by Hutterer et al. (2005), “barbastelles are sedentary bats but may occasionally migrate or disperse”.
The present conclusions were conditioned by the limited survey effort performed and the interruptions in recording at GSB. Getting a clearer comprehension of bat migration at the study sites would require more surveys, extending the recording period (weather conditions and accessibility allowing) to October, operating from more recording points for each site and (to take into account possible annual variations) for more years.
Enlarging the data set would allow to gain an increased understanding of the influence of weather variables on migration, and particularly the role of wind speed combined with wind direction, which can have important implications for bat migration (Shamoun-Baranes et al. 2007; Smith and McWilliams 2016, Hüppop and Hill 2016).
Nowadays, the possibility to record bat calls using bat detectors that operate in real-time and can be left unattended for several days gives the opportunity to collect useful information on bat migration in a non-invasive way, including at sites, such as high mountain areas, where direct (manual) surveys may prove difficult because of accessibility and environmental factors. The problem of the correct identification of many species through acoustic analysis is still unsolved (Russo and Voigt 2016); nevertheless, even adopting a very conservative approach as here—despite not being able to identify many species—it is possible to get useful information to improve the knowledge of bat migration.
References
Adams AM, Jantzen MK, Hamilton RM, Fenton MB (2012) Do you hear what I hear? Implications of detector selection for acoustic monitoring of bats. Methods Ecol Evol 3(6):992–998. https://doi.org/10.1111/j.2041-210X.2012.00244.x
Aellen V (1962) Le baguement des chauves-souris au col de Bretolet (Valais)
Ahlén I (1997) Migratory behaviour of bats at south Swedish coasts. Z Säugetierkd 62:375–380
Ahlén I, Bach L, Baagøe HJ, Pettersson J (2007) Bats and offshore wind turbines studied in southern Scandinavia. The Swedish Environmental Protection Agency, Stockholm. Report 5571. https://www.naturvardsverket.se/Documents/publikationer/620-5571-2.pdf
Alma PJ (1970) A study of the activity and behaviour of the winter moth, Operophtera brumata (L.) (Lep., Hydriomenidae). Entomol Mon Mag 105(1265/1267):258–265
Ancillotto L, Cistrone L, Jones G, Russo D (2012) Forest bats in forestless landscapes: the case of barbastelle bats in clay badlands. Hystrix, It. J. Mamm. (n.s.) Supp. 2012 (VIII Congr. It. Teriologia): 49
Ancillotto L, Russo D (2015) Reassessing the breeding range limits for two long-distance migratory vespertilionid bats, Pipistrellus nathusii and Nyctalus leisleri in the Italian Peninsula. Mammalia 79(2):245–248. https://doi.org/10.1515/mammalia-2014-0009
Arnett EB, Brown WK, Erickson WP, Fiedler JK, Hamilton BL, Henry TH, Jain A, Johnson GD, Kerns J, Koford RR, Nicholson CP, O’Connel TJ, Piorkowski MD, RDjr T (2008) Patterns of bat fatalities at wind energy facilities in North America. J Wildl Manag 72(1):61–78. https://doi.org/10.2193/2007-221
Baerwald EF, Barclay RMR (2011) Patterns of activity and fatality of migratory bats at a wind energy facility in Alberta. Canada J Wildl Manag 75:1103–1114. https://doi.org/10.1002/jwmg.147
Barataud M (2012) Ecologie acoustique des chiroptères d’Europe. Identification des espèces, étude de leurs habitats et comportements de chasse. Biotope Editions, Muséum national d’Histoire naturelle, Paris
Bocca M, Maffei G (1997) Gli uccelli della Valle d’Aosta. Indagine bibliografica e dati inediti: Ristampa con aggiornamento al 1997 e check-list degli uccelli valdostani. Regione Autonoma Valle d’Aosta, Servizio Tutela dell’Ambiente Naturale e delle Foreste
Bocca M, Bovio M, Passerin d’Entrèves P, Poggio L, Tutino S (2016) Natura 2000 in Valle d’Aosta. Regione Autonoma Valle d’Aosta
Bontadina F, Beck A, Dietrich A, Dobner M, Eicher C, Frey-Ehrenbold A, Krainer K, Loercher F, Maerki K, Mattei-Roesli M, Mixanig H, Plank M, Vorauer A, Wegleitner S, Widerin S, Wieser D, Wimmer B, Reiter G (2014) Massive bat migration across the Alps: implications for wind energy development. XIIIth European Bat Research Symposium, 1-5 September 2014, Sibenik, Croatia. Abstract 41. https://doi.org/10.13140/2.1.4560.2569
Cockbain AJ (1961) Low temperature thresholds for flight in Aphis fabae Scop. Entomol Exp Appl 4(3):211–219. https://doi.org/10.1111/j.1570-7458.1961.tb02136.x
Cryan PM, Brown AC (2007) Migration of bats past a remote island offers clues toward the problem of bat fatalities at wind turbines. Biol Conserv 139(1):1–11. https://doi.org/10.1016/j.biocon.2007.05.019
Dietz C, Kiefer A (2014) Die Fledermäuse Europas: kennen, bestimmen, schützen. Franckh-Kosmos Verlags-GmbH & Co, KG, Stuttgart
Debernardi P, Patriarca E (2008) Prima segnalazione di Myotis bechsteinii, Myotis daubentonii, Myotis nattereri, Nyctalus leisleri, Pipistrellus pygmaeus, Plecotus macrobullarise Tadarida teniotisin Valle d’Aosta. Aggiornamento dell’inventario dei chirotteri noti per la regione. Rev. Valdôtaine Hist. Nat (61-62):5-27
Dondini G, Rutkowski T, Vergari S, Wojtaszyn G (2012) Long distance migration of female Leisler’s bat (Nyctalus leisleri) from Italy to Poland. Hystrix, It. J. Mamm 23(2):95–96. https://doi.org/10.4404/hystrix-23.2-8602
Erickson JL, West SD (2002) The influence of regional climate and nightly weather conditions on activity patterns of insectivorous bats. Acta Chiropterol 4:17–24. https://doi.org/10.3161/001.004.0103
Erkert HG (1982) Ecological aspects of bat activity rhythms. In: Kunz TH (ed) Ecology of bats. Plenum Press, New York, pp 201–242
Fleming TH, Eby P (2003) Ecology of bat migration. In: Kunz TH, Fenton MB (eds) Bat ecology. Chicago & London (The University of Chicago Press), pp 156–208
Furmankiewicz J, Kucharska M (2009) Migration of bats along a large river valley in southwestern Poland. J Mammal 90(6):1310–1317. https://doi.org/10.1644/09-MAMM-S-099R1.1
Hachler M, Bloesch B, Mittaz C (2002) Migration des lépidoptères nocturnes: observations au col du Grand-Saint-Bernard. Revue Suisse d’Agriculture 3:137–144
Hardesty-Moore M, Deinet S, Freeman R, Titcomb GC, Dillon EM, Stears K, Klope M, Bui A, Orr D, Young HS, Miller-ter Kuile A, Hughey LF, McCauley DJ (2018) Migration in the Anthropocene: how collective navigation, environmental system and taxonomy shape the vulnerability of migratory species. Phil Trans R Soc B 373:20170017. https://doi.org/10.1098/rstb.2017.0017
Horn JW, Arnett EB, Kunz TH (2008) Behavioral responses of bats to operating wind turbines. J Wildlife Manag 72:123–132. https://doi.org/10.2193/2006-465
Hüppop O, Hill R (2016) Migration phenology and behaviour of bats at a research platform in the south-eastern North Sea. Lutra 59(1–2):5–22 http://www.zoogdierwinkel.nl/sites/default/files/imce/nieuwesite/Winkel/pdf%20download/Lutra%2059(1-2)_H%C3%BCppop%20%26%20Hill_2016.pdf
Hutterer R, Ivanova T, Meyer-Cords C, Rodrigues L (2005) Bat migrations in Europe. A review of banding data and literature. Naturschutz und Biologische Vielfalt, 28. BfN, federal Agency for Nature Conservation, Bonn
Jarzembowski T (2003) Migration of the Nathusius’ pipistrelle Pipistrellus nathusii (Vespertilionidae) along the Vistula Split. Acta Theriol 48(3):301–308
Jones G, Duvergé PL, Ransome RD (1995) Conservation biology of an endangered species: field studies of greater horseshoe bats. Symp Zool Soc Lond 67:309–324
Korner-Nievergelt F, Brinkmann R, Niermann I, Behr O (2013) Estimating bat and bird mortality occurring at wind energy turbines from covariates and carcass searches using mixture models. PLoS One 8(7):e67997. https://doi.org/10.1371/journal.pone.0067997
Lanza B (2012) Mammalia V. Chiroptera. Fauna d’Italia. Il Sole 24 Ore, Edagricole
Lewis T, Taylor LR (1965) Diurnal periodicity of flight by insects. Ecol Entomol 116(15):393–435
Mercalli L, Cat Berro D, Montuschi S, Castellano C, Ratti M, Di Napoli G, Mortara G, Guindani N (2003) Atlante climatico della Valle d’Aosta. Regione Autonoma Valle d’Aosta, Società Meteorologica Italiana onlus. Collana “Memorie dell’Atmosfera” 2
Middleton N, Froud A, French K (2014) Social calls of the bats of Britain and Ireland. Pelagic Publishing, Exeter
Miller BW (2001) A method for determining relative activity of free flying bats using a new activity index for acoustic monitoring. Acta Chiropterol 3(1):93–105
Niederfriniger O (2001) I pipistrelli in Alto Adige. Museo Scienze naturali Alto Adige, Bolzano
Oppliger J (2004) La migration des chiroptères aux cols de Jaman et de Bretolet. Bulletin de la Société des Enseignants Neuchâtelois de Sciences 28, Décembre 2004, Ethologie:1–27. http://www.sens-neuchatel.ch/bulletin/no28/art2.pdf
Patriarca E, Debernardi P (2014) A checklist of bats (Mammalia: chiroptera) of Aosta Valley (NW Italy). In: Imperio S, Mazzaracca S, Preatoni DG (eds) IX Congr. It. Teriologia. Hystrix, It. J. Mamm. 25 (Supplement):127
Patriarca E, Debernardi P, Garzoli L (2018) The bats of Gran Paradiso National Park: a preliminary characterization based on summer surveys. Journal of Mountain Ecology 11:1–58 http://www.mountainecology.org/index.php/me/article/download/205/175
Petersons G (2004) Seasonal migrations of north-eastern populations of Nathusius’ bat Pipistrellus nathusii (Chiroptera). Myotis 41(42):29–56
Pfalzer G (2002) Inter-und Intraspezifische Variabilität der Soziallaute Heimischer Fledermausarten (Chiroptera: Vespertilionidae). Mensch-und-Buch, Berlin
Pfalzer G, Kusch J (2003) Structure and variability of bat social calls: implications for specificity and individual recognition. J Zool, London 261:21–33
Pfalzer G (2007) Verwechslungsmöglichkeiten bei der akustischen Artbestimmung von Fledermäusen anhand ihrer Ortungs- und Sozialrufe. Nyctalus (N.F.). Berlin 12(1):3–14
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Racey PA, Speakman JR (1987) The energy costs of pregnancy and lactation in heterothermic bats. Symp Zool Soc Lond 57:107–125
Reynolds DS (2006) Monitoring the potential impact of a wind development site on bats in the northeast. J Wildl Manag 70:1219–1227. https://doi.org/10.2193/0022-541X(2006)70[1219:MTPIOA]2.0.CO;2
Russo D, Jones G (1999) The social calls of Kuhl’s pipistrelles Pipistrellus kuhlii (Kuhl, 1819): structure and variation (Chiroptera: Vespertilionidae). J Zool, London 249:476–481. https://doi.org/10.1111/j.1469-7998.1999.tb01219.x
Russo D, Jones G (2000) The two cryptic species of Pipistrellus pipistrellus (Chiroptera: Vespertilionidae) occur in Italy: evidence from echolocation and social calls. Mammalia 64:187–197. https://doi.org/10.1515/mamm.2000.64.2.187
Russo D, Voigt CC (2016) The use of automated identification of bat echolocation calls in acoustic monitoring: a cautionary note for a sound analysis. Ecol Indic 66:598–602. https://doi.org/10.1016/j.ecolind.2016.02.036
Rydell J, Ottvall R, Green M, Pettersson S (2017) The effects of wind power on birds and bats – an updated synthesis report 2017. Naturvårdsverket. Svedish EPA, report 6791
Shamoun-Baranes J, van Loon E, Liechti F, Bouten W (2007) Analyzing the effect of wind on flight: pitfalls and solutions. J Exp Biol 210(1):82–90. https://doi.org/10.1242/jeb.02612
Serra-Cobo J, Lopez-Roig M, Marques-Bonet T, Lahuerta E (2000) Rivers as possible landmarks in the orientation flight of Miniopterus schreibersii. Acta Theriol 45(3):347–352
Skiba R (2009) Europäische Fledermäuse–Kennzeichen, Echoortung und Detektoranwendung. 2, aktualisierte und erweiterte Auflage von 2009. VerlagsKG Wolf
Smith AD, McWilliams SR (2016) Bat activity during autumn relates to atmospheric conditions: implications for coastal wind energy development. J Mamma l97(6):1565–1577. https://doi.org/10.1093/jmammal/gyw116
Speakman JR, Rydell J, Webb PI, Hayes JP, Hays GC, Hulbert IAR, McDevitt RM (2000) Activity patterns of insectivorous bats and birds in northern Scandinavia (69 N), during continuous midsummer daylight. Oikos 88(1):75–86. https://doi.org/10.1034/j.1600-0706.2000.880109.x
Strelkov PP (1969) Migratory and stationary bats (Chiroptera) of the European part of the Soviet Union. Acta Zool Cracov 16:393–439
Taylor LR (1963) Analysis of the effects of temperature on insects in flight. J Anim Ecol 32:99–117
Thaxter CB, Buchanan GM, Carr J, Butchart SH, Newbold T, Green RE, Tobias JA, Foden WB, O’Brien S, Pearce-Higgins JW (2017) Bird and bat species’ global vulnerability to collision mortality at wind farms revealed through a trait-based assessment. Proc R Soc B Biol Sci 284:20170829. https://doi.org/10.1098/rspb.2017.0829
Venables WN, Ripley BD (2002) Modern applied statistics with S. Fourth Edition. Springer, New York. ISBN 0-387-95457-0
Vergari S, Dondini G (2011) Long-term monitoring of Nyctalus leisleri at an Italian mating site. Hystrix, It. J. Mamm. 22(1):93–98. https://doi.org/10.4404/hystrix-22.1-4476
Voigt CC, Lehnert LS, Petersons G, Adorf F, Bach L (2015) Wildlife and renewable energy: German politics cross migratory bats. Eur J Wildl Res 61:213–219. https://doi.org/10.1007/s10344-015-0903-y
Walter G, Matthes H, Joost M (2005) Fledermausnachweis bei Offshore-Untersuchungen im Bereich von Nord-und Ostsee. Natur-und Umweltschutz (Zeitschrift Mellumrat) 3(2):8–12
Walters CL, Freeman R, Collen A, Dietz C, Brock Fenton M, Jones G, Obrist MK, Puechmaille SJ, Sattler T, Siemers BM, Parsons S, Jones KE (2012) A continental-scale tool for acoustic identification of European bats. J Appl Ecol 49:1064–1074. https://doi.org/10.1111/j.1365-2664.2012.02182.x
Williams CB (1961) Studies in the effect of weather conditions on the activity and abundance of insect populations. Philos Trans R Soc 244:331–370
Zambelli N, Mattei-Roesli M, Moretti M (2008) Nottola di Leisler (Nyctalus leisleri, Chiroptera), regina delle selve castanili. Bollettino della Società Ticinese di Scienze Naturali 96:49–59
Widerin K, Reiter G (2017) Bat activity at high altitudes in the Central Alps, Europe. Acta Chiropterol 19(2):379–387
Zingg PE, Bontadina F (2016) Migrating bats cross top of Europe. PeerJ Preprints4:e2557v1 https://doi.org/10.7287/peerj.preprints.2557v1
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Gail M, Krickeberg K, Samet JM, Tsiatis A, Wong W (eds). New York, NY: Spring Science and Business Media
Acknowledgments
This work was carried out in the context of the chiropterological surveys funded by Regione Autonoma Valle d’Aosta – Assessorato Agricoltura e Risorse naturali – Servizio Aree protette. We are particularly grateful to S. Tutino, who actively promotes bat research and conservation in the region. The weather data were provided by F. Brunier (Regione Autonoma Valle d’Aosta - Centro funzionale regionale) and A. Ponti (Osservatorio meteorologico del Piccolo San Bernardo).
Logistic support was provided by Associazione “Jardin historique du Col du Petit St. Bernard – La Chanousia” and Associazione Onlus Gran San Bernardo (Lodi). We thank D. Chamberlain for his suggestions and language revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 766 kb)
Rights and permissions
About this article
Cite this article
Caprio, E., Patriarca, E. & Debernardi, P. Bat activity and evidence of bat migration at two high elevation passes in the Western Alps. Eur J Wildl Res 66, 63 (2020). https://doi.org/10.1007/s10344-020-01402-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-020-01402-0